Abstract
Background
Rituximab is a monoclonal antibody directed at CD20 positive B-lymphocytes and a potential therapeutic option in the treatment of multiple sclerosis. The safety of recurrent dosing is not established.
Objectives
The objective of this work was to report the experience of long-term rituximab administration in a comprehensive multiple sclerosis care clinic.
Methods
This was a single-center retrospective observational analysis of patients receiving rituximab for the treatment of multiple sclerosis from 2004 to 2015. Different dosing regimens were reviewed to determine whether frequency or dose may affect safety. CD19 and CD20 counts were collected to evaluate B-cell suppression during therapy. Relapses, magnetic resonance imaging activity and rituximab-related adverse events were collected by chart review and prospective database entry.
Results
Of 107 patients included, the average duration of treatment was 33.2 months. Seventy-seven patients received recurrent rituximab dosing after initiation. CD19/20 reconstitution occurred in approximately 20% of patients at 6 months, regardless of dosing strategy. Despite CD19/20 counts of 0, three patients had relapses or magnetic resonance imaging activity. Mostly mild side effects in relation to therapy were seen, with the exception of three patients requiring hospitalization for urinary tract infections.
Conclusions
In our clinic population, rituximab was well tolerated and safe with recurrent administration.
Keywords: Multiple sclerosis, rituximab, safety, long-term
Introduction
Evidence has accumulated suggesting that B cells are involved either directly or indirectly in the pathophysiology of multiple sclerosis (MS). Rituximab is a monoclonal antibody directed at CD20 positive B-lymphocytes resulting in cell-mediated apoptosis.1 A phase II double blinded randomized controlled trial of rituximab in a relapsing MS population demonstrated robust efficacy on clinical measures and magnetic resonance imaging (MRI) with an acceptable safety profile.2 Ocrelizumab was recently shown to be effective and safe in two phase III trials conducted in relapsing–remitting patients showing an annualized relapse rate reduction of 46/47%, 43/37% risk reduction in confirmed disability progression and 94/95% reduction in total number of gadolinium enhancing lesions in the OPERA I and II studies, respectively.3 Ofatumumab was also successful in phase II.4 These monoclonal antibodies all deplete CD20 B cells, but differ in their chimeric (rituximab), humanized (ocrelizumab), or fully human (ofatumumab) molecular composition as well as their ability to stimulate complement and antibody dependent cellular cytotoxic effector mechanisms.
The safety profile of recurrent long-term dosing is not yet established in an MS population. Rituximab has been used extensively in many patient populations after its approval in 1997 for non-Hodgkin’s lymphoma. Although overall patient exposure is unknown, over 300,000 patients with rheumatoid arthritis are known to have been treated with rituximab. A recent study demonstrated that rituximab was safe in a follow-up of a rheumatoid arthritis trial population of 1246 patients with over 5 years of recurrent exposure.5 The recommended dosing regimen of rituximab in rheumatoid arthritis patients is 1000 mg intravenous infusions separated by 2 weeks with subsequent re-dosing at 6 months.6 Although safety complications, including progressive multifocal leukoencephalopathy, have arisen, it is unclear how this applies to an MS patient population – a generally younger cohort with fewer co-morbidities than other rituximab treated groups. No significant safety signals have been evident in the phase II trials of rituximab, ocrelizumab and ofatumumab, although long-term follow-up data are lacking for these trials.2–4
In addition, no established dosing schedule exists for MS.7 In the rituximab phase II trial, patients were dosed with rituximab 1000 mg with a subsequent 1000 mg dose 15 days later and were followed for 48 weeks.2 No longer term follow-up of this cohort has been published. Some data do exist for neuro-myelitis optica, however. Pellkofer and colleagues reviewed rituximab experience for 10 neuromyelitis optica patients, and based on their results concluded that a fixed dosing schedule every 6–9 months was advisable.8 Greenberg and colleagues also retrospectively reviewed rituximab dosing in a neuromyelitis optica clinical cohort and concluded that patients should be re-dosed prior to evidence of B-cell reconstitution by CD19/20 counts, and also reported evidence of reconstitution in a small population of patients less than 6 months (17%) after a 1000 mg rituximab dose was administered.9 Kim et al. reported that measuring CD27+ memory B cells was a better measure of B-cell repopulation and that 83% of patients had evidence of reconstitution by 6 months.10 In other disease states like rheumatoid arthritis and myasthenia gravis there is also no consensus about the proper dosing schedule.11,12 In addition, Gottenberg et al. looked for evidence of a link between B-cell reconstitution and clinical events in rheumatoid arthritis and could find no relationship.13 The objective of this observational study is to evaluate the safety of long-term rituximab administration and review different dosing strategies at our comprehensive MS care clinic.
