Abstract
Background. The ideal management of SAH patients with negative initial DSA findings remains unresolved. Objective. (i) To present risk factors, clinical courses, and outcomes in different types of SAH patients with negative DSA findings; (ii) to explore the differences of basal vein between aSAH patients and NASAH patients; and (iii) to evaluate the value of repeated DSA for these patients. Methods. All SAH patients with negative initial DSA findings between 2013 and 2015 in our hospital were enrolled and were further categorized as perimesencephalic SAH (PMN-SAH) or nonperimesencephalic SAH (nPMN-SAH). Risk factors, clinical courses, outcomes, and the basal vein drainage patterns were compared. Results. A total of 137 patients were enrolled in the present study. The PMN-SAH group had better GOS and mRS values at 1-year follow-up. Moreover, the nPMN-SAH group had a higher rate of complications. The basal vein drainage pattern showed significant difference when comparing each of the NASAH subtypes with aSAH groups. There was a significant higher rate of a responsible aneurysm in nPMN-SAH group upon repeated DSA. Conclusions. SAH patients with negative initial DSA findings had benign clinical courses and outcomes. Repeated DSA studies are strongly advised for patients with the nPMN-SAH pattern.
1. Introduction
Spontaneous subarachnoid hemorrhage (SAH), characterized by bleeding into the subarachnoid space in the absence of trauma, is most often caused by the rupture of an intracranial aneurysm [1, 2]. However, according to the previous studies [3, 4], even though there is now widespread use of digital subtraction angiogram (DSA) to aid in the diagnosis of spontaneous SAH, nearly fifteen percent of cases remain idiopathic [4]. This category of SAH has been termed nonaneurysmal SAH (NASAH), which typically follows a benign clinical course and has a generally favorable prognosis when compared with aneurysmal subarachnoid hemorrhage (aSAH) [5, 6].
According to the distribution pattern of the subarachnoid blood, these NASAH patients are usually divided into two subcategories, perimesencephalic (PMN-SAH) and nonperimesencephalic hemorrhage (nPMN-SAH) [7]. Although it is generally recognized that NASAH has a more preferable outcome than aSAH [5], recent studies suggest that the management of the nPMN-SAH subgroup should be more rigorous in light of its more severe clinical courses and outcomes [8, 9]. As nPMN-SAH is a diagnosis of exclusion, some controversial issues remain regarding the management of these patients that have negative initial DSA findings [10, 11]. Despite numerous studies utilizing multiple imaging modalities, the bleeding source of NASAH has not been elucidated. Though most authors support the hypothesis that the culprit of the bleeding is of venous origin, the precise mechanism of the bleeding source remains unknown [12]. Furthermore, given the low detection rate of a responsible intracranial lesion in NASAH patients, the need for serial DSAs in these cases remains an open question.
Here we retrospectively analyzed the data of all SAH patients with initial negative DSA findings in our center between 2013 and 2015. The risk factors, clinical courses, and outcomes of these patients were evaluated in our study. In addition, the venous drainage patterns in both subgroups were compared. Finally, the necessity of repeated DSA examinations was also evaluated based on our study and the review of related literature.
2. Methods
2.1. Patients and Procedure
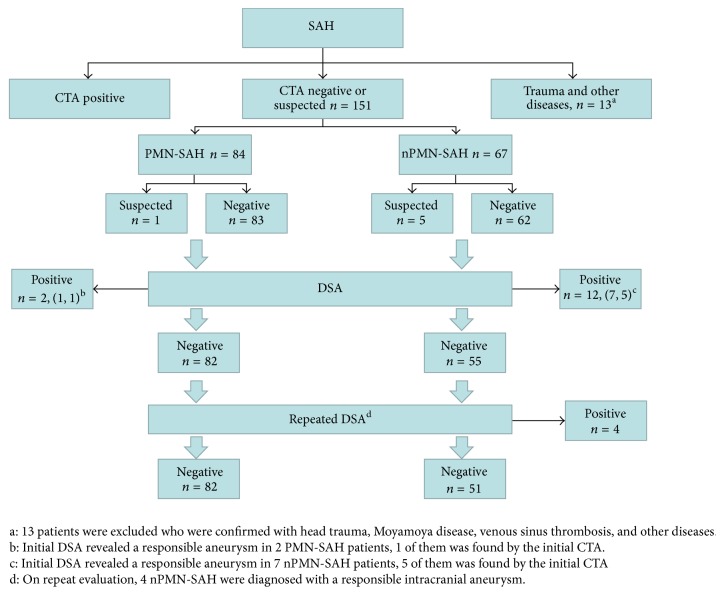
The data of patients who presented with SAH between 2013 and 2015 in our center (Second Affiliated Hospital, School of Medicine, Zhejiang University) were retrospectively analyzed. According to standard management of SAH patients [13, 14], all patients were screened by CTA upon admission, followed by emergent DSA examination. There were 164 SAH patients who had an initial CTA that failed to definitively demonstrate a culprit lesion. Of these, 13 were excluded due to a history of traumatic brain injury or another definitive cause of SAH, leaving 151 patients with negative CTA findings. Among them, 14 patients had positive initial DSA results (2 with a perimesencephalic pattern and 12 with a nonperimesencephalic pattern). This left 137 SAH patients with negative initial DSA findings (hereafter referred to as NASAH patients) who were enrolled in the present study. Based on the distribution of the subarachnoid blood, NASAH patients were divided into PMN-SAH (n = 82 patients) and nPMN-SAH groups (n = 55) [15]. To further exclude intracranial aneurysm as the source of hemorrhage [16], all of them underwent a repeated DSA examination either 10–14 days after admission or one month after discharge. In total, 57 patients agreed to undergo a repeated DSA during hospitalization or follow-up. Four patients were found to have a culprit intracranial aneurysm on the second angiogram, all of whom were patients with the nonperimesencephalic pattern (Figure 1).
