Abstract
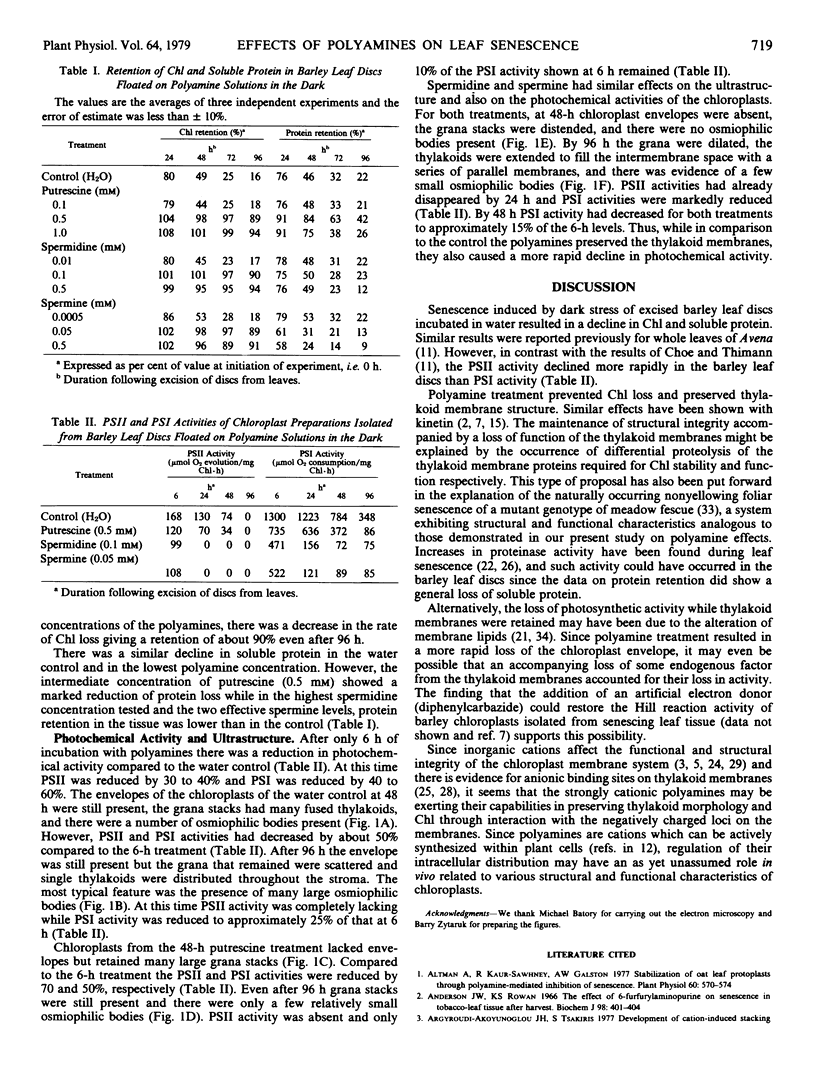
The polyamines putrescine, spermidine, and spermine prevent the loss of chlorophyll normally associated with senescence of excised leaf tissue maintained in darkness on water (control). Retention of chlorophyll in barley leaf discs was in the range of 90% 4 days after excision and placement on effective polyamine solutions. In contrast, the loss of soluble protein was hastened with 0.5 millimolar spermidine and spermine treatments but it was retarded by 0.5 millimolar putrescine.