Methods
Patients
This was a single-center retrospective observational analysis of medical records and available comprehensive longitudinal investigation in MS database (CLIMB) information for patients treated with rituximab at a tertiary academic medical center between 2004 and 2015. Brigham and Women’s Hospital institutional review board approval was obtained prior to initiation of the study (protocol 1999-P-010435). We included patients for analysis if they were 18 years old or older and had a confirmed diagnosis of MS by 2001 McDonald’s criteria. Patients were excluded if the start date of rituximab therapy could not be determined or if they were treated with a concomitant disease modifying agent other than steroids.
Clinical assessment
Baseline demographic information, duration of treatment, relapse incidence, MRI data and CD19/CD20 laboratory results for all patients were collected and analyzed. Rituximab has been reported to deplete B cells from the circulation within 1 month after administration and sustain suppression for up to 8 months.14 We followed patients for 9 months after their last rituximab infusion or the end of the study period, whichever was sooner, resulting in a minimum duration of treatment of 9 months. Relapses were included if there was documentation of a relapse or exacerbation in the patient chart and/or the patient received at least three consecutive days of intravenous methylprednisolone therapy. Neuroradiologist MRI reports were reviewed to determine the presence of T2 or fluid-attenuated inversion recovery (FLAIR) change when compared to previous MRI. MRI scans were collected at the discretion of the provider and based on clinical need. All scans were acquired and interpreted at Brigham and Women’s Hospital. Over the duration of the study period, MRI scan acquisition varied as protocols continued to be improved. MRIs of the brain and/or spinal cord were included for analysis only if a previous baseline MRI had been performed while on rituximab. Both brain and spinal cord MRIs were included for analysis. In addition, the presence of gadolinium enhancing T1 lesions was collected to determine disease activity. No prior MRI was required if an enhancing lesion was identified. No quantitative analysis was performed due to the variability in the acquisitions of MRI. CD19 and CD20 laboratory results were collected per clinician discretion while the patient was on rituximab therapy. CD19 and CD20 counts were divided into three cohorts in accordance with previous reports of rituximab use monitoring strategies for autoimmune disorders such as neuromyelitis optica: CD19 and CD20 counts equal to 0 are <2%, or are >2%.15 The tolerability of long-term rituximab therapy and reports of adverse effects were determined by chart review.
Results
Patient characteristics
A total of 107 patients were identified as meeting our inclusion criteria for this study, representing 296 patient years. Baseline characteristics are summarized in Table 1. The timing of re-dosing of rituximab was at the discretion of the prescribing clinician. The majority of patients (90%) received a rituximab starting dose of 1000 mg at week 0 and a subsequent repeat 1000 mg dose approximately 14 days later. The remaining patients at initiation either received a single dose of rituximab 1000 mg (9%) or a single dose of rituximab 100 mg (1%). In total, 28% (30/107) of patients only received a starting dose of rituximab therapy with no subsequent administrations, resulting in 72.0% (77/107) of patients having received recurrent rituximab dosing. Subsequent doses were observed to have intra and inter-patient variability, ranging from 500 mg × one dose, 1000 mg × one dose, or 1000 mg × two doses 14 days apart. In total, 501 doses were administered (including the initial dosing regimen), with 446 of these doses 1000 mg (either at initiation or follow-up). Fifty-four follow-up doses were 500 mg, and a single 100 mg initiating dose was given.
Table 1.