Figure 1.
The demographic data includes patient sex, age, smoking history, alcohol use, hypertension, diabetes, and history of anticoagulant use. The Glasgow Coma Score (GCS), Hunt-Hess (HH) grade, and the modified Fisher Scale (mFS) were used for evaluation upon admission [5, 33]. The length of hospital stay (LOS) and in-hospital complications, namely, hydrocephalus, cerebral vasospasm, and rebleeding, were compared between the two groups. All patients were followed up by telephone interview or outpatient clinic at three months and one year after discharge. The outcomes were evaluated using the modified Rankin Scale (mRS) and Glasgow Outcome Scale (GOS).
In order to find a potential venous source of bleeding, we compared the basal vein of Rosenthal (BVR) anatomy among the different groups. Excluding 4 patients who were later found to have a definite bleeding source, the venous configurations of 133 NASAH patients (82 PMN-SAH and 51 nPMN-SAH) were evaluated and compared to a total of 133 consecutive aSAH patients during the same period. The classification of the BVR was performed as described by Ramazan Buyukkaya et al. [12]. Briefly, the drainage pattern of unilateral BVR was divided into three types:
Type A (normal continuous): a continuous BVR drains mainly into the vein of Galen.
Type B (normal discontinuous): in a discontinuous BVR, the anterior part drains into the sphenoparietal sinus or cavernous sinus through an uncal vein and posterior part drains to the vein of Galen.
Type C (primitive variant): this variant drains mainly to the dural sinuses, not via the vein of Galen (i.e., the perimesencephalic veins drain into the superior petrosal sinus or the BVR drains directly into the transverse or straight sinus).
2.2. Statistical Analysis
Categorical variables of different groups, such as the parameter of demographic data, GCS, HH grade, mFS, mRS, and GOS, were compared using the Chi-squared test or Fisher's exact test. Continuous variables, such as the number of patients in each group, were compared using Student's t-test. p < 0.05 was considered as significant statistical difference.
3. Results
3.1. Demographics
The demographics of all NASAH patients with negative initial DSA findings are summarized in Table 1. There were no significant differences in sex, age, smoking history, alcohol abusing history, diabetes, hypertension, or anticoagulation medication history between PMN-SAH and nPMN-SAH groups (p > 0.05 for each parameter).
Table 1.
Demographics of patients with nonaneurysmal subarachnoid hemorrhage.
| NASAH | PMN-SAH | nPMN-SAH | p value | |
|---|---|---|---|---|
| Sum (%) | 137 (100%) | 82 (59.9%) | 55 (40.1%) | NS |
| Male-female ratio | 76/61 (1.2/1) | 42/40 (1.05/1) | 33/22 (1.5/1) | 0.357 |
| Age (range) | 56.0 ± 10.4 (30–80) | 55.4 ± 9.6 (34–76) | 56.7 ± 13.7 (30–80) | 0.156 |
| Smoker (%) | 18 (13.1%) | 11 (13.4%) | 7 (12.7%) | 0.907 |
| Alcohol abusing (%) | 21 (15.3%) | 13 (15.9%) | 8 (14.5%) | 0.835 |
| Diabetes (%) | 7 (5.1%) | 3 (3.7%) | 4 (7.3%) | 0.438 |
| Hypertension (%) | 30 (21.9%) | 15 (18.3%) | 15 (27.3%) | 0.213 |
| Anticoagulant Using (%) | 4 (2.9%) | 2 (2.4%) | 2 (3.6%) | 1 |
NASAH: nonaneurysmal subarachnoid hemorrhage, PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage, nPMN-SAH: nonperimesencephalic nonaneurysmal subarachnoid hemorrhage; NS means can not be analyzed.
