Abstract
Background
Preclinical studies have demonstrated that MIS416, a bacterially derived immune modulator, targets myeloid cells following systemic delivery. MIS416 stimulated myeloid cells have the capacity to regulate innate inflammation, a potential therapeutic target for progressive multiple sclerosis.
Objectives
To determine the safety, tolerability, pharmacodynamics and maximum tolerated dose and/or recommended Phase 2 dose of MIS416.
Methods
An open-label, non-randomized, phase II, dose-escalation study, in patients with progressive multiple sclerosis: dose-escalation phase, with MIS416 administered once weekly for four weeks to determine maximum tolerated dose; and dose-confirmation phase, administered once weekly for up to 12 weeks.
Results
The safety profile indicates the majority of adverse events were mild or moderate, tolerable, self-limiting and consistent with the known bioactivity of MIS416 (acute flu-like symptoms). Maximum tolerated dose was not reached. A dose of 500 µg/week was recommended for the Phase 2 dose.
Conclusion
MIS416 is well tolerated at a dose of 500 µg/week. The adverse event profile is consistent with the mechanism of action of MIS416, indicating bioactivity within the signal transduction pathways and supported by induction of a known MIS416 pharmacodynamic marker. It is recommended that safety and efficacy of MIS416 is investigated further in a larger randomized controlled trial.
http://clinicaltrials.gov reference NCT01191996
Keywords: Multiple sclerosis, MIS416, immune modulator, myeloid cells, safety, pharmacodynamic, pattern recognition receptor, PRR, NOD-2, TLR-9
Introduction
Over the past two decades a number of novel and disease-modifying therapies for relapsing remitting multiple sclerosis (RRMS) have become available.1,2 Evaluation of these same agents in patients with chronic progressive forms of MS has failed to yield therapeutic benefits.3
It is increasingly recognized that multiple sclerosis (MS) has both autoimmune and inflammatory components, and that progressive forms of disease may reflect a shift towards innate inflammation being a significant cause of continuing neurodegeneration.4 Accordingly, novel strategies that can target innate inflammation may be therapeutically relevant. There are a broad spectrum of myeloid cell associated anti-inflammatory activities that are critical to immune homeostasis as well as stimulating wound healing/tissue repair pathways that are established following injury or infection.5–7 An increased understanding of the molecular cues that regulate these activities provides novel opportunities to exploit myeloid-directed immune regulation.8
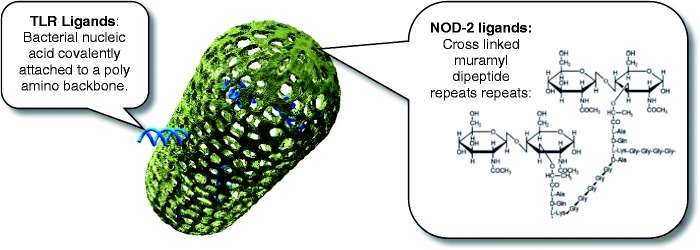
MIS416 is a myeloid targeted therapy, consisting of a micro-particulate formulation of gram positive Propionibacterium acnes bacteria, which have been biochemically modified to retain the bacterial DNA within the bacterial cell wall skeleton as a source of naturally occurring toll-like receptor-9 (TLR-9) and nucleotide oligomerization domain-2 (NOD-2) immune stimulatory ligands9 (see Figure 1). TLR-9 and NOD-2 are pattern recognition receptor (PRR) ligands fundamental to the recognition of, and host response to, molecules associated with infectious agents. Activation of NOD-2 and TLR-9 induces the expression of a variety of genes involved in immune responses associated with both the inflammatory as well as resolution phases of immune responses, and NOD-2 signaling in particular has been implicated as a homeostatic regulator of pro-inflammatory signaling pathways.10,11 NOD-2 and TLR9 signaling have also been implicated in the regulation of MS, and consistent with this observation, systemic MIS416 therapy in mouse models of MS has been shown to be effective.12 This effectiveness correlates with altered peripheral myeloid subset expansion as well as increased myeloid IL-10 secretion and expansion of regulatory T cells (Tregs) within the systemic immune system. Together these data suggest that MIS416 has the capacity to upregulate myeloid directed anti-inflammatory activities.
Figure 1.
Graphical representation of MIS416 which comprises 0.5 × 2.0 micron rod-shaped particles of bacterial cell wall skeleton containing muramyl dipeptide (L-alanine D-isoglutamine dipeptide), as part of the amino chain that crosslinks the peptidoglycan sugar backbone.
Based on the important unmet medical need for patients with secondary progressive MS (SPMS) and primary progressive MS (PPMS), coupled with the novel immunomodulatory and anti-inflammatory mechanisms of MIS-416,12 it is hypothesized that MIS-416 may provide a unique therapeutic approach to this population.
Prior to this study, MIS416 had been used as an unapproved experimental medicine (‘compassionate use’), under sections 25 and 29 of the New Zealand Medicine’s Act, 1981, in a small number of patients, with partly inflammatory medical conditions, including SPMS, PPMS, and Alzheimer’s disease. This experience formed the basis for the clinical development of MIS416 as an immune modulator for progressive MS and helped establish a desirable dose range of 2–14 µg/kg based on induction of a transient pro-inflammatory response as evidenced by development of acute flu-like symptoms.
