Abstract
Multiple sclerosis (MS) is an autoimmune disease characterised by lymphocytic infiltration of the central nervous system and subsequent destruction of myelin and axons. On the background of a genetic predisposition to autoimmunity, environmental triggers are assumed to initiate the disease. The majority of MS research has focused on the pathological involvement of lymphocytes and other immune cells, yet a paucity of attention has been given to erythrocytes, which may play an important role in MS pathology. The following review briefly summarises how erythrocytes may contribute to MS pathology through impaired antioxidant capacity and altered haemorheological features. The effect of disease-modifying therapies on erythrocytes is also reviewed. It may be important to further investigate erythrocytes in MS, as this could broaden the understanding of the pathological mechanisms of the disease, as well as potentially lead to the discovery of novel and innovative targets for future therapies.
Keywords: Erythrocytes, multiple sclerosis, antioxidant enzymes, haemorheology
Introduction
Multiple sclerosis (MS) is a debilitating inflammatory disease of the central nervous system (CNS), affecting around 2.5 million individuals worldwide. It is more prevalent in females, typically diagnosed in the third and fourth decades of life, and reduces life expectancy by 5 to 10 years.1 Lymphocytes are known to play a role in MS pathology, but erythrocytes may also be involved. The role of erythrocytes in MS pathophysiology is poorly understood, yet erythrocytes may contribute to the disease through impaired antioxidant capacity and altered haemorheology.
Erythrocytes – a brief overview
Mature erythrocytes are observed as anucleate biconcave disks that do not contain organelles. Their main function is gas transport and consequently around 97% of the erythrocyte is occupied by haemoglobin. Nevertheless, erythrocytes contain antioxidant enzymes and structural proteins. Due to the lack of mitochondria, erythrocytes rely on anaerobic glycolysis for energy production. Erythrocytes originate in the bone marrow from pluripotent haematopoietic stem cells and are part of the myeloid lineage. Homeostatic erythropoiesis is essential to ensure adequate blood viscosity and prevent hypoxia.2 Mature erythrocytes have an average lifespan of 100–120 days;2 yet reduced erythrocyte lifespans may be observed in individuals with elevated levels of oxidative stress.3
Erythrocytes may contribute to the pathophysiological mechanisms of MS through impaired antioxidant capacity and altered haemorheology, leading to increased oxidative stress in the periphery and potential ischaemic tissue damage respectively.
Oxidative stress in MS
In addition to the inflammatory component, MS may also be affected by oxidative stress in both the CNS and periphery; a topic that has been recently reviewed.4 Peripheral oxidative stress in MS has been evidenced by increased levels of erythrocyte lipid peroxidation and erythrocyte advanced oxidation protein products in MS patients compared to healthy controls.5–7 Oxidative stress increases between patients with clinically isolated syndrome (CIS) and patients with relapsing–remitting MS (RRMS).5 The age of the CIS patients (17–57, median: 37.5 years) recruited for this study did not significantly differ from the age of RRMS patients (23–58, median: 40 years), it is therefore unlikely that age was a confounder. The documented differences in oxidative stress may be attributed to ongoing inflammation.5 Further, oxidative stress correlates with higher Expanded Disability Status Scale (EDSS) scores, lesion load, and disease duration in RRMS.5 Malondialdehyde, a by-product of lipid peroxidation, was also positively correlated with disease duration, EDSS scores, and lesion load.5 These correlations suggest a link between oxidative stress in MS and disease severity; however, oxidative stress is not disease specific and has been observed as a part of ageing.8 Increased levels of oxidative stress may be the result of inflammation not necessarily intrinsic to MS pathology. This is supported by a study that compared RRMS patients in relapse and in remission. Oxidative stress appears to be increased in relapse, but unchanged during remission compared to healthy controls. Although the sample size of the above-mentioned study was small (18 RRMS patients and 7 healthy controls),9 the same patients were analysed in relapse and remission, strengthening the reliability of the findings. However, a larger cohort should be assessed. The presence of oxidative stress in the periphery in secondary progressive MS (SPMS) patients,7 in whom the disease is thought to be more neurodegenerative and confined to the CNS,1 supports the hypothesis that oxidative stress is part of the pathogenesis and not merely a result of tissue destruction. This study also had a small number of SPMS (n = 16) and healthy controls (n = 13).7 To determine if oxidative stress actually affects MS pathology, further studies with larger cohorts and appropriate pathological and age-matched controls are required.