Baseline characteristics.
| Baseline characteristics | % (n = 107) |
|---|---|
| Men | 25.2 (27/107) |
| Women | 74.8 (80/107) |
| Average age at the start of therapy in years (range) | 46 (20–76) |
| Ethnicity | |
| Caucasian | 85.0 (91/107) |
| African American | 11.2 (12/107) |
| Hispanic | 0.9 (1/107) |
| Not reported/other | 2.8 (3/107) |
| Duration of rituximab treatment (months)a | 33.2 ± 24.43 |
| Multiple sclerosis diagnosis | |
| Relapsing–remitting | 50.5 (54/107) |
| Secondary progressive | 34.5 (37/107) |
| Primary relapsing | 5.6 (6/107) |
| Primary progressive | 4.7 (5/107) |
| Unclassified | 4.7 (5/107) |
| RRMS patients baseline characteristics (n = 54) | |
| Average age at the start of therapy in years (range) | 42.2 (19–71) |
| Duration of rituximab treatment (months)a | 32.9 ± 25.0 |
| EDSS score at baselinea | 2.9 ± 1.6 |
| EDSS score at the end of treatmenta | 2.5 ± 1.8 |
Data presented as average ± standard deviation.
RRMS: relapsing–remitting multiple sclerosis; EDSS: Expanded Disability Status Scale.
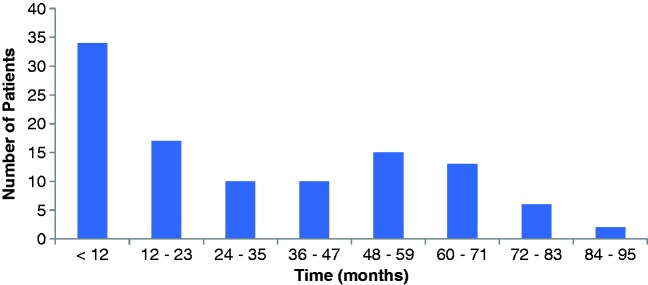
For those that received subsequent doses after initiation, the average duration of rituximab treatment was 43.0 months (range 9.4–86.0 months) and is described in Figure 1. Seventy-three patients (68.2%) were treated with rituximab for at least 12 months, with the remaining 31.8% receiving rituximab treatment for less than 1 year. The average interval between rituximab doses was 11 months (range 2.6–42.2 months). Approximately half, 48.7% (97/199), of rituximab doses were administered 6–9 months after the previous dose, with 10% administered between 10 and 12 months and 37% administered more than 12 months between doses.
Figure 1.
Duration of rituximab therapy.
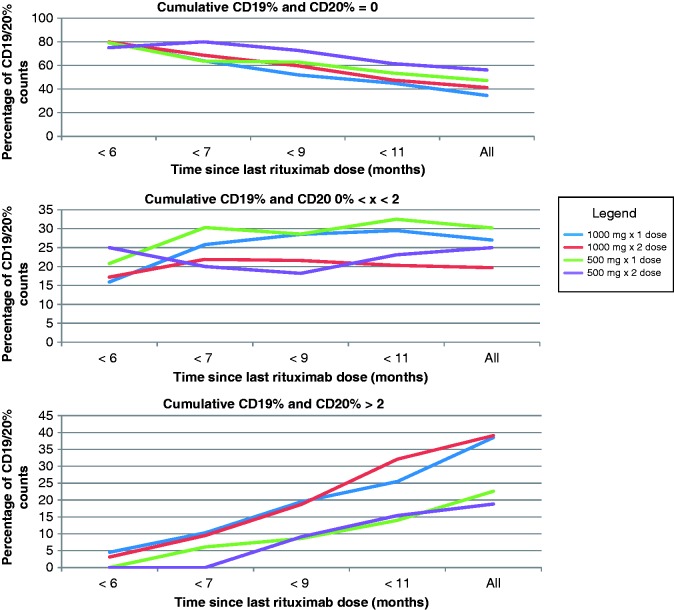
CD19 and CD20 reconstitution
Of 107 patients evaluated, 105 (98.1%) had at least one follow-up CD19 and CD20 count after the first rituximab administration. CD19 and CD20 follow-up levels occurred an average of 138.3 ± 121.4 days apart. CD19 and CD20 counts of patients who received 1000 mg with a recurrent 1000 mg dose 2 weeks later were above 0 by 6 months in 20% of patients, and 3% of patients had a CD19/20 percentage above 2%. Of patients receiving a single rituximab 1000 mg dose, 20% had a CD19/20 count above 0 by 6 months, and 5% above 2% by 6 months. Further details are available in Figure 2.