Materials and method
Patients
A total of 34 patients (20 females) 18 years or older were enrolled, 19 in the dose escalation (DE) and 15 in the dose confirmation (DC) phase. All had a diagnosis of MS (McDonald’s criteria13), either PPMS or SPMS,14 evidence of worsening clinical status over the previous two years, and Expanded Disability Status Scale (EDSS) of 2.5–7.0 at screening. Enrollment in the DC phase was limited to patients with SPMS to support a planned Phase II trial in this more homogeneous population. All patients provided informed consent prior to screening. Exclusion criteria included receipt of any immunomodulatory therapy in the previous six months or vaccine/systemic corticosteroid in the previous 60 days, other diseases that could confound the diagnosis or evaluation of MS and previous exposure to MIS416.
Study design
This was an open-label, non-randomized, multiple ascending dose study, conducted in two phases: a DE phase, to evaluate the safety, tolerability, maximum tolerated dose (MTD) and pharmacodynamics (PD) of MIS416 administered intravenously (IV) once weekly for four doses (Cycle 1), and a DC phase, with cohort expansion at or below the MTD (i.e., the recommended phase 2 dose (RP2D)) of MIS416, dosed once weekly for up to 12 doses (Cycles 1–3). An adaptive ‘3 + 3’ study design15 was employed with the first cohort of three patients receiving drug at the starting dose (125 µg/week). The safety profile of the DE phase was intended to inform the dose going into the DC phase (RP2D).
Dose limiting toxicity (DLT) was defined as an adverse event (AE) of Grade 3 or 4, assessed as at least possibly related to the study drug and unrelated to disease progression, concurrent illness, or concomitant medications. Toxicity was graded according to the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventative Vaccine Clinical Trials,16 modified to include a systemic line item for rigors/chills and an amendment to the classification of pyrexia for applicability to the expected AE profile associated with MIS416.
The study was conducted at a single site, Primorus Clinical Trials Ltd, Christchurch, New Zealand, in accordance with the Declaration of Helsinki,17 and was approved by the Upper South A Health and Disability Ethics Committee (URA/10/01/011), Christchurch, New Zealand. Written informed consent was obtained from all study participants prior to the conduct of any trial procedures.
All patients underwent brain magnetic resonance imaging (MRI) at baseline and end of study for purposes of assessing safety. Additional safety assessments during the DC phase included ophthalmologic and echocardiographic examinations. Ophthalmologic examination was incorporated, based on an observation of leucocyte infiltration in the eye in a newly reported pre-clinical chronic toxicity study.18 Echocardiographic examination was introduced as a screening procedure, following exposure of one subject in cohort DE4, with previously undiagnosed hypertrophic obstructive cardiomyopathy, who experienced an SAE of symptomatic ST depression. Clinical status was assessed before, during and after the DC phase with repeat EDSS, Multiple Sclerosis Functional Composite (MSFC), Fatigue Severity Scale (FSS) and Short Form Health Survey–36 questions (SF-36). Repeated blood samples for exploratory immunological testing were taken from all patients.
Safety and pharmacodynamic immune marker analysis
Heparin anti-coagulated peripheral blood from the 15 patients in the DC phase was processed to obtain plasma that was frozen and stored at −80℃ until analysis. Plasma cytokines associated with inflammatory activity were measured for safety (TNFα, IL-1β, IL-12p70, IL-2, IL-17A) and neopterin was measured as a MIS416-inducible pharmacodynamic marker. All analytes were quantified using flow cytometry fluorescent bead-based multiplex assay (Becton Dickinson CBA™) according to the manufacturer’s instructions.
Statistical analysis
There was no formal hypothesis testing in this study and the sample size was dependent upon the observed safety profile within the adaptive ‘3 + 3’ clinical trial design. Summary statistics are presented for the Safety Evaluable (SE) and Clinical Activity (CA) populations. All statistical analyses were performed using SPSS version 19. The analyses of safety endpoints were conducted on the SE population. Statistical tests for safety were not performed. Analyses of clinical status endpoints were conducted on the CA population. The secondary outcome summary statistics were presented for baseline measurements and changes from baseline at each dose level for the MSFC score (and three component sub-scores), the FSS score, the SF-36 PCS and MCS scores (and eight sub-scales), the EDSS score and clinical exacerbations.
The validated QualityMetric software was used to transform SF-36v2 data into t-scores. t-scores were standardized using the means and standard deviations from the 1998 and the 2009 US general population. MSFC data were presented as raw scores and also transformed into z-scores using baseline visit data from all patients in a particular study cohort.
Pharmacodynamic analysis of safety-related immune soluble proteins was conducted. Absolute amounts of analyte were compiled in relation to the MIS416 dose level and the time point (24 hours and seven days) post each dose. For many of the analytes there was either a measurable baseline level, or the level was below the lower limit of sensitivity for the assay. Analytes were therefore compared based on absolute values as opposed to being normalized relative to baseline values.