Impaired erythrocyte antioxidant capacity in MS
Decreased erythrocyte antioxidant capacity in MS may be explained by lower erythrocyte antioxidant enzyme activities in MS patients compared to healthy controls. Relevant antioxidant enzymes may include glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase.
Erythrocyte GPx
GPx protects the body from oxidative damage by catalysing the reduction of lipid hydroperoxides to their corresponding alcohols, as well as the reduction of hydrogen peroxide to water.10 Multiple studies found erythrocyte GPx activity to be decreased in MS patients (n = 24–57) compared to healthy controls (n = 24–69)11–13 and patients with other neurological diseases, including cerebrovascular syndrome (n = 8), lumbar cervical discopathy (n = 6), amyotrophic lateral sclerosis (n = 4), Guillain-Barré syndrome (n = 3) and others (n = 9)14 (Figure 1). Mehlert et al. did not find a decrease in GPx activity in MS patients (n = 12) compared to healthy controls (n = 11) or controls with other neurological diseases (n = 12, neurological diseases not disclosed).15 However, the sample recruited by Mehlert et al.15 was smaller than the sample recruited by studies that reported significant differences11–14 and sample size may explain the discrepancy. Erythrocyte GPx activity was lower in RRMS patients than in CIS patients, and lower in CIS patients than in healthy controls.13 Additionally, erythrocyte GPx activity was lower in SPMS patients than in healthy controls.7 In contrast, lymphocytes, granulocytes and platelets appear to have normal GPx activity in MS.16 Thus, reduced activity of this enzyme supports the hypothesis of impaired erythrocyte antioxidant capacity in MS; nonetheless, failure to control for selenium levels may have impacted on study results as selenium is vital for GPx activity.10 Future investigations should consider measuring patients’ selenium levels and controlling for this confounder.
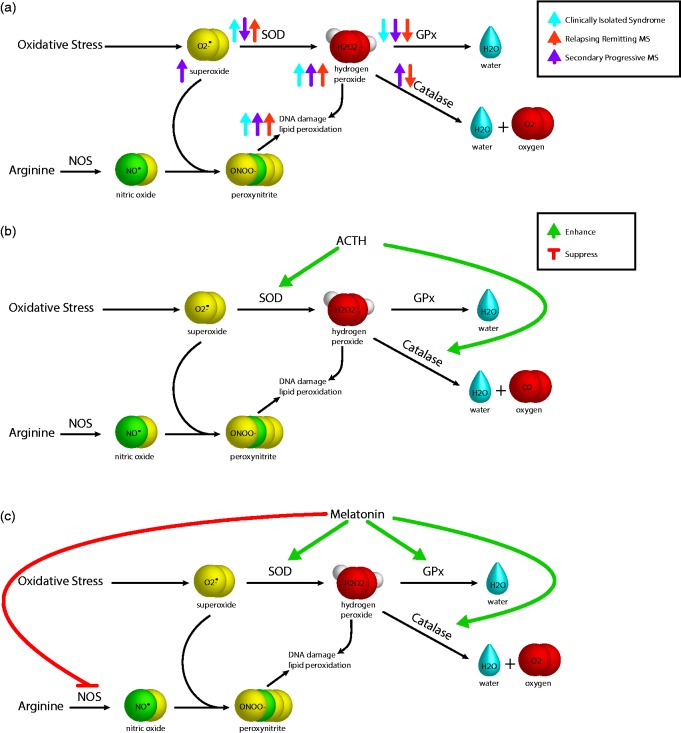
Figure 1.
Erythrocyte antioxidant enzymes in multiple sclerosis. The figure shows (a) three of the erythrocyte antioxidant enzymes – superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase – and the reaction they catalyse to combat the effects of oxidative stress. Activities of erythrocyte SOD, GPx and catalase are altered in clinically isolated syndrome, relapsing–remitting MS, and secondary progressive MS compared to healthy controls. Nitric oxide synthase (NOS) catalyses the synthesis of nitric oxide, which may contribute to oxidative stress following the reaction with superoxide resulting in peroxynitrite. (b) Adrenocorticotropic hormone (ACTH) and (c) melatonin enhance and suppress various erythrocyte enzymes.