Figure 2.
Comparison of dosing strategies and CD19/CD20 reconstitution.
Adverse events
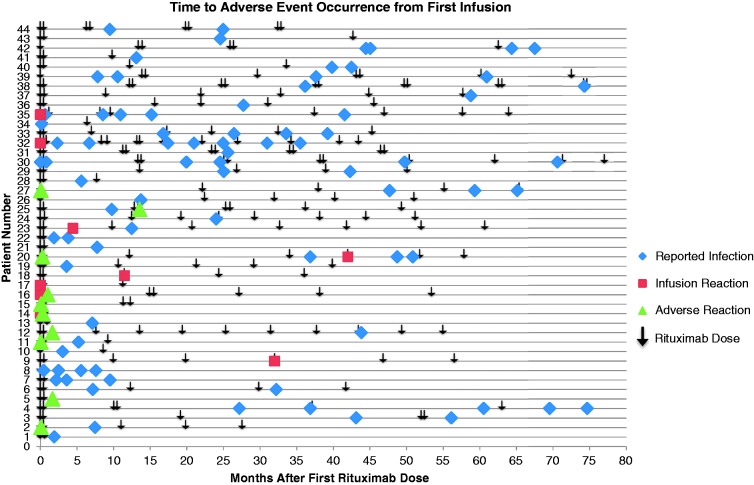
Rituximab therapy was well tolerated in our patient population, with 18% (19/107) of patients reporting mild side effects related to therapy. No clear relationship was seen between duration of therapy and adverse events (Figure 3). Infusion-related reactions despite pre-treatment prophylaxis occurred in 8% (8/107) of patients. No serious infusion reactions occurred. Infections were reported in 36% (38/107) of patients. The most common infections were urinary tract infections (13%) and upper respiratory tract infections (12%). Three patients diagnosed with a urinary tract infection required hospitalization. Patient 1 (baseline Expanded Disability Status Scale (EDSS) 6) was diagnosed with pyelonephritis approximately 43.8 months after starting rituximab treatment, and had last received a dose of rituximab 2 weeks prior to hospitalization. Patient 2 (baseline EDSS 4) had two hospitalizations related to recurrent urinary tract infections. The first hospitalization was for an infection limited to the urinary tract 49.8 months after initiation of therapy, while the second hospitalization was for urosepsis 70.6 months after initiation of therapy. Approximately 11 and 9 months had lapsed since the previous dose of rituximab and hospitalizations 1 and 2, respectively. The third patient (baseline EDSS 6) also required two hospitalizations for recurrent urinary tract infections, with the first being limited to the urinary tract and the second having progressed to urosepsis. The first hospitalization for this patient occurred 37.6 months after initiation of rituximab therapy, while the second occurred 61.0 months after initiation of therapy. Patient 3 had a history of recurrent urinary tract infections prior to starting rituximab treatment. The patient had last received rituximab approximately 8 months and 3 weeks prior to hospitalizations 1 and 2, respectively. For each of these patients CD19/20 counts were obtained during hospitalization and were 0. No malignancies were observed secondary to rituximab treatment.
Figure 3.
Time to adverse event occurrence from first infusion.