Results
Demographics
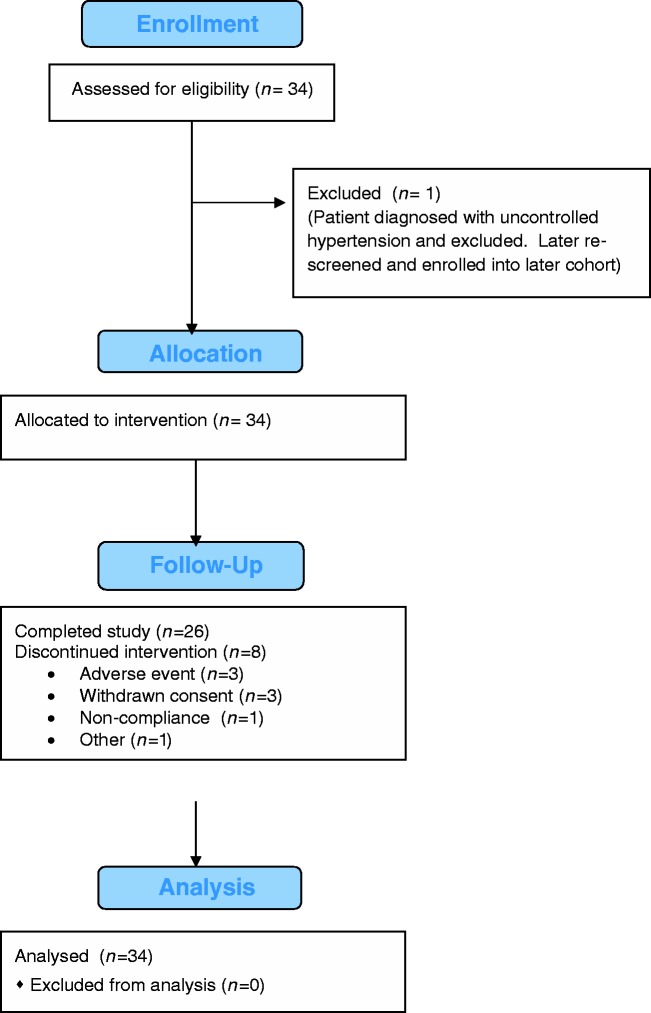
A total of 34 patients were enrolled in this study, with 19 in the DE phase and 15 in the DC phase. Patient disposition is summarized in Figure 2.
Figure 2.
CONSORT 2010 flow diagram.
The dose groups were demographically similar to each other in terms of age (overall mean 52.1 years), weight (mean 71 kg), BMI (mean 24.8 kg/m2) and EDSS scores (mean 6.1). Twenty (58.8%) patients were female. The mean duration of MS from diagnosis was 11 years (Table 1). In the DE phase, 15 patients (79%) had a diagnosis of PPMS. All 15 patients in the DC phase had a diagnosis of SPMS (Table 2). No patients were receiving disease modifying therapy at the time of enrolment.
Table 1.
Demographic statistical summaries.
| Total population | |
|---|---|
| N | 34 |
| Age | |
| Mean | 52.1 |
| Range | 36–69 |
| BMI | |
| Mean | 24.8 |
| Range | 16.5–37.7 |
| Baseline EDSS | |
| Mean | 6.0 |
| Median | 6.0 |
| Range | 4.0–7.0 |
| Sex | |
| Female | 20 |
| Male | 14 |
| Race | |
| Native Hawaiian/Other Pacific Islander | 4 |
| White | 30 |
| Diagnosis | |
| PPMS | 15 (44%) |
| SPMS | 19 (56%) |
PPMS: primary progressive MS; SPMS: secondary progressive MS.
Table 2.
Individual weekly infusions per patient and cumulative dose (µg) – DE and DC phases.
| Doses (µg/week) administered |
|||||||
|---|---|---|---|---|---|---|---|
| Cohort (N) Subject No. | Diagnosis | 125 | 250 | 375 | 500 | 600 | Cumulative dose (µg) (Total number of doses) |
| DE1 (3) | |||||||
| DE101 | SPMS | 4 | 0 | 0 | 0 | 0 | 500 (4) |
| DE102 | PPMS | 4 | 0 | 0 | 0 | 0 | 500 (4) |
| DE103 | PPMS | 4 | 0 | 0 | 0 | 0 | 500 (4) |
| DE2 (7) | |||||||
| DE201 | PPMS | 0 | 1 | 0 | 0 | 0 | 250 (1) |
| DE202 | PPMS | 0 | 2 | 0 | 0 | 0 | 500 (2) |
| DE203 | PPMS | 0 | 4 | 0 | 0 | 0 | 1000 (4) |
| DE204 | PPMS | 0 | 4 | 0 | 0 | 0 | 1000 (4) |
| DE206 | PPMS | 0 | 4 | 0 | 0 | 0 | 1000 (4) |
| DE207 | PPMS | 0 | 4 | 0 | 0 | 0 | 1000 (4) |
| DE208 | PPMS | 0 | 3 | 0 | 0 | 0 | 750 (3) |
| DE3 (3) | |||||||
| DE301 | PPMS | 0 | 0 | 4 | 0 | 0 | 1500 (4) |
| DE302 | PPMS | 0 | 0 | 4 | 0 | 0 | 1500 (4) |
| DE303 | SPMS | 0 | 0 | 4 | 0 | 0 | 1500 (4) |
| DE4 (6) | |||||||
| DE401 | PPMS | 0 | 0 | 0 | 0 | 4 | 2400 (4) |
| DE402 | PPMS | 0 | 0 | 0 | 0 | 4 | 2400 (4) |
| DE403 | PPMS | 0 | 0 | 0 | 0 | 4 | 2400 (4) |
| DE404 | SPMS | 0 | 0 | 0 | 4 | 0 | 2000 (4) |
| DE405 | PPMS | 0 | 0 | 0 | 4 | 0 | 2000 (4) |
| DE406 | SPMS | 0 | 0 | 0 | 4 | 0 | 2000 (4) |
| DC (15) | |||||||
| DC01 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC02 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC03 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC04 | SPMS | 1 | 1 | 0 | 5 | 0 | 2875 (7) |
| DC05 | SPMS | 2 | 2 | 0 | 8 | 0 | 4750 (12) |
| DC06 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC07 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC08 | SPMS | 1 | 1 | 0 | 0 | 0 | 375 (2) |
| DC09 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC10 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC11 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC12 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC13 | SPMS | 1 | 1 | 0 | 1 | 0 | 875 (3) |
| DC14 | SPMS | 1 | 1 | 0 | 10 | 0 | 5375 (12) |
| DC15 | SPMS | 1 | 1 | 0 | 0 | 0 | 375 (2) |
PPMS: primary progressive MS; SPMS: secondary progressive MS.