In addition, erythrocyte glutathione, one of the cofactors of GPx,10 was found to be decreased in RRMS patients and patients with CIS compared to healthy controls,13 negatively correlating to disease severity and lesion load both in RRMS and CIS patients. This study also found erythrocyte glutathione levels were negatively correlated with disease duration in CIS patients.13 Interestingly, decreased glutathione levels have also been identified through magnetic resonance spectroscopic imaging both in the grey matter and white matter of MS patients compared to healthy controls.17
Erythrocyte SOD
SOD catalyses the dismutation of the superoxide radical to either hydrogen peroxide or oxygen, acting as an antioxidant enzyme that protects against oxidative stress.18 Compared to healthy controls, erythrocyte SOD activity, measured spectrophotometrically, is lower in SPMS patients,7 yet greater in RRMS and CIS patients5,6 than in healthy controls. Erythrocyte SOD activity is greatest in CIS patients5 (Figure 1). Erythrocyte SOD activity is negatively correlated with EDSS scores across studied disease subtypes.5,7 While the studies had small numbers, appropriate age-matching of controls and MS subtypes excludes age as a confounding factor and strengthens the findings. Decreased activity of this enzyme in SPMS supports impaired erythrocyte antioxidant capacity in the chronic neuro-inflammatory stage of disease, but not in CIS or RRMS. This potentially indicates that initial increases in oxidative stress can be compensated for by SOD, but SOD antioxidant mechanisms gradually fail as the disease progresses, leading to ongoing neuronal damage. This is supported by the negative correlations between erythrocyte SOD activity and multiple markers of disease severity (disease duration, lesion load, and EDSS scores).5 Erythrocyte SOD activity may be improved with adrenocorticotropic hormone (ACTH) therapy19 (Figure 1), a historic treatment for MS.
Erythrocyte catalase
Catalase protects against oxidative damage by converting hydrogen peroxide to water and oxygen.20 Interestingly, erythrocyte catalase activity was found to be increased in patients with SPMS compared to healthy controls,7 yet decreased in RRMS19 (Figure 1). An increase in this enzyme in SPMS may indicate a compensation mechanism to combat lower levels of erythrocyte GPx, as both enzymes protect against hydrogen peroxide.10,20 However, this requires further investigation. Treatment with ACTH also improves catalase activity19 (Figure 1). Potential benefits in progressive MS should be further investigated.
Treatment to combat oxidative stress in MS
Melatonin is a powerful antioxidant.21 Melatonin increased erythrocyte SOD activity and erythrocyte GPx activity in SPMS patients supplemented with 10 mg melatonin daily for 30 days7 (Figure 1). Melatonin supplementation also resulted in a decrease in erythrocyte membrane lipid peroxidation in SPMS patients.7 Furthermore, melatonin has been shown to contribute to the seasonality of MS relapses, as well as ameliorating symptoms of the MS experimental model, experimental autoimmune encephalomyelitis.22 Interestingly, these findings suggest that melatonin supplementation may improve antioxidant capacity in SPMS patients. This is particularly significant, as there are currently no treatments available for SPMS. Supplementation with antioxidant vitamins may also be beneficial in MS and should be more thoroughly investigated, as decreased antioxidant vitamin levels have been reported in MS patients compared to healthy controls.23 Whole-body cryotherapy has also been found to reduce oxidative stress in MS patients. Whole-body cryotherapy consists of exposure to extremely low temperatures, during which less oxygen is required, reducing oxidative stress.24
Other erythrocyte enzymes
Other erythrocyte enzymes have been studied in MS. For example, erythrocyte adenosine deaminase (ADA) activity was lower in MS patients than in healthy controls. ACTH treatment restored ADA activity to healthy levels, and increased the enzyme’s activity above that of healthy controls.25 The authors hypothesised that increased ADA activity following ACTH treatment may be due to a reduction in oxidative stress and consequently less damage to the erythrocyte membrane and membrane-associated enzymes such as erythrocyte ADA.25
While there appear to be differences in the activities of several enzymes, possibly reflecting changes in concentration or enzyme damage, a previous study identified no differences between MS patients and control participants erythrocyte proteins, demonstrating that all proteins found in control erythrocytes were also found in MS erythrocytes and vice-versa.26 There are conflicting studies reporting on activity in erythrocyte 2’, 3’-cyclic nucleotide 3-phosphohydrolase (CNP).27,28 Decreased CNP activity in MS erythrocyte membranes was observed only in the preliminary study, for which only five MS patients and five healthy controls were recruited.28 Consequently, observed differences in CNP activity between MS patients and healthy controls are unlikely to be significant. No significant differences were found in erythrocyte pyrimidine 5'-nucleotidase activity.29