3.2. Clinical Course and Risk Factors for Hydrocephalus
The patients' GCS, HH scale, and mFS were evaluated upon arrival at our emergency room. Despite the interval from ictus to evaluation, the nPMN-SAH group presented with higher GCS and mFS on their initial CT scan (p < 0.05). However, there was no significant difference in the HH grade between the two groups (p = 0.157) (Table 2).
Table 2.
The clinical characteristics of nonaneurysmal subarachnoid hemorrhage.
| All NASAH (n = 137) | PMN-SAH (n = 82) | nPMN-SAH (n = 55) | p value | |
|---|---|---|---|---|
| LOS, mean (range) | 8.5 ± 8.5 (2–75) | 7.7 ± 4.0 (2–16) | 12.9 ± 11.9 (2–75) | 0.001 |
| GCS | ||||
| Mild (13–15) | 132 (97.0%) | 82 (100%) | 50 (90.9%) | 0.009 |
| Middle (9–12) | 1 (0.7%) | 0 | 1 (1.8%) | |
| Severe (3–8) | 4 (2.3%) | 0 | 4 (7.3%) | |
| H-H grade | ||||
| Good (I-II) | 132 (96.4%) | 81 (98.8%) | 51 (92.7%) | 0.157 |
| Poor (III-IV) | 5 (3.6%) | 1 (1.2%) | 4 (7.3%) | |
| mFS | ||||
| 0-1 | 78 (56.9%) | 70 (85.4%) | 8 (14.5%) | <0.001 |
| 2–4 | 59 (43.1%) | 12 (14.6%) | 47 (85.5%) | |
| Complication | ||||
| Hydrocephalus | 14 (10.2%) | 3 (3.7%) | 11 (20.0%) | 0.001 |
| Cerebral vasospasm | 7 (5.1%) | 1 (1.2%) | 6 (10.9%) | 0.017 |
| Rebleeding | 1 (0.7%) | 0 | 1 (1.8%) | 0.401 |
| Pulmonary infections | 3 (2.2%) | 1 (1.2%) | 2 (3.6%) | 0.564 |
NASAH: nonaneurysmal subarachnoid hemorrhage, PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage, nPMN-SAH: nonperimesencephalic nonaneurysmal subarachnoid hemorrhage, GCS: Glasgow coma scale, H-H grade: Hunt-Hess grade, mFS: modified Fisher Scale; LOS: length of hospital stay.
Complications that developed during hospitalization, hydrocephalus, cerebral vasospasm, and rebleeding were compared. The nPMN-SAH group was associated with higher incidence of hydrocephalus and symptomatic cerebral vasospasm (p = 0.001 and 0.017, resp.). Only one patient experienced rebleeding but no definite source of bleeding was confirmed even though a repeat DSA was conducted. There was no significant difference in the rebleeding rate during hospitalization between the two groups (p = 0.401) (Table 2).
There were 14 patients (10.2%) who developed hydrocephalus. The risk factors for hydrocephalus in these patients were compared. There were significant differences in the GCS and mFS between the hydrocephalus group and nonhydrocephalus group (p < 0.001). Furthermore, patients who developed hydrocephalus were associated with significantly higher proportion of a diffuse bleeding pattern on imaging studies (e.g., nPMN-SAH and intraventricular hemorrhage) (p = 0.002 and p < 0.001, resp., Table 3).
Table 3.
Clinical characteristics of hydrocephalus patients in NASAH group.
| No hydrocephalus (n = 123) | Hydrocephalus (n = 14) | p | |
|---|---|---|---|
| Demography | |||
| Age | 56.4 ± 10.0 | 52.9 ± 13.3 | 0.147 |
| Gender (female) | 57 (46.3%) | 4 (28.6) | 0.205 |
| Smoker | 16 (13.0%) | 2 (14.3%) | 0.893 |
| Alcohol abuse | 18 (14.6%) | 3 (21.4%) | 0.504 |
| Hypertension | 26 (21.1%) | 4 (28.6%) | 0.524 |
| Diabetes | 5 (4.1%) | 2 (14.3%) | 0.100 |
| Clinical grade | |||
| GCS < 13 | 0 (0%) | 4 (28.6%) | <0.001 |
| Modified Fisher Scale > 1 | 56 (45.5%) | 11 (78.6%) | <0.001 |
| Bleed Pattern | |||
| PMN-SAH | 79 (63.4%) | 3 (21.4%) | 0.002 |
| nPMN-SAH | 44 (35.8%) | 11 (78.6%) | |
| Anterior circulationa | 96 (78.0%) | 12 (85.7%) | 0.506 |
| Posterior circulationb | 27 (21.9%) | 2 (14.3%) | |
| Intraventricular hemorrhage | 8 (6.5%) | 10 (71.4%) | <0.001 |
| Cortical hemorrhage | 22 (17.9%) | 3 (21.4%) | 0.745 |
EVD: external ventricular drain, GCS: Glasgow coma scale.
aAnterior circulation was characterized by blood mainly locating at anterior of the brain.
bPosterior circulation was characterized by blood mainly locating at posterior of the brain.