Dosing in the DE phase commenced at 125 µg/week (DE1). The cumulative dose per patient in cycle 1 ranged from 250 µg to 2400 µg. Dose escalation into DE3 was reduced (to 375 µg/week) following a single DLT of pyrexia in DE2. Following review of safety data, the Safety Review Team recommended dose escalation into DE4 at 600 µg/week. This cohort was further expanded with dosing in the three additional patients at 500 µg/week. MTD was not reached, but a RP2D of 500 µg/week was determined, with a further modification to up-titrate to RP2D over the first three weeks in the DC phase. Patients in the DC phase received between two and 12 weekly infusions each. The cumulative dose per patient in cycles 1–3 ranged from 375 µg to 5375 µg. The number of infusions and total cumulative dose (µg) is shown in Table 2.
Safety
Analysis of safety was conducted on the SE population (n = 34). A total of 34 (100%) patients reported Treatment Emergent Adverse Events (TEAEs). At least one of the TEAEs in each of 33 (97%) patients was considered by the investigator to be related to study drug (i.e., possibly, or probably related). Of a total of 665 TEAEs, 655 (98.5%) were mild to moderate in intensity.
The most frequent TEAEs (those with an incidence of ≥20%) were pyrexia (28 patients (82.4%)), headache (27 (79.4%)), fatigue (19 (55.9%)), muscular weakness (15 (44.1%)), tachypnea (11 (32.4%)), muscle spasms (9 (26.5%)) and dizziness (9 (26.5%)). These occurred at similar rates across all cohorts with the exception of muscle spasms, which occurred more frequently in higher dose cohorts (DE4 (500–600 µg/week) and DC (500 µg/week)), and tachypnea, which was not observed in the DC group. The severity and frequency of AEs showed significant inter-subject variation. Table 3 provides the profile of AEs occurring at an incidence of >20% in any group.
Table 3.
Frequency of adverse events >20% by preferred term and system organ class.
| DE1 |
DE2 |
DE3 |
DE4 |
DC |
Overall |
|
|---|---|---|---|---|---|---|
| SOC | n = 3 | n = 7 | n = 3 | n = 6 | n = 15 | n = 34 |
| Dose (µg/week) | 125 | 250 | 375 | 500, 600 | 125–500 | 125–600 |
| Cardiac disorders | 0 (0%) | 0 (0%) | 1 (33.3%) | 5 (83.3%) | 1 (6.7%) | 7 (20.6%) |
| – Palpitations | 0 (0%) | 0 (0%) | 0 (0%) | 4 (66.7%) | 1 (6.7%) | 5 (14.7%) |
| – Tachycardia | 0 (0%) | 0 (0%) | 1 (33.3%) | 3 (50%) | 0 (0%) | 4 (11.7%) |
| Gastrointestinal disorders | 0 (0%) | 3 (42.9%) | 0 (0%) | 5 (83.3%) | 6 (40%) | 14 (41.2%) |
| – Nausea | 0 (0%) | 3 (42.9%) | 0 (0%) | 0 (0%) | 4 (26.7%) | 7 (20.6%) |
| – Vomiting | 0 (0%) | 1 (14.3%) | 0 (0%) | 2 (33.3%) | 0 (0%) | 3 (8.8%) |
| General disorders and administration site conditions | 2 (66.7%) | 7 (100%) | 3 (100%) | 6 (100%) | 15 (100%) | 33 (97.1%) |
| – Asthenia | 0 (0%) | 1 (14.3%) | 2 (66.7%) | 1 (16.7%) | 1 (6.7%) | 5 (14.7%) |
| – Chills | 0 (0%) | 2 (28.6%) | 0 (0%) | 2 (33.3%) | 4 (26.7%) | 8 (17.6%) |
| – Fatigue | 1 (33.3%) | 6 (85.7%) | 2 (66.7%) | 3 (50%) | 7 (46.7%) | 19 (55.9%) |
| – Hunger | 0 (0%) | 1 (14.3%) | 1 (33.3%) | 0 (0%) | 0 (0%) | 2 (5.9%) |
| – Lethargy | 1 (33.3%) | 1 (14.3%) | 0 (0%) | 1 (16.7%) | 3 (20%) | 6 (17.7%) |
| – Oedema peripheral | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33.3%) | 1 (6.7%) | 3 (8.9%) |
| – Pyrexia | 0 (0%) | 4 (57.1%) | 3 (100%) | 6 (100%) | 15 (100%) | 28 (82.4%) |
| Infections and infestations | 0 (0%) | 0 (0%) | 1 (33.3%) | 4 (66.7%) | 3 (20%) | 8 (20.6%) |
| Injury, poisoning and procedural complications | 0 (0%) | 1 (14.3%) | 0 (0%) | 1 (16.7%) | 4 (26.7%) | 6 (17.7%) |
| Investigations | 0 (0%) | 4 (57.1%) | 0 (0%) | 5 (83.3%) | 8 (53.3%) | 17 (50%) |
| – Alanine aminotransferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) | 5 (33.3%) | 8 (23.5%) |