Haemorheology – a brief overview
Haemorheology is the study of blood fluidity. Blood fluidity is not constant throughout the circulatory system. At higher shear rates observed in arteries, blood viscosity decreases, while venous blood subjected to lower shear rates is more viscous. This distinct haemodynamic characteristic can be attributed to erythrocyte deformability at higher shear rates, and erythrocyte aggregation at lower shear rates. Erythrocyte deformability is facilitated by the distinct plasma membrane composition, as well as lack of a nucleus and organelles, permitting transit of biconcave erythrocytes to ellipsoidal structures at higher shear rates. At lower shear rates, erythrocytes regain their biconcave shape and aggregate, forming reversible rouleaux.30
Alterations in haemorheology MS
Total blood viscosity was found to be increased at higher and lower shear rates in MS patients compared to healthy individuals31 and pathological controls suffering from other neuro-inflammatory and neurodegenerative disorders.32 Several alterations in erythrocyte haemorheology have been studied and reported in MS; however, results are conflicting and the mechanisms responsible for increased blood viscosity in MS patients are poorly understood.31,32
Impaired flow in the cerebral microcirculation due to an increase in blood viscosity may lead to stasis of blood in these vessels, which causes ischaemic tissue damage. An inflammatory response to this ischaemic tissue damage then results in impairment of the vessel wall, increasing its permeability and potentially enabling inflammatory cells and mediators to infiltrate the CNS.33,34 The recent discovery of lymphatic vessels in the brain challenges the paradigm that the CNS is ‘immune-privileged’.35 Thus, disruption of blood vessel walls may not be required for immune infiltration to occur. While there is some evidence that decreased blood fluidity is associated with decreased tissue perfusion, measurements of rheological parameters fail to take into account in vivo compensation mechanisms, such as vasomotor control. Despite questions about the implications of haemorheological alterations in vivo, strong associations have been reported between altered blood fluidity and disease processes.36
By ruling out alterations in haematocrit,31 extreme leucocytosis37 and increased plasma viscosity32 alterations in blood viscosity are likely to be accounted for by altered erythrocyte aggregation and/or deformability.36
Erythrocyte deformability
Simpson et al.31 indicated impaired erythrocyte deformability by early filtration experiments. Impaired erythrocyte deformability may be causing increased blood viscosity in MS patients. However, Pollock et al. did not find impaired erythrocyte deformability in MS patients.38 Both studies31,38 were of small size, but Simpson et al. failed to control for fasting blood lipids and blood sugar, and some MS patients were receiving steroids at the time of sample collection.31 As leucocyte contamination can cause blockage of the filter pores used for early blood filtration experiments and new and improved technology has been developed since the original studies, impaired erythrocyte deformability in MS should be reinvestigated. Morphological changes to erythrocytes, (macrocytes and echinocytes) are positively correlated to MS disease severity31,39,40 and may impair erythrocyte deformability.41
Red cell distribution width (RDW) is a measure of the size and volume of erythrocytes and influences deformability.42 One study found that RRMS patients (n = 109) have increased RDW compared to healthy controls (n = 130). This may underlie altered erythrocyte deformability in MS. Elevated RDW was found to be positively related to EDSS scores.42 This study had a reasonable sample size, but failed to control for other potential confounders that influence RDW and lacked appropriate pathological controls. Further investigation in the use of RDW as a biomarker is required.
Oxidative stress in the peripheral circulation of MS patients24,43 may further impede erythrocyte deformability through erythrocyte membrane lipid peroxidation.7,36 Nonetheless, more research into impaired erythrocyte deformability in MS patients is needed as better technology has been developed since the original studies.
Erythrocyte aggregation
MS is inflammatory driven;1 inflammatory markers, including fibrinogen (essential for blood clot formation), are known to influence haemorheology.36 Thus, there is a possibility that increased erythrocyte aggregation is associated with the haemorheological changes seen in MS.