3.3. Outcome
A total of 21 patients (12 PMN-SAH and 9 nPMN-SAH) were lost to follow-up after discharge. The mRS and GOS were used to evaluate the outcomes for all patients. We found that patients with PMN-SAH had a better clinical outcome at 3 months after ictus with respect to both mRS (p < 0.001) and GOS (p = 0.003) (Table 4). Though there was no significant difference of GOS between the two groups at 1-year follow-up (p = 0.09), the nPMN-SAH group had a trend towards a higher incidence of mild disability (mRS = 2 or 3, p = 0.006, Table 4).
Table 4.
The prognosis of patient after charged from hospital.
| All NASAH (n = 116)∗ | PMN-SAH (n = 70) | nPMN-SAH (n = 46) | p value | |
|---|---|---|---|---|
| mRS at 3 months | ||||
| 0-1 | 96 (82.8%) | 66 (94.3%) | 30 (65.2%) | <0.001 |
| 2-3 | 17 (14.7%) | 4 (5.7%) | 13 (28.2%) | |
| 4–6 | 3 (2.5%) | 0 | 3 (6.6%) | |
| GOS at 3 months | ||||
| 5 | 110 (94.8%) | 70 (100%) | 40 (87.0%) | 0.003 |
| 4 | 3 (3.1%) | 0 | 3 (6.5%) | |
| 1–3 | 3 (3.1%) | 0 | 3 (6.5%) | |
| mRS at 1 year | ||||
| 0-1 | 78 (86.7%) | 46 (95.8%) | 32 (76.2%) | 0.006 |
| 2-3 | 10 (11.1%) | 2 (4.2%) | 8 (19.0%) | |
| 4–6 | 2 (2.2%) | 0 | 2 (4.8%) | |
| GOS at 1 year | ||||
| 5 | 87 (96.7%) | 48 (100%) | 39 (92.8%) | 0.09 |
| 4 | 1 (1.1%) | 0 | 1 (2.4%) | |
| 1–3 | 2 (2.2%) | 0 | 2 (4.8%) |
NASAH: nonaneurysmal subarachnoid hemorrhage; PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage; nPMN-SAH: nonperimesencephalic nonaneurysmal subarachnoid hemorrhage; GOS: Glasgow outcome scale, mRS: modified Rankin Scale.
∗21 patients (12 perimesencephalic and 9 nonperimesencephalic patients) were lost to follow-up after discharge.
3.4. BVR Pattern
The venous phase images on DSA for 3 NASAH patients were unavailable due to technical reasons. The distribution of BVR subtypes of three groups are listed in Table 5. Compared with aSAH patients, there was a significant difference of BVR distribution in both of the PMN-SAH group (p = 0.003) and nPMN-SAH group (p = 0.021). However, there was no statistically significant difference of unilateral BVR development between PMN-SAH and nPMN-SAH groups (p = 0.950). To further analyze the correlation of hemorrhage type and BVR development, the bilateral BVRs were classified as four subgroups, AA, AB/BB, AC/BC, and CC, respectively. A significant statistical difference was only found between PMN-SAH and aSAH groups (p = 0.028, Table 6).
Table 5.
Type of BVR in nonaneurysmal subarachnoid hemorrhage and aneurysmal subarachnoid hemorrhage patients.
| BVR type | PMN-SAH | nPMN-SAH | aSAH | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| Unilateral | n = 160 | n = 100 | n = 266 | 0.003 | 0.021 | 0.950 |
|
| ||||||
| A | 61 (38.1%) | 39 (39.0%) | 147 (55.3%) | |||
| B | 48 (30.0%) | 31 (31.0%) | 61 (22.9%) | |||
| C | 51 (31.9%) | 30 (30.0%) | 58 (22.8%) | |||
|
| ||||||
| Bilateral | n = 80 | n = 50 | n = 133 | 0.028 | 0.066 | 0.882 |
|
| ||||||
| AA | 15 (18.8%) | 9 (18.0%) | 50 (37.6%) | |||
| AB, BB | 26 (32.5%) | 16 (32.0%) | 38 (28.6%) | |||
| AC, BC | 26 (32.5%) | 19 (38.0%) | 32 (24.1%) | |||
| CC | 13 (16.2%) | 6 (12.0%) | 13 (9.8%) | |||
BVR: basal vein of Rosenthal; PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage; nPMN-SAH: nonperimesencephalic nonaneurysmal subarachnoid hemorrhage.
p1: statistical analysis was proceeded between PMN-SAH and aSAH group.
p2: statistical analysis was proceeded nPMN-SAH and aSAH group.
p3: statistical analysis was proceeded PMN-SAH and nPMN-SAH group.