| – Aspartate aminotransferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33.3%) | 4 (26.7%) | 6 (17.7%) |
| – Blood pressure diastolic increased | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) | 0 (0%) | 3 (8.9%) |
| – Body temperature increased | 0 (0%) | 3 (42.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (8.9%) |
| – Gamma-glutamyltransferase increased | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) | 5 (33.3%) | 8 (23.5%) |
| Musculoskeletal and connective tissue disorders | 0 (0%) | 4 (57.1%) | 2 (66.7%) | 5 (83.3%) | 13 (86.7%) | 24 (64.7%) |
| – Muscle spasms | 0 (0%) | 1 (14.3%) | 0 (0%) | 2 (33.3%) | 6 (40%) | 9 (26.5%) |
| – Musculoskeletal stiffness | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 5 (33.3%) | 6 (17.6%) |
| – Myalgia | 0 (0%) | 1 (14.3%) | 1 (33.3%) | 0 (0%) | 6 (40%) | 8 (23.5%) |
| Nervous system disorders | 1 (33.3%) | 7 (100%) | 2 (66.7%) | 6 (100%) | 14 (93.3%) | 30 (88.2%) |
| – Headache | 1 (33.3%) | 5 (71.4%) | 1 (33.3%) | 6 (100%) | 14 (93.3%) | 27 (79.4%) |
| – Muscular weakness | 0 (0%) | 4 (57.1%) | 0 (0%) | 4 (66.7%) | 7 (46.7%) | 15 (44.1%) |
| – Vision blurred | 0 (0%) | 0 (0%) | 0 (0%) | 2 (33.3%) | 0 (0%) | 2 (5.9%) |
| Renal and urinary disorders | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 2 (13.3%) | 3 (8.9%) |
| – Pollakiuria | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 1 (6.7%) | 2 (5.9%) |
| Respiratory, thoracic and mediastinal disorders | 0 (0%) | 5 (71.4%) | 2 (66.7%) | 5 (83.3%) | 3 (20%) | 15 (44.1%) |
| – Tachypnoea | 0 (0%) | 4 (57.1%) | 2 (66.7%) | 5 (83.3%) | 0 (0%) | 11 (32.4%) |
| Skin and subcutaneous tissue disorders | 0 (0%) | 3 (42.9%) | 0 (0%) | 2 (33.3%) | 4 (26.7%) | 9 (26.5%) |
| Vascular disorders | 2 (66.7%) | 2 (28.6%) | 1 (33.3%) | 5 (83.3%) | 8 (53.3%) | 18 (52.5%) |
| – Diastolic hypertension | 1 (33.3%) | 1 (14.3%) | 0 (0%) | 3 (50%) | 0 (0%) | 5 (14.7%) |
| – Dizziness | 1 (33.3%) | 2 (28.6%) | 0 (0%) | 2 (33.3%) | 4 (26.7%) | 9 (26.5%) |
| – Flushing | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 2 (13.3%) | 3 (8.9%) |
| – Hypertension | 0 (0%) | 0 (0%) | 0 (0%) | 3 (50%) | 0 (0%) | 3 (8.9%) |
| – Systolic hypertension | 0 (0%) | 1 (14.3%) | 0 (0%) | 4 (66.7%) | 0 (0%) | 5 (14.7%) |
To note, AEs did not appear with significantly increased frequency in the DC group alone, suggesting that in any given individual, the AE profile of MIS416 will be defined within the first month of treatment and there did not appear to be any cumulative toxicity. Also, the incidence of AEs in the DC group (500 µg/week) was appreciably lower than in the DE4 group (500–600 µg/week), notably in several important system organ classes, namely Cardiac, Gastrointestinal, Investigations and Vascular (and in particular, a reduction in the incidence of increased blood pressure and hypertension).
No deaths were reported. A total of three Serious Adverse Events (SAEs) were reported. These included electrocardiogram change (n = 2) and muscular weakness (n = 1), all possibly related to MIS416. The electrocardiogram changes included ST depression in patients with pre-existing cardiac conditions (one with ischaemic heart disease and the other with previously undiagnosed hypertrophic obstructive cardiomyopathy with systolic anterior motion). Neither were associated with elevation in Troponin I levels. A retrospective review of all ECGs was conducted by an independent cardiologist who reported that a number of non-significant ST changes occurring in several patients were possibly related to post-dose fever and associated tachycardia. No other clinically significant changes were observed.
No relevant concomitant medications were reported during the course of the study.