Despite inflammatory events, RRMS patients do not appear to have elevated fibrinogen levels in the remitting phase.44 However, D-dimer levels, turnover markers of cross-linked fibrin, are elevated44,45 and low fibrinogen levels during remission (MS patients vs. controls) do not exclude the possibility of increased fibrinogen levels during relapse, especially considering the role of fibrinogen in MS pathology, where fibrin is involved in the release of cytokines and activation of microglia in the CNS.46,47
Potential interference of platelets
Platelets play an essential role in the coagulation cascade and are abundant in MS lesions.48 While platelets themselves do not directly impact on erythrocyte aggregation, they influence thrombotic processes, which may interfere with haemorheological studies.36,49 Blood clot formation in the cerebral microcirculation due to increased platelet counts supports the idea of ischaemic tissue injury.33
Erythrocyte NOS – potential implications for haemorheology
Erythrocytes may influence blood fluidity through expression of erythrocyte NOS. Erythrocytes contain messenger RNA (mRNA) for NOS and other proteins,50 as well as functional NOS in both their membrane and cytoplasm.51 NOS activity in MS patients has not yet been investigated; however, the nitric oxide (NO) produced by NOS acts as a vasodilator under hypoxic conditions.52,53 Therefore, decreased NOS expression during erythrocyte development in MS may lead to decreased NO levels and hence cerebral vasoconstriction, resulting in ischaemic tissue injury.33 Moreover, erythrocyte-derived NOS has been shown to regulate erythrocyte deformability and platelet aggregation. Kleinbongard et al. hypothesised that NOS influences erythrocyte deformability through altering functional characteristics of the erythrocyte membrane.51 Therefore, a reduction in this enzyme might further lead to impaired erythrocyte deformability and enhanced platelet aggregation, two features that may also contribute to ischaemic tissue injury. Alternatively, if erythrocyte NOS activity were increased in MS, it may be contributing to the oxidative stress observed in MS through NO (Figure 1). However, until erythrocyte NOS activity is measured in MS, no conclusions can be drawn in this area.
Erythrocyte complement receptor (CR1)
MS patients’ erythrocytes appear to have lower CR1 expression than healthy controls.54 Erythrocyte CR1 mediates immune-adherence clearance of immune inflammatory particles (immune complexes, apoptotic debris and microbes).55 Consequently, decreased levels of this CR1 may result in increased accumulation of inflammatory particles in the circulation, leading to potential inflammatory damage to the vasculature and surrounding tissues.
Serum myeloperoxidase (MPO) and MS
MPO, a haeme containing enzyme mainly synthesised by neutrophils, is released in response to inflammation. It catalyses the generation of reactive oxygen species (ROS), as well as the consumption of NO,56 thereby contributing to oxidative damage and potentially ischaemic damage through the consumption of the vasodilator NO. Erythrocytes have been found to bind MPO,57,58 which impaired their deformability58 and altered vascular resistance systemically.57 While increased levels of MPO have been observed in the lesions of MS patients59 and serum of an Asian MS cohort,60 no data are available regarding erythrocyte-bound MPO in multiethnic MS cohorts.
Disease-modifying therapies (DMTs) and erythrocytes
Depending on their mechanism of action, DMTs have the potential to affect circulating erythrocytes. Treatment with natalizumab results in detection of previously undetected erythroblasts in the peripheral blood of RRMS patients. Other haematopoietic precursors, namely neutrophil precursors, are also increased after natalizumab treatment.61–63 Mitoxantrone, fingolimod, and dimethyl fumarate all have the potential to cause eryptosis (erythrocyte-specific apoptosis).64–66 Interferon-ß treatment appeared to reduce RDW and thus may counteract the increased RDW observed in RRMS patients.42
Conclusions and future directions
As previous research has shown, erythrocytes are altered in the various subtypes of MS and potentially contribute to the oxidative stress in MS through impaired antioxidant capacity. Furthermore, altered haemorheological features observed in MS may be the result of altered erythrocyte phenotypes. Differences in erythrocyte RDW between MS patients and controls42 could be associated with altered erythrocyte deformability and hence altered haemorheology. Additionally, increased erythrocyte aggregation due to greater levels of inflammation in the periphery of MS patients may further contribute to altered haemorheology in MS patients. Both MS pathology and DMTs appear to affect circulating erythrocytes. Further research in this area is needed to establish the implications of altered erythrocytes for MS patients. Many of the studies on erythrocytes in MS were of low power and lacked pathological controls, which may confound the results.
It will be beneficial to confirm the results in larger cohorts, with appropriately matched (age, gender, physical activity level, body composition and diet) healthy and pathological controls. Activities of erythrocyte antioxidant enzymes as well as potential mechanisms of altered erythrocyte enzyme activities in MS subtypes need to be investigated. Alterations in blood viscosity in MS subtypes need to be confirmed and potential mechanisms, including but not limited to erythrocyte involvement, need to be studied in further detail. NOS activity should also be investigated in MS and compared to healthy and pathological controls as it may affect both haemorheological characteristics and antioxidant capacity of erythrocytes in MS.