Table 6.
Type of BVR in nonaneurysmal subarachnoid hemorrhage and aneurysmal subarachnoid hemorrhage patients.
| Author/year | Unilateral BVR type | Bilateral BVR type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMN-SAH (%) | aSAH (%) | PMN-SAH (%) | aSAH (%) | |||||||||||
| A | B | C | A | B | C | AA | AB BB |
AC BC |
CC | AA | AB BB |
AC BC |
CC | |
| Watanabe et al. [17]/2002 | 3 (25) | 2 (17) | 7 (58) | 79 (41) | 70 (37) | 42 (22) | 0 | NS | NS | NS | 22 (22) | NS | NS | NS |
| Alén et al. [18]/2008∗ | 36 (24) | 66 (44) | 47 (32) | 116 (59) | 51 (26) | 31 (15) | 12 (13) | 41 (45) | 38 (42) | 54 (48) | 34 (30) | 24 (21) | ||
| van der Schaaf et al. [19]/2008 | 21 (19) | 48 (43) | 21 (37) | 49 (58) | 26 (31) | 9 (11) | 4 (7) | NS | NS | 29 (53) | 15 (36) | NS | NS | 8 (19) |
| Yamakawa et al. [20]/2008 | 10 (29) | 7 (20) | 18 (51) | 111 (57) | 61 (31) | 23 (12) | 2 (11) | NS | NS | NS | 32 (29) | NS | NS | NS |
| Daenekindt et al. [21]/2008 | 49 (42) | 34 (30) | 32 (28) | 50 (46) | 31 (29) | 27 (25) | 13 (22) | 18 (31) | 28 (47) | 10 (17) | 28 (47) | 21 (36) | ||
| Song et al. [22]/2010 | NS | NS | NS | NS | NS | NS | 10 (31) | 8 (25) | 8 (25) | 6 (19) | 34 (60) | 12 (21) | 9 (16) | 2 (4) |
| Kawamura et al. [23]/2011 | 6 (32) | 8 (42) | 5 (26) | 33 (49) | 27 (35) | 7 (10.4) | 0 | 5 (50) | 3 (30) | 1 (10) | 14 (42) | 12 (36) | 7 (21) | |
| Sabatino et al. [24]/2014∗ | 36 (46) | 31 (39) | 12 (15) | 50 (66) | 8 (11) | 18 (24) | NS | NS | NS | NS | NS | NS | NS | NS |
| Buyukkaya et al. [12]/2014 | NS | NS | NS | NS | NS | NS | 14 (35) | 3 (9) | 12 (34) | 6 (17%) | 26 (74) | 5 (14) | 3 (9) | 1 (3) |
PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage; aSAH: aneurysmal subarachnoid hemorrhage; NS means no data was recorded. ∗The idiopathic subarachnoid hemorrhage has not been described in detail; no group was divided.
3.5. Repeated DSA Findings
To evaluate the utility of repeated DSA examination in patients with initially negative DSA findings, we compared the detection rate of a responsible intracranial lesion by the repeated DSA in both of the subgroups and also included data from related literature for analysis. A total of 60 patients underwent a repeated DSA examination in our series. On repeat evaluation, 4 of 31 patients with nPMN-SAH were diagnosed with a responsible intracranial aneurysm, resulting in a detection rate of 12.9% on repeat DSA examination; however none of the PMN-SAH patients had positive findings on their repeated DSA (Table 7). According to the data from recent studies, the detection rate of a responsible intracranial aneurysm by repeated DSA was 12.5% in the nPMN-SAH subgroup and 1.2% in the PMN-SAH subgroup (Table 7).
Table 7.