Review of safety laboratory results and vital signs failed to detect any safety signal. The most common laboratory abnormalities included GGT increase (eight patients (38.1%)), ALT increase (eight patients (38.1%)), and AST increase (six patients (28.6%)). Transaminase elevations were most often noted early in the treatment period, were asymptomatic and generally abated despite continued treatment. No cases meeting the criteria of Hy’s Law were observed.19
Baseline MRI brain scans showed multiple white matter lesions typical for MS in all patients. The majority of patients (n = 22) exhibited no gadolinium lesion enhancement at either baseline or in follow-up and no change in the number or character of white matter lesions. Two patients demonstrated new enhancing lesions at follow-up that were not apparent at baseline. Reassuringly however, in four patients enhancing lesions at baseline either no longer enhanced or showed reduced enhancement at follow-up. Thus there was no evidence from this study that repeated MIS416 infusions exacerbate existing or induce new MS-related inflammatory lesions in the brain.
No significant ophthalmologic changes from baseline to end of study were observed in any patients in the DC phase.
Pharmacodynamics
Statistical analysis of plasma PD biomarkers was conducted on the ITT population. A summary of the findings for safety related inflammatory biomarkers sampled in the DC phase is shown in Table 4.
Table 4.
Summary of mean (range) changes detected in the amounts of TNF-α, IL-1β, IL-17A, IL-2 and IL-12p70 detected in peripheral blood plasma samples at baseline, 24 hours post MIS416 dosing and seven days post MIS416 dosing, for four doses (1 dose/week for a four-week cycle – DC phase) (n = 15; dose = 500 µg/week). Longer term samples were obtained on seven days post dose 7 and 11. Values below 20 pg/mL are below the lower limit of sensitivity of the assay.
| Safety cytokines (pg/mL) |
PD marker (pg/mL) |
|||||
|---|---|---|---|---|---|---|
| Sample timepoint | TNF-α | IL-1β | IL-12p70 | IL-17A | IL-2 | Neopterin |
| Baseline |
1.75 (0–4.37) | 0 | 0 | 0 | 0 (0–60.5) | 1240 (617–3604) |
| 24 hr post dose 1 |
0 (0–3.6) | 0 | 0 (0–19.6) | 0 | 0 (0–55.2) | 2072 (1476–4026) |
| 7 days post dose 1 |
0.96 (0–4.2) | 0 | 0 | 0 | 0 (0–49.0) | 1819 (942–4655) |
| 24 hr post dose 2 |
2.18 (0–3.9) | 0 | 0 | 0 | 0 (0–30.6) | 3377 (1520–4574) |
| 7 days post dose 2 |
0 (0–3.4) | 0 | 0 | 0 | 0 (0–33.0) | 1276 (810–2554) |
| 24 hr post dose 3 |
2.98 (0–5.6) | 0 | 0 (0–20.5) | 0 | 0 (0–45,7) | 3203 (1581–4294) |
| 7 days post dose 3 |
1.49 (0–3.4) | 0 | 0 | 0 | 0 (0–27.9) | 1546 (1032–3342) |
| 24 hr post dose 4 |
1.94 (0–3.3) | 0 (0–0.9) | 0 | 0 | 0 (0–34.6) | 2641 (1844–5237) |
| 7 days post dose 7 |
2.16 (0–3.8) | 0 | 0 | 0 | 0 (0–27.2) | 1270 (846–1960) |
| 7 days post dose 11 | 1.01 (0–3.9) | 0 (0–1.61) | 0 | 0 | 0 (0–46.9) | 1281 (598–2007) |
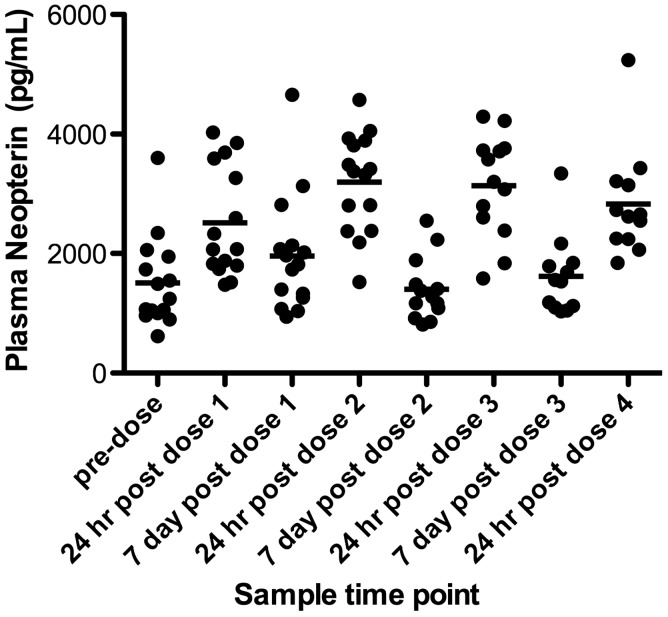
Notably, none of the patients showed a significant baseline, or MIS416-induced level of plasma TNF-α, IL-1β and IL-12p70, indicating the absence of overt systemic inflammation. Cytokines associated with T cell activity such as IL-2 and IL-17A were also not induced above pre-treatment levels by MIS416 treatment. Neopterin, an interferon gamma inducible protein associated with MIS416 proximal signaling pathways was transiently upregulated at 24 hrs after each dose of MIS416 (Figure 3), confirming that the dose of MIS416 was sufficient to stimulate MIS416-dependent pathways.