Investigation of the role of erythrocytes in MS may lead to the discovery of further specific differences that could be exploited as biomarkers of the disease and aid patient stratification. Additionally, research on the role of erythrocytes in MS may broaden the understanding of the pathological mechanisms of this complex and heterogeneous disease and this in turn may lead to the discovery of novel and innovative targets for future therapies that may greatly improve patients’ quality of life.
Acknowledgements
The authors would like to acknowledge Jared Q Coleman-Stark for his editing contributions to this review, as well as Associate Professor Lotti Tajouri, whose curiosity for the involvement of erythrocytes in MS pathology inspired this review. KA Sanders is funded by a scholarship from Multiple Sclerosis Research Australia and the Trish Multiple Sclerosis Research Foundation. VE Maltby is funded by fellowships from Multiple Sclerosis Research Australia and the Canadian institutes of Health Research. K Groen is funded by a partial scholarship from Bond University.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest.
Associate Professor J Lechner-Scott’s institution receives non-directed funding, as well as honoraria for presentations and membership on advisory boards, from Sanofi Aventis, Biogen Idec, Bayer Health Care, Merck Serono, Teva and Novartis Australia. The other authors have nothing to declare.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Marieb EN, Hoehn K. Human anatomy & physiology, 9th Boston, MA: Pearson, 2013. [Google Scholar]
- 3.Smith J. Exercise, training and red blood cell turnover. Sports Med 1995; 19: 9–31. [DOI] [PubMed] [Google Scholar]
- 4.Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp Neurol 2016; 277: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljubisavljevic S, Stojanovic I, Cvetkovic T, et al. Erythrocytes’ antioxidative capacity as a potential marker of oxidative stress intensity in neuroinflammation. J Neurol Sci 2014; 337: 8–13. [DOI] [PubMed] [Google Scholar]
- 6.Polidoro G, Di Ilio C, Arduini A, et al. Superoxide dismutase, reduced glutathione and TBA-reactive products in erythrocytes of patients with multiple sclerosis. Int J Biochem 1984; 16: 505–509. [DOI] [PubMed] [Google Scholar]
- 7.Miller E, Walczak A, Majsterek I, et al. Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunolol 2013; 257: 97–101. [DOI] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 9.Fiorini A, Koudriavtseva T, Bucaj E, et al. Involvement of oxidative stress in occurrence of relapses in multiple sclerosis: The spectrum of oxidatively modified serum proteins detected by proteomics and redox proteomics analysis. PLoS One 2013; 8: e65184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhabak KP, Mugesh G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidants. Acc Chem Res 2010; 43: 1408–1419. [DOI] [PubMed] [Google Scholar]
- 11.Shukla VK, Jensen GE, Clausen J. Erythrocyte glutathione peroxidase deficiency in multiple sclerosis. Acta Neurol Scand 1977; 56: 542–550. [DOI] [PubMed] [Google Scholar]
- 12.Syburra C, Passi S. Oxidative stress in patients with multiple sclerosis. Ukr Biokhim Zh (1999) 1999; 71: 112–115. [PubMed] [Google Scholar]
- 13.Ljubisavljevic S, Stojanovic I, Cvetkovic T, et al. Glutathione homeostasis disruption of erythrocytes, but not glutathione peroxidase activity change, is closely accompanied with neurological and radiological scoring of acute CNS inflammation. Neuroimmunomodulation 2014; 21: 13–20. [DOI] [PubMed] [Google Scholar]
- 14.Szeinberg A, Golan R, Ben Ezzer J, et al. Decreased erythrocyte glutathione peroxidase activity in multiple sclerosis. Acta Neurol Scand 1979; 60: 265–271. [DOI] [PubMed] [Google Scholar]
- 15.Mehlert A, Metcalfe RA, Diplock AT, et al. Glutathione peroxidase deficiency in multiple sclerosis. Acta Neurol Scand 1982; 65: 376–378. [DOI] [PubMed] [Google Scholar]
- 16.Szeinberg A, Golan R, Ben-Ezzer J, et al. Glutathione peroxidase activity in various types of blood cells in multiple sclerosis. Acta Neurol Scand 1981; 63: 67–75. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, et al. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging 2010; 28: 163–170. [DOI] [PubMed] [Google Scholar]