Diagnostic yield of a repeated DSA investigation.
| Author/year | Study type | PMN-SAH | Positive | Misdiagnose rate | nPMN-SAH | Positive | Misdiagnose rate |
|---|---|---|---|---|---|---|---|
| Topcuoglu et al. [10]/2003 | Retrospective | 31 | 0 | 0 | 36 | 3 | 8.3% |
| Jung et al. [25]/2006 | Retrospective | 65 | 1 | 1.5% | 37 | 17 | 45.9% |
| Huttner et al. [11]/2006 | Prospective | 38 | 0 | 0 | NS | NS | NS |
| Little et al. [26]/2007 | Retrospective | 23 | 1 | 4.3% | 59 | 5 | 8.5% |
| Gupta et al. [3]/2009 | Retrospective | 18 | 0 | 0 | 43 | 2 | 4.7% |
| Carvi y Nievas and Archavlis [27]/2009 | Retrospective | 8 | 0 | 0 | 3 | 1 | 33.3% |
| Agid et al. [9]/2010 | Retrospective | 28 | 0 | 0 | 28 | 4 | 14.3% |
| Fontanella et al. [28]/2011 | Retrospective | 23 | 0 | 0 | 72 | 9 | 12.5% |
| Maslehaty et al. [29]/2011 | Retrospective | 34 | 1 | 2.9% | 120 | 13 | 10.8% |
| Kelliny et al. [30]/2011 | Retrospective | 35 | 0 | 0 | 37 | 6 | 16.2% |
| Delgado Almandoz et al. [31]/2012 | Prospective | 29 | 1 | 3.4% | 39 | 2 | 5.1% |
| Lin et al. [32]/2012 | Retrospective | 27 | 0 | 0 | 41 | 2 | 4.9% |
| DW et al. [8]/2012 | Retrospective | 6 | 0 | 0 | 12 | 2 | 16.7% |
| Present study | Retrospective | 31 | 0 | 0 | 29 | 4 | 13.8% |
| Total | 365 | 4 | 1.1% | 556 | 70 | 12.6% | |
PMN-SAH: perimesencephalic nonaneurysmal subarachnoid hemorrhage; nPMN-SAH: nonperimesencephalic nonaneurysmal subarachnoid hemorrhage; NS means no data was recorded.
4. Discussion
SAH patients with negative initial DSA findings, usually defined as NASAH, can be a management challenge as the optimal management scheme for these patients remains controversial. The present study analyzes the data of SAH patients with negative initial DSA findings from our center. We divided those patients into the two subgroups of PMN-SAH and nPMN-SAH. The development of BVR type in each group was compared with an aSAH control group to help elucidate the source of bleeding. The necessity of repeated DSA examinations was evaluated in each group by calculating the positive finding rate from second angiogram.
It is wildly accepted that NASAH patients have a more favorable clinical course and a lower incidence of complications when compared to those with aSAH [34]. The subgroup of PMN-SAH was first described by van Gijn et al. [35] as a benign entity, characterized by the distribution of the subarachnoid hemorrhage mainly or only in the perimesencephalic cisterns. However, the nPMN-SAH group has a more diffuse distribution of the subarachnoid hemorrhage that is more similar to aSAH [15]. A diagnosis of nPMN-SAH is associated with higher rate of complications, such as hydrocephalus, cerebral vasospasm, and cerebral infarction [5]. Hydrocephalus is one of the most common complications of NASAH, normally identified by symptoms of elevated intracranial pressure (progressive headache, altered sensorium) or findings on head CT [36]. The incidence of hydrocephalus from the present cohort was 14.3%, which is in agreement with previous reports [34]. We also found that, in the nonperimesencephalic pattern, the presence of intraventricular hemorrhage or poorer clinical grade on presentation (GCS < 13 and modified Fisher Scale > 2) were associated with the development of hydrocephalus.
Interestingly, despite the repeated imaging studies that have been performed on NASAH patients, the etiology of bleeding remains obscure. The potential pathogenesis may include a venous system variant [20], capillary abnormality, intracranial basilar dissection, ruptured perforating artery [37], cavernous malformation [38], or capillary telangiectasia [15]. Although both arterial and venous origins for the SAH have been proposed, most of the studies favor a venous source of PMN-SAH [39]. It was first hypothesized by Watanabe et al. [17] that a large portion of the BVRs in PMN-SAH patients vary from a normal configuration with drainage into dural sinuses instead of the vein of Galen. Similarly, van der Schaaf et al. [19] reported that primitive venous drainage was more common in patients with PMN-SAH and that the venous drainage variation was ipsilateral to the side of bleeding. Most of the subsequent studies supported the theory that the drainage into the deep venous drain system (the vein of Rosenthal variant) was related to the PMN-SAH [12, 18–20, 22–24]. However, a study from Daenekindt et al. [21] suggested otherwise (Table 6), but this may be explained by its relatively small patient series, short follow-up period, or differences in diagnostic criteria. Similar to prior publications, our present study demonstrated a significant difference in BVR anatomy between the PMN-SAH and aSAH groups, regardless of unilateral or bilateral BVR type (p = 0.003, 0.028) and that the primitive venous configuration was ipsilateral to the source of the bleeding. However, it remains unclear exactly how the venous drainage variation contributes to PMN-SAH.