Relapses and MRI change
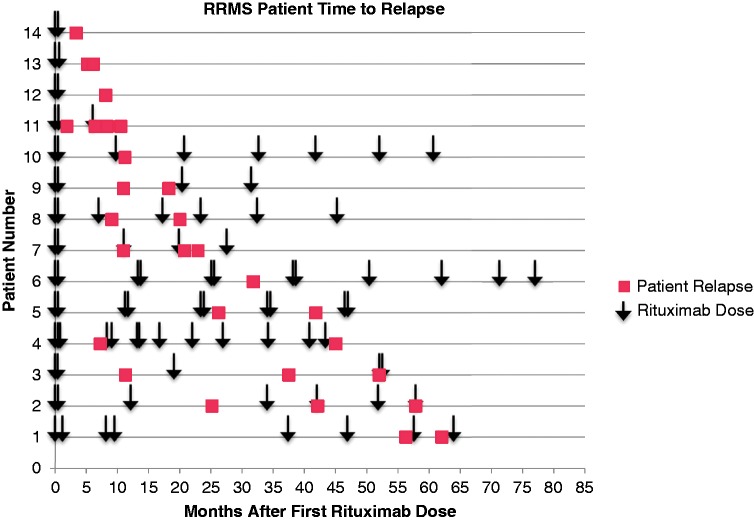
In the relapsing–remitting MS patients (n = 54), 29 relapses were reported among 14 patients after initiation of therapy. Of these, 83% of patients received intravenous methylprednisolone infusions after symptom report. In the relapsing–remitting MS patient population, patients on average received rituximab therapy every 10.9 ± 4.6 months (Figure 4). Of patients who relapsed, 55.2% of patients received a dose less than 6 months from their relapse, 20.7% 6–9 months from their relapse, and 24.1% were dosed over 9 months from when their relapse occurred. Fifteen CD19 and CD20 counts were collected within 30 days of patient relapse and 26.6% of counts were equal to 0, 20.0% 2 or less, and 53.3% of counts collected were over 2%. Seven relapses occurred with patients having CD19/20 counts less than 2%, four of these patients had CD19/20 counts of 0. Five patients without overt evidence of reconstitution (CD 19/20 < 2%) who experienced a relapse were continued on therapy. One of these five patients went on to another relapse during our study. Of interest, no enhancement was seen on T1 post-contrast imaging patients scanned within 30 days of relapse (n = 10), although three patients had MRIs with new T2/FLAIR change on subsequent MRI. However, enhancement may not have been observed because all patients received 3 days of intravenous methylprednisolone for the treatment of MS relapse prior to imaging.
Figure 4.
Relapsing–remitting multiple sclerosis patient relapse.
A total of 122 MRI scans in 43 patients were collected for the overall relapsing–remitting MS patient population, with a mean scan interval of 10 months (Supplemental Figure e2). There was heterogeneity in whether MRIs were obtained at regular intervals or in response to patient relapse. Approximately 3% (3/122) exhibited gadolinium enhancing T1 lesions and 11% (13/122) displayed T2/FLAIR lesion changes when compared to previous on-rituximab scan. Two patients with MRI showing enhancement on T1 sequences had CD19/20 drawn within 30 days. In both cases CD19/20 counts were 0. Of patients experiencing activity on MRI 76.9% received a dose less than 6 months prior to MRI change, 15.3% 6–9 months prior to MRI change, and 7.7% dosed over 9 months prior to MRI change. One patient only received an initial starting dose of rituximab.
An additional 61 MRI scans were performed in patients classified as having progressive MS. Of these, 3/61 (4.9%) scans showed MRI activity, taken from two patients. The first patient had three successive MRIs with new gadolinium enhancing T1 lesions and concurrent T2/FLAIR change, the second patient had one MRI with T2/FLAIR change and had a worsening disability over time.
Three patients experienced relapses and MRI activity, 11 patients with relapses only, and five with MRI activity only, so a total of 19 patients had clinical evidence of breakthrough disease.
Discussion
In our patient population rituximab was relatively well tolerated, although urinary tract infections were more frequently seen, similar to what was reported in the past with B-cell agent trials.2–5 Infections did not occur more frequently in patients who had been receiving rituximab for a longer period of time. Three patients were hospitalized due to infection. These patients had more disability, but the rituximab may have contributed given that CD19/20 counts were 0. No opportunistic infections were seen. It should be noted that CD19 and CD20 levels may not accurately estimate a patient’s risk for infection while on rituximab therapy. While IgG levels were not monitored in our patient population, low IgG levels before the initiation of rituximab treatment have been associated with a high risk of severe infection.13 Recurrent rituximab dosing has resulted in an increased risk of low IgG levels in patients in clinical trials and an increase in infections in this patient population, although non-significant.16,17
Based on the design of our study we are not able to reach a conclusion with regard to proper dosing frequency. In the absence of definitive trial data in this regard the best surrogate may be a study published by Mease et al. in rheumatoid arthritis.18 Investigators dosed patients with 1000 mg rituximab spaced 2 weeks apart and then randomly assigned them to either placebo or a follow-up 1 g dose at week 24. They found that patients with rheumatoid arthritis who were re-dosed at 24 weeks had a similar safety profile and better response than those dosed yearly, with patients that received a single dose beginning to show clinical breakthrough at 28 weeks.