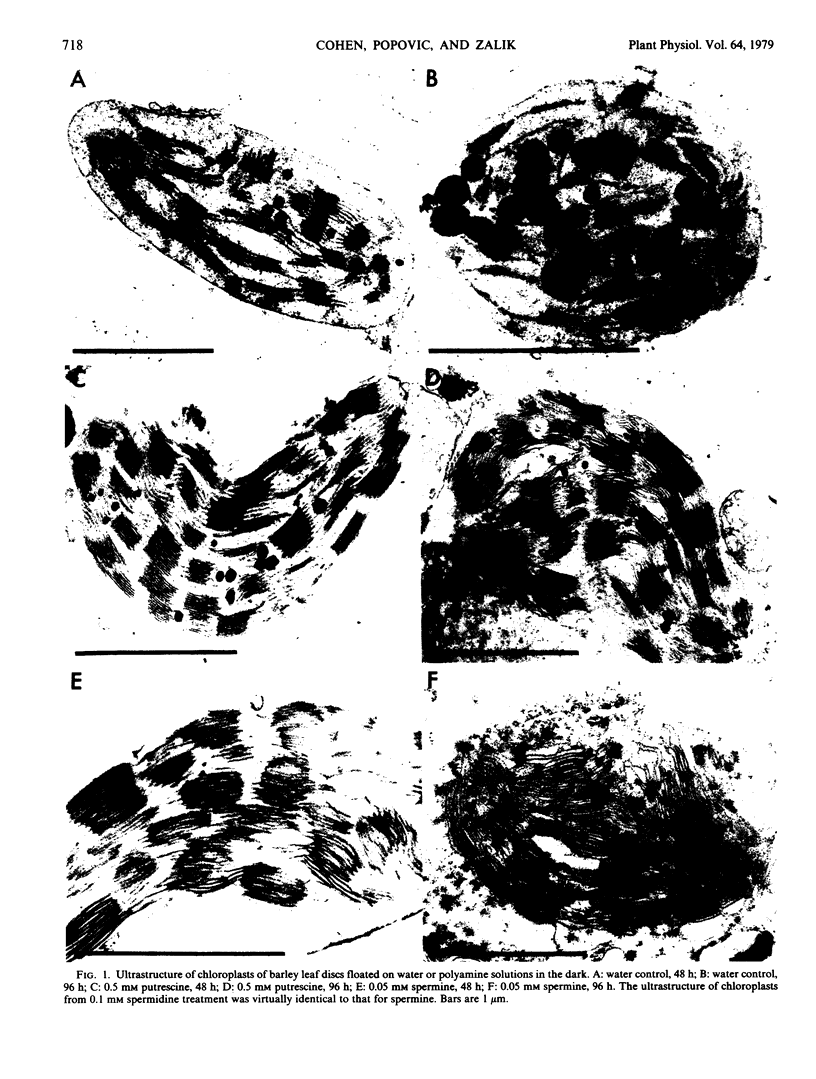
Photosystem I and II activities of chloroplasts from polyamine-treated leaf discs declined more rapidly as compared to the control. Chloroplast ultrastructural changes resulting from the polyamine treatments included the apparent destruction of the envelope, preservation of thylakoid membrane structure, and reduced accumulation of osmiophilic bodies. The influence of polyamines on senescence-related processes may be due to their cationic nature.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Rowan K. S. The effect of 6-furfurylaminopurine on senescence in tobacco-leaf tissue after harvest. Biochem J. 1966 Feb;98(2):401–404. doi: 10.1042/bj0980401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Ditto C. L. Effects of cations upon chloroplast membrane subunit. Interactions and excitation energy distribution. Biochim Biophys Acta. 1976 Nov 9;449(2):259–274. doi: 10.1016/0005-2728(76)90138-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caldarera C. M., Rossoni C., Casti A. Involvement of polyamines in ribonucleic acid synthesis as a possible biological function. Ital J Biochem. 1976 Jan-Feb;25(1):33–55. [PubMed] [Google Scholar]
- HAROLD F. M. STABILIZATION OF STREPTOCOCCUS FAECALIS PROTOPLASTS BY SPERMINE. J Bacteriol. 1964 Nov;88:1416–1420. doi: 10.1128/jb.88.5.1416-1420.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J., Forrester J. A. Surface charges on chloroplast membranes as studied by particle electrophoresis. Biochim Biophys Acta. 1978 Oct 11;504(1):215–225. doi: 10.1016/0005-2728(78)90019-1. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R. Stepwise generation of the natural oxidant in a reconstituted chloroplast system. Plant Physiol. 1976 Feb;57(2):203–208. doi: 10.1104/pp.57.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]