However, unlike with the PMN-SAH group, the relationship between venous drainage and nPMN-SAH was rarely described previously. It is generally hypothesized that the nPMN pattern may have resulted from an arterial source to account for its relatively “malignant” clinical presentation and extensive blood distribution into the cisterns and parenchyma. Consequently, this pattern of SAH is also referred to as aneurysm-like SAH. Interestingly, our study revealed that there was also a significant difference in the unilateral BVR type between the nPMN-SAH and aSAH groups (p = 0.021). However, for bilateral venous drainage, there was no significant difference between the two groups (p = 0.066). The inconsistent findings between the unilateral and bilateral BVR types could be explained by a contribution of true aSAH that were misclassified as nPMN-SAH because of an initial negative DSA. Although a higher portion of nPMN-SAH with negative initial DSA findings were eventually confirmed to have resulted from ruptured intracranial aneurysms, compared to PMN-SAH, most sources of nPMN-SAH remain idiopathic. Therefore, we conclude that if a ruptured intracranial aneurysm can be absolutely excluded, the bleeding source of a true nPMN-SAH may be similar to that found in PMN-SAH.
Another main concern in the management of NASAH is to evaluate the necessity of repeated DSA examinations for each individual. While DSA is currently the standard method to diagnose an intracranial aneurysm for patients suffering from SAH, there are risks to the procedure. Some studies have shown that catheter angiography has up to a 2.6% risk of permanent neurologic complications in NASAH [40]. Recently, numerous reports have proposed the use of noninvasive techniques to diagnose an intracranial aneurysm, such as CTA and magnetic resonance angiography (MRA) [41, 42]. The accuracy of the DSA result can be affected by numerous factors: the resolution of the DSA device, the quality of the acquired scan, the 3D reconstruction capabilities, the interval between the onset of the symptoms and the examination, and the experience of the technician. Furthermore, a small or dissecting aneurysm, hemorrhage or vasospasm concealing the aneurysm, or technical deficiencies can lead to a false-negative result upon the initial examination [43]. Even though improvements in imaging technology have decreased the incidence of misdiagnosis, there are still nearly 15% of SAH patients who have negative findings upon their initial DSA examination [43, 44]. Since missing an aneurysmal source on DSA would expose patients to the extensive morbidity and mortality of rebleed, most practitioners carry out repeat DSA exams on SAH patients with a negative initial DSA. Consequently, it would be of value to select the specific individuals who would benefit from repeated DSA exams.
According to our present study, a total of 60 patients underwent a repeat DSA examination. Four of 31 patients with nPMN-SAH were ultimately diagnosed with a ruptured intracranial aneurysm, resulting in a detection rate of 12.9% for a repeat DSA. However none of the PMN-SAH patients in our study had positive findings on their repeat DSA. To further address this finding, a thorough review of data from past studies was evaluated. There are 13 studies in the past 15 years that discuss a positive finding on repeat DSA exams in SAH patients with negative initial DSA findings. For the pooled data of PMN-SAH patients, a repeat DSA detected a culprit aneurysm in only 4 of 151 patients [2, 3, 8, 27, 28, 30, 32] (Table 7) [25, 26, 29, 31]. Combined with our data, the overall misdiagnosis rate is only 1.1%, which is likely lower than the risk of the DSA procedure itself. We therefore propose that it may be acceptable to follow up PMN-SAH patients by noninvasive image studies rather than DSA. Some authors go further, even raising the possibility that it is reasonable to manage PMN-SAH patients completely with noninvasive cerebral vascular imaging. The preference at our institution is that unless the initial CT scan with high quality vascular imaging is completed within several hours after the onset of symptoms, all PMN-SAH patients need at least one DSA to exclude a ruptured intracranial aneurysm. However, for centers without available neurointerventionalists, the DSA can be delayed because PMN-SAH is usually associated with a benign clinical course and good prognosis. Unlike the extremely low rate of aneurysm detection in PMN-SAH patients, our data revealed that 12.6% of nPMN-SAH patients were ultimately found to have a responsible aneurysm on the second DSA. In previous studies, the misdiagnosis rate of the initial DSA in nPMN-SAH patients varied greatly (misdiagnose rate from 4.7% to 45.9%), which is likely due to several factors, as previously mentioned. After pooling all the published studies, the overall misdiagnose rate was 12.5% (66 out of 527, Table 7), which is similar to the findings at our institution. Therefore, we strongly recommend a repeat DSA examination for patients with nPMN-SAH who had negative initial findings. Additionally, it is advisable to use 3D image acquisition for both internal carotid arteries and the vertebral arteries in the initial and repeat DSA to optimize the chances of finding small lesions. Since the aneurysms discovered on repeat imaging tend to be miniature in size and are found at the bifurcation of small perforating arteries, we also suggest consultation with an experienced neuroradiological or/and neurointervention physician to establish the diagnosis.