We did find that a subpopulation of our patients (20%) experienced reconstitution of CD19/20 earlier than 6 months, similar to what has been reported in the neuromyelitis optica literature.15
Also as reported in the neuromyelitis optica cohorts, we have identified a population of patients who have either relapsed or experienced new MRI activity without evidence of reconstitution by CD19/20.8,9 Three possible explanations exist for these occurrences. The first is that some or all of these patients were ‘non-responders’ to rituximab. A second possibility is that they developed antibodies to rituximab. A third possibility is that peripheral CD19/20 measures are inadequate to capture what is occurring with B-cell populations in different tissues. A number of patients that could have been considered ‘non-responders’ due to relapse or MRI activity despite CD19/20 counts of 0 received further doses of rituximab without evidence of recurrent disease activity. It is possible that these patients have been not been followed long enough forward to conclude definitively that with recurrent rituximab they will remain therapeutic and that they are in fact ‘non-responders’ as previously suggested. It is also possible, however, that they have the potential to respond to rituximab and that CD19/20 counts in the periphery do not mark the beginnings of B-cell reconstitution. We know, for example, that B-cell depletion is less effective in lymphatic tissue and differs due to microenvironmental factors.19 Although many theories abound for how B-cell depleting agents have an effect in MS, the true mechanism is currently unclear. If one accepts that some of these patients were sub-therapeutic on rituximab despite having CD19/20 counts of 0 then one might conclude that re-dosing to keep patients at 0 may not be sufficient to be assured that the patient is adequately therapeutic. As such, a fixed dosing schedule similar to what has been done in rheumatoid arthritis may be advisable. Further strengthening this argument is what was seen in this real-life chart review. Many patients were non-compliant with follow-up blood draws so that the average patient in our group was re-dosed at 11 months, with many patients having CD19/20 counts above 2% when checked prior to re-infusion. Taken together we assert that given extant data the best approach would be to check CD19/20 counts monthly for the first 6 months after dosing in order to identify early reconstitution and prevent possible relapses and then re-dose 1000 mg regardless of the CD19/20 count at 6 months. This recommendation is similar to that given by investigators who have explored dosing in neuromyelitis optica.8,9 An even better approach may be to assay for CD27 + B memory cell re-emergence in peripheral blood mononuclear cells or to identify subpopulations at higher genetic risk for insufficient memory B-cell depletion, but these options are not currently clinically available.10,20
During this study, the timing of rituximab re-dosing was at the discretion of the treating neurologist and was not standardized. As a result, a wide variety of dosing frequencies was observed and a large proportion of patients were re-dosed more than 10 months apart. A standardized fixed dosing schedule would help avoid this scenario.
Limitations
This was a single-center retrospective chart and database review with a small sample size (73 patients were dosed over a year). We focused our study on safety and dosing strategy and did not collect treatment history prior to starting rituximab or rationale for use in particular patients. CD19/CD20 counts and MRIs were obtained at clinician discretion, and were not systematically collected. This may have introduced unmeasured confounders, which may have impacted our results. MRI scan information is helpful to assess disease activity. In our study it should be interpreted with caution given that the MRIs were collected based on clinical need and that scanners and protocols varied over time.
Conclusion
Long-term treatment with rituximab did not increase the risk of safety events in our cohort. A few patients with CD19/20 counts of 0 experienced relapse or new MRI events, but remained stable with subsequent retreatment. A subpopulation of patients experienced reconstitution as measured by CD19/20 counts prior to 6 months. Based on our clinical review and review of the literature we believe a monthly monitoring for early reconstitution followed by a fixed 1000 mg dose at 6 months may be the best strategy for long-term dosing.