- 18.Van Raamsdonk JM, Hekimi S. Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci U S A 2012; 109: 5785–5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kopff M, Zakrzewska I, Czernicki J, et al. Red blood cell superoxide dismutase and catalase activities in patients suffering from multiple sclerosis treated with adrenocorticotropic hormone. Pol J Pharmacol 1996; 48: 441–445. [PubMed] [Google Scholar]
- 20.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci 2004; 61: 192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardeland R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005; 27: 119–130. [DOI] [PubMed] [Google Scholar]
- 22.Farez MF, Mascanfroni ID, Méndez-Huergo SP, et al. Melatonin contributes to the seasonality of multiple sclerosis relapses. Cell 2015; 162: 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besler HT, Comoğlu S, Okçu Z. Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci 2002; 5: 215–220. [DOI] [PubMed] [Google Scholar]
- 24.Miller E, Mrowicka M, Malinowska K, et al. Effects of whole-body cryotherapy on a total antioxidative status and activities of antioxidative enzymes in blood of depressive multiple sclerosis patients. World J Biol Psychiatry 2011; 12: 223–227. [DOI] [PubMed] [Google Scholar]
- 25.Kopff M, Zakrzewska I, Klem J, et al. Activity of adenosine deaminase in red blood cells of patients suffering from multiple sclerosis treated with adrenocorticotropic hormone. Pol J Pharmacol 1995; 47: 525–530. [PubMed] [Google Scholar]
- 26.Wheeler TT, Ford HC. A search for protein abnormalities in erythrocyte membranes and platelets from patients with multiple sclerosis using double-label two-dimensional electrophoresis. J Neurol Sci 1988; 88: 151–159. [DOI] [PubMed] [Google Scholar]
- 27.Rastogi SC, Clausen J. Membrane-bound 2',3',-cyclic nucleotide 3'-phosphohydrolase activity of lymphocytes, granulocytes and erythrocytes in multiple sclerosis. Acta Neurol Scand 1985; 71: 303–308. [DOI] [PubMed] [Google Scholar]
- 28.Dreiling CE, Norrgran C, Smith E. Reduced 2',3'-cyclic nucleotide 3'-phosphohydrolase activity in erythrocyte membranes of patients with multiple sclerosis. Metab Pediatr Syst Ophthalmol 1985; 8: 69–74. [PubMed] [Google Scholar]
- 29.Mendz GL, Middlehurst CR, Kuchel PW, et al. Determination of erythrocyte pyrimidine 5'-nucleotidase activity by 31P nuclear magnetic resonance: Comparison of normal controls and multiple sclerosis patients. Experientia 1986; 42: 1016–1018. [DOI] [PubMed] [Google Scholar]
- 30.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost 2003; 29: 435–450. [DOI] [PubMed] [Google Scholar]
- 31.Simpson LO, Shand BI, Olds RJ, et al. Red cell and hemorheological changes in multiple sclerosis. Pathology 1987; 19: 51–55. [DOI] [PubMed] [Google Scholar]
- 32.Brunetti A, Ricchieri G, Patrassi G, et al. Rheological and fibrinolytic findings in multiple sclerosis. J Neurol Neurosurg Psychiatry 1981; 44: 340–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol Dis 2004; 16: 1–13. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg GA. Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab 2012; 32: 1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmonds MJ, Meiselman HJ, Baskurt OK. Blood rheology and aging. J Geriatr Cardiol 2013; 10: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sagar HJ, Allonby ID. Lymphocyte subpopulations in multiple sclerosis: Serial studies and clinical correlations. J Neurol Sci 1979; 43: 133–148. [DOI] [PubMed] [Google Scholar]
- 38.Pollock S, Harrison M, O’Connell G. Erythrocyte deformability in multiple sclerosis. J Neurol Neurosurg Psychiatry 1982; 45: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crellin R, Bottiglieri T, Reynolds E. Multiple sclerosis and macrocytosis. Acta Neurol Scand 1990; 81: 388–391. [DOI] [PubMed] [Google Scholar]
- 40.Prineas J. Red blood cell size in multiple sclerosis. Acta Neurol Scand 1968; 44: 81–90. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y, Kim K, Park Y. Measurement techniques for red blood cell deformability: Recent advances, Rijeka, Croatia: INTECH Open Access Publisher, 2012. [Google Scholar]