Figure 3.
Plasma from blood samples collected at 24 hours and seven days post MIS416 dosing from each patient was assayed for neopterin using flow cytometry bead-based ELISA technology. The data shown are the mean values (pg/mL) + SD (n = 15). (DC Cohort; 500 µg/week.).
Clinical status assessments
Statistical analysis of clinical status was conducted on the CA population (500 µg/week). Percentage changes from baseline (PCFB) to end of dosing showed that nine (82%) patients had a 20% or greater improvement in at least one measure of clinical status. No single tool showed a positive PCFB for all patients, however nine (82%) participants recorded positive SF-36 PCS changes ranging from 2.7% to 68.8%. The number of participants that showed a PCFB (based on raw scores) of +20% or greater are shown in Table 5. There were no self-reported or clinically confirmed MS exacerbations.
Table 5.
Clinical status percentage changes from baseline (PCFB) at end of dosing (DC cohort; n = 11) (dose 500 µg/week).
| Assessment Tool | PCFB ≥ +20% |
|---|---|
| SF36 - PCS | 5 (45%) |
| EDSS | 2 (18%) |
| FSS | 4 (36%) |
| PASAT | 4 (36%) |
| SF36-MCS | 3 (27%) |
A summary of results for each of the efficacy parameters is given in Table 6. FSS mean scores decreased (improved) modestly over the course of the study (49.9 at baseline vs 46.7 at end of dosing). No consistent trends in the MSFC total z-scores were observed over the dosing period. SF-36 sub-scores and summary scores all showed slight improvements over the treatment period. Among the eight sub-scores, four showed mean improvements over baseline of 10% or more, with the largest improvement in Role Physical being 8.4 units (28.2%). The two summary scores, Physical Components Score (PCS) and Mental Components Score (MCS), showed mean increases of 24.8% and 3.9%, respectively. EDSS scores improved slightly from a baseline mean of 5.8 to 5.3 at end of dosing.
Table 6.
Summary of scores for efficacy parameters in CA population (DC cohort; n = 11) (dose 500 µg/week).
| Visit 1 (Cycle 1 Day 1) | Visit 5 (Cycle 2 Day 1) | Visit 9 (Cycle 3 Day 1) | End of Dosing | |
|---|---|---|---|---|
| MSFC z-score | ||||
| 9-hole Peg Test | ||||
| Mean | −0.09 | −0.33 | −0.26 | −0.17 |
| Min | −1.76 | −1.62 | −1.61 | −1.59 |
| Max | 1.18 | 1.00 | 0.91 | 0.95 |
| Timed 25-foot walk | ||||
| Mean | −0.17 | −0.15 | 0.07 | −0.30 |
| Min | −2.93 | −2.56 | −1.37 | −4.07 |
| Max | 0.78 | 0.77 | 0.79 | 0.87 |
| PASAT | ||||
| Mean | −0.04 | −0.01 | 0.2 | 0.27 |
| Min | −1.30 | −1.30 | −1.38 | −1.30 |
| Max | 1.58 | 1.34 | 1.58 | 1.5 |
| Total MSFC | ||||
| Mean | −0.13 | −0.19 | −0.02 | −0.08 |
| Min | −1.13 | −1.07 | −0.72 | −1.52 |
| Max | 0.66 | 0.54 | 0.68 | 0.69 |
| FSS | ||||
| Mean | 49.9 | 55 | 51.9 | 46.7 |
| Min | 9.0 | 44.0 | 31.0 | 24.0 |
| Max | 63.0 | 63.0 | 63.0 | 63.0 |
| SF-36 score | ||||
| PCS | ||||
| Mean | 31.3 | – | – | 37.2 |
| Min | 13.1 | 22.1 | ||
| Max | 42.3 | 46.3 | ||
| MCS | ||||
| Mean | 49.1 | – | – | 49.1 |
| Min | 36.6 | 36.6 | ||
| Max | 66.9 | 66.9 | ||
| EDSS | ||||
| Mean | 5.7 | 5.7 | 5.6 | 5.3 |
| Min | 4.0 | 4.0 | 4.0 | 3.0 |
| Max | 7.0 | 7.0 | 7.0 | 7.0 |
Discussion
The primary objective of the study was to determine the safety and tolerability of ascending multiple doses of MIS416, administered intravenously to patients with SPMS or PPMS. DLT, MTD and the RP2D were to be defined to support longer-term exposure in a future planned efficacy trial.
Safety results suggest that repeat MIS416 administration at 500 µg/week is generally well tolerated in this patient group. The majority of AEs were of mild to moderate intensity, self-limiting, tolerable in a chronic dosing regimen and did not require modification or termination of dosing. While the MTD was not achieved, the RP2D dose of 500 µg/week was determined based on the observed safety profile during the DE phase and clinical experience within the compassionate use program. The nature of the most common AEs (i.e., pyrexia, headache, fatigue, weakness, transaminase elevations, myalgia and muscle stiffness) is consistent with the known mechanism of action of MIS416, indicating likely uptake by the reticuloendothelial system and subsequent activation of signal transduction pathways. Significant inter-patient variation was observed with no clear dose-response relationship and there was also a tendency for the severity and frequency of AEs to diminish with repeat dosing, indicating a potential development of tolerance to exposure in the judgement of the investigators. In the DC group alone, no AEs appear with significantly increased frequency, suggesting that in any given individual, the AE profile will be defined within the first month of dosing. The absence of any AEs occurring with significantly increased frequency in the DC group alone provides support for initial dose titration up to RP2D over the first three weeks of treatment.