Conflicts of interest.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: James Stankiewicz has received honorarium for past consuting from genentech, sanofi, biogen-idec, teva neuroscience, bayer, novartis, and EMD serono. Tanuja Chitnis has received consulting fees from Biogen-Idec, Sanofi Aventis, Novartis, and Alexion; and she has received grant support from Merch-Serono and Novartis for unrelated activities. Megan Barra and Dhruv Soni report no disclosures.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Petereit HF, Moeller-Hartmann W, Reske D, et al. Rituximab in a patient with multiple sclerosis – effect on B cells, plasma cells and intrathecal IgG synthesis. Acta Neurol Scand 2008; 117: 399–403. [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008; 358(7): 676–688. [DOI] [PubMed]
- 3.Genentech. Genentech’s Ocrelizumab First Investigational Medicine to Show Positive Pivotal Study Results in Both Relapsing and Primary Progressive Forms of Multiple Sclerosis.www.gene.com/media/press-releases/14609/2015-10-08/genentechs-ocrelizumab-first-investigati (2015, accessed 13 August 2016).
- 4.Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing–remitting multiple sclerosis: a phase 2 study. Neurology 2014; 82(7): 573–581. [DOI] [PubMed] [Google Scholar]
- 5.van Vollenhoven RF, Fleischmann RM, Furst DE, et al. Longterm safety of rituximab: final report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 years. J Rheumatol 2015; 42(10): 1761–1776. [DOI] [PubMed] [Google Scholar]
- 6.RITUXAN Rituximab injection [package insert]. South San Francisco, CA: Genentech USA, Inc., 2016.
- 7.Krumbholz M, Derfuss T, Hohlfeld R, et al. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 2012; 8(11): 613–623. [DOI] [PubMed] [Google Scholar]
- 8.Pellkofer HL, Krumbholz M, Berthele A, et al. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 2011; 76(15): 1310–1315. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler 2012; 18(7): 1022–1026. [DOI] [PubMed] [Google Scholar]
- 10.Kim SH, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011; 68: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 11.Dudler J, Finckh A, Kyburz D, et al. Swiss consensus statement: recommendations for optimising re-treatment with MabThera (rituximab) in rheumatoid arthritis. Swiss Med Wkly 2010; 140: w13073. [DOI] [PubMed] [Google Scholar]
- 12.Iorio R, Damato V, Alboini PE, et al. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J Neurol 2015; 262: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 13.Gottenberg JE, Ravaud P, Bardin T, et al. AutoImmunity and Rituximab registry and French Society of Rheumatology. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum 2010; 62: 2625–2632. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC. Invited article: inhibition of B cell functions: implications for neurology. Neurology 2008; 70: 2252–2260. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler 2012; 18: 1022–1026. [DOI] [PubMed] [Google Scholar]
- 16.Keystone E, Fleishmann R, Emergy P, et al. Safety and efficacy of additional courses of rituximab in patients with active rheumatoid arthritis: an open-label extension analysis. Arthritis Rheum 2007; 56: 3896–3908. [DOI] [PubMed] [Google Scholar]
- 17.Van Vollenhoven RF, Emergy R, Bingham CO, III, et al. Long term safety of rituximab: follow-up of the RA clinical trials and re-treatment population [abstract]. Ann Rheum Dis 2009; 68(Suppl 3): 76. [Google Scholar]
- 18.Mease PJ, Cohen S, Gaylis NB, et al. Efficacy and safety of retreatment in patients with rheumatoid arthritis with previous inadequate response to tumor necrosis factor inhibitors: results from the SUNRISE trial. J Rheumatol 2010; 37: 917–992. [DOI] [PubMed] [Google Scholar]
- 19.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol 2006; 24: 467–496. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Jeong IH, Hyun JW, et al. Treatment outcomes with rituximab in 100 patients with neuromyelitis optica: influence of FCGR3A polymorphisms on the therapeutic response to rituximab. JAMA Neurol 2015; 72: 989. [DOI] [PubMed] [Google Scholar]