- 42.Peng YF, Cao WY, Zhang Q, et al. Assessment of the relationship between red cell distribution width and multiple sclerosis. Medicine (Baltimore) 2015; 94: e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gironi M, Borgiani B, Mariani E, et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res 2014; 2014: 961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aksungar FB, Topkaya AE, Yildiz Z, et al. Coagulation status and biochemical and inflammatory markers in multiple sclerosis. J Clin Neurosci 2008; 15: 393–397. [DOI] [PubMed] [Google Scholar]
- 45.Arpaia G, Bavera PM, Caputo D, et al. Effects of elastic compression on hypomobility edema and fibrinolysis activation in multiple sclerosis. Panminerva Med 2011; 53(3 Suppl 1): 71–74. [PubMed] [Google Scholar]
- 46.Adams RA, Bauer J, Flick MJ, et al. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 2007; 204: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 2012; 3: 1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langer HF, Choi EY, Zhou H, et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res 2012; 110: 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamlin SK, Benedik PS. Basic concepts of hemorheology in microvascular hemodynamics. Crit Care Nurs Clin North Am 2014; 26: 337–344. [DOI] [PubMed] [Google Scholar]
- 50.Kabanova S, Kleinbongard P, Volkmer J, et al. Gene expression analysis of human red blood cells. Int J Med Sci 2009; 6: 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood 2006; 107: 2943–2951. [DOI] [PubMed] [Google Scholar]
- 52.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells evidence for an s-nitrosothiol-based signal. Circ Res 2008; 103: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortese-Krott MM, Kelm M. Endothelial nitric oxide synthase in red blood cells: Key to a new erythrocrine function? Redox Biol 2014; 2: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nowak J, Wender M. Reduced expression of erythrocyte complement receptor (C3bR) in MS. Acta Neurol Scand 1994; 89: 266–269. [DOI] [PubMed] [Google Scholar]
- 55.Melhorn MI, Brodsky AS, Estanislau J, et al. CR1-mediated ATP release by human red blood cells promotes CR1 clustering and modulates the immune transfer process. J Biol Chem 2013; 288: 31139–31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klebanoff SJ. Myeloperoxidase: Friend and foe. J Leukoc Biol 2005; 77: 598–625. [DOI] [PubMed] [Google Scholar]
- 57.Adam M, Gajdova S, Kolarova H, et al. Red blood cells serve as intravascular carriers of myeloperoxidase. J Mol Cell Cardiol 2014; 74: 353–363. [DOI] [PubMed] [Google Scholar]
- 58.Gorudko IV, Sokolov AV, Shamova EV, et al. Binding of human myeloperoxidase to red blood cells: Molecular targets and biophysical consequences at the plasma membrane level. Arch Biochem Biophys 2015; 591: 87–97. [DOI] [PubMed] [Google Scholar]
- 59.Gray E, Thomas TL, Betmouni S, et al. Elevated activity and microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol 2008; 18: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minohara M, Matsuoka T, Li W, et al. Upregulation of myeloperoxidase in patients with opticospinal multiple sclerosis: Positive correlation with disease severity. J Neuroimmunol 2006; 178: 156–160. [DOI] [PubMed] [Google Scholar]
- 61.Bridel C, Beauverd Y, Samii K, et al. Hematologic modifications in natalizumab-treated multiple sclerosis patients: An 18-month longitudinal study. Neurol Neuroimmunol Neuroinflamm 2015; 2: e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jing D, Oelschlaegel U, Ordemann R, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone Marrow Transplant 2010; 45: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 63.Lesesve JF, Debouverie M, Decarvalho Bittencourt M, et al. CD49d blockade by natalizumab therapy in patients with multiple sclerosis increases immature B-lymphocytes. Bone Marrow Transplant 2011; 46: 1489–1491. [DOI] [PubMed] [Google Scholar]
- 64.Arnold M, Bissinger R, Lang F. Mitoxantrone-induced suicidal erythrocyte death. Cell Physiol Biochem 2014; 34: 1756–1767. [DOI] [PubMed] [Google Scholar]
- 65.Ghashghaeinia M, Bobbala D, Wieder T, et al. Targeting glutathione by dimethylfumarate protects against experimental malaria by enhancing erythrocyte cell membrane scrambling. Am J Physiol Cell Physiol 2010; 299: 791–804. [DOI] [PubMed] [Google Scholar]
- 66.Eberhard M, Ferlinz K, Alizzi K, et al. FTY720-induced suicidal erythrocyte death. Cell Physiol Biochem 2010; 26: 761–766. [DOI] [PubMed] [Google Scholar]