A comparison of MRI brain scans at the end of dosing with baseline was reassuring, with no evidence of exacerbation of MS.
Analysis of plasma for inflammatory mediators showed an absence of any systemic inflammatory process at baseline in any of the patients and, more importantly, failed to demonstrate any MIS-416-induced systemic inflammation (as shown by values of TNF-α, IL-1β and IL-17A at or close to zero). A transient low-level peak in IL-12p70 at 24 hours post the first dose was noted but the levels were below the lower limit of sensitivity for the assay. The repeated transient elevation of neopterin (a protein known to be induced by MIS416), following each dose, confirmed that the safety cytokines measured were in the context of MIS416 pharmacodynamic activity (Figure 3).
Although this trial was not designed to demonstrate efficacy of MIS416 in patients, standard MS clinical assessment tools were used to provide preliminary efficacy data as a basis for the design of future efficacy trials. No progression or exacerbations of disease state were observed over the course of the study. Being mindful of the small population at a single investigative site, the short duration of observation (three months) and the open-label design of this study, promising observations were made particularly in the SF-36–PCS and the EDSS scores. These observations, in concert with the safety and tolerability profile, suggest that further investigation into the safety and efficacy of MIS416 in progressive forms of MS is justified in a larger randomized placebo-controlled Phase II clinical trial of longer duration.
Acknowledgements
The authors wish to thank Associate Professor Chris Frampton, Biostatistician, Christchurch School of Medicine, University of Otago for his statistical support of the clinical trial and Professor Benjamin M. Segal, Staff Neurologist and Director of the MS Clinic VA, Ann Arbor Healthcare System for his participation and advice as a member of the Safety Review Team.
No writing assistance was sought in the preparation of this article.
Innate Immunotherapeutics provided all direct and indirect costs for the conduct of the clinical trial.
Conflict of interest.
Alison M Luckey and Michael H Silverman are paid consultants to Innate Immunotherapeutics. Tim Anderson was a paid consultant to Innate Immunotherapeutics during the conduct of the trial.
References
- 1.Hausar SL and Goodin DS. Multiple sclerosis and other demyelinating diseases. In: Harrison's Textbook of Internal Medicine. 18th ed. New York: McGraw Hill. Available at: http://accessmedicine.mhmedical.com/content.aspx?bookid=331§ionid=40727196 (accessed 24 January 2015).
- 2.Harrison DM. In the clinic: Multiple sclerosis. Ann Internal Med 2014; 160: ITC4–2-16. [DOI] [PubMed] [Google Scholar]
- 3.Langer-Gould AM, Anderson WE, Armstrong MJ, et al. The American Academy of Neurology's Top Five Choosing Wisely recommendations. Neurology 2013; 81: 1004–1011. [DOI] [PubMed] [Google Scholar]
- 4.Stys PK, Zamponi GW, van Minnen J, et al. Will the real multiple sclerosis please stand up? Nat Rev Neurosci 2012; 13: 507–514. [DOI] [PubMed] [Google Scholar]
- 5.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 2009; 6(7): e1000113. DOI: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed]
- 7.Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Ann Rev Immunol 2012; 30: 491–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer ‘if' but ‘how'. J Pathology 2013; 229: 332–346. [DOI] [PubMed] [Google Scholar]
- 9.Girvan RC, Knight DA, O'Loughlin CJ, et al. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine 2011; 29: 545–557. [DOI] [PubMed] [Google Scholar]
- 10.Lee K-H, Biswas A, Liu Y-J, et al. Proteasomal degradation of Nod2 protein mediates tolerance to bacterial cell wall components. J Biol Chem 2012; 287: 39800–39811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedl M, Abraham C. Secretory mediators regulate Nod2-induced tolerance in human macrophages. Gastroenterology 2011; 140: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White M, Webster G, O'Sullivan D, et al. Targeting innate receptors with MIS416 reshapes Th responses and suppresses CNS disease in a mouse model of multiple sclerosis. PLoS One 2014; 9(1): e87712 DOI: 10.1371/journal.pone.0087712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 14.Lublin FD, Reingold SC and National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the course of multiple sclerosis: Results of an international survey. Neurology 1996; 46: 907–911. [DOI] [PubMed]
- 15.Le Tourneau C, Lee JJ, Sui LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst 2009; 101: 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials, http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/%20Guidances/Vaccines/ucm074775.htm (2009, accessed 10 October 2012).
- 17.Recommendations guiding physicians in biomedical research involving human subjects, Helsinki 1964, including all amendments up to and including the Scotland revision, 2008.
- 18.MPI Research. MIS416: A 26-week intravenous toxicity study in rabbits. Unpublished report, 2012.
- 19.U.S. Department of Health and Human Services. Guidance for Industry: Drug-Induced Liver Injury: Premarketing Clinical Evaluation,http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM174090.pdf (2009, accessed 2 October 2014).