5. Conclusion
Managing SAH patients with negative initial DSA findings can be challenging. Based on the results of our present study and a review of the pertinent literature, the PMN-SAH subgroup usually has a benign clinical course and a repeat DSA very seldom reveals a ruptured intracranial aneurysm. More importantly, nPMN-SAH patients are associated with higher complication rate and incidence of a ruptured aneurysm. Therefore, we strongly recommend a repeat DSA in patients with nPMN-SAH pattern on initial imaging.
Acknowledgments
The authors thank Rudi Scharnweber for revising the manuscript. This study was funded by the National Natural Science Foundation of China (no. 81671131 to Jing Xu).
Conflicts of Interest
The authors report no conflicts of interest concerning the materials or methods used in this study or findings specified in this paper.
Authors' Contributions
Drs Liang Xu and Yuanjian Fang contributed equally to this work.
References
- 1.Lucke-Wold B., Logsdon A., Manoranjan B., et al. Aneurysmal subarachnoid hemorrhage and neuroinflammation: a comprehensive review. International Journal of Molecular Sciences. 2016;17(4, article 497) doi: 10.3390/ijms17040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa Y., Suzuki H., Uekawa K., Kawano T., Kim-Mitsuyama S. Characteristics of cerebrovascular injury in the hyperacute phase after induced severe subarachnoid hemorrhage. Translational Stroke Research. 2015;6(6):458–466. doi: 10.1007/s12975-015-0423-9. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S. K., Gupta R., Khosla V. K., et al. Nonaneurysmal nonperimesencephalic subarachnoid hemorrhage: is it a benign entity? Surgical Neurology. 2009;71(5):566–572. doi: 10.1016/j.surneu.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Rinkel G. J., Wijdicks E. F., Vermeulen M., et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR American Journal of Neuroradiology. 1991;12(5):829–834. [PMC free article] [PubMed] [Google Scholar]
- 5.Kong Y., Zhang J. H., Qin X. Perimesencephalic subarachnoid hemorrhage: risk factors, clinical presentations, and outcome. Acta Neurochirurgica Supplement. 2011;110, part 1:197–201. doi: 10.1007/978-3-7091-0353-1_34. [DOI] [PubMed] [Google Scholar]
- 6.Du C. P., Ke K. F. The clinical analysis of nonaneurysmal subarachnoid hemorrhage. Zhonghua Nei Ke Za Zhi. 2011;50:408–410. [PubMed] [Google Scholar]
- 7.Herrmann L. L., Zabramski J. M. Nonaneurysmal subarachnoid hemorrhage: a review of clinical course and outcome in two hemorrhage patterns. The Journal of Neuroscience Nursing : Journal of The American Association of Neuroscience Nurses. 2007;39(3):135–142. doi: 10.1097/01376517-200706000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Yu D. W., Jung Y. J., Choi B. Y., Chang C. H. Subarachnoid hemorrhage with negative baseline digital subtraction angiography: is repeat digital subtraction angiography necessary? Journal of Cerebrovascular and Endovascular Neurosurgery. 2012;14(3):210–215. doi: 10.7461/jcen.2012.14.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agid R., Andersson T., Almqvist H., et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: when is digital subtraction angiography still needed? American Journal of Neuroradiology. 2010;31(4):696–705. doi: 10.3174/ajnr.A1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topcuoglu M. A., Ogilvy C. S., Carter B. S., Buonanno F. S., Koroshetz W. J., Singhal A. B. Subarachnoid hemorrhage without evident cause on initial angiography studies: diagnostic yield of subsequent angiography and other neuroimaging tests. Journal of Neurosurgery. 2003;98(6):1235–1240. doi: 10.3171/jns.2003.98.6.1235. [DOI] [PubMed] [Google Scholar]
- 11.Huttner H. B., Hartmann M., Köhrmann M., et al. Repeated digital substraction angiography after perimesencephalic subarachnoid hemorrhage? Journal of Neuroradiology. 2006;33(2):87–89. doi: 10.1016/S0150-9861(06)77236-4. [DOI] [PubMed] [Google Scholar]
- 12.Buyukkaya R., Yildirim N., Cebeci H., et al. The relationship between perimesencephalic subarachnoid hemorrhage and deep venous system drainage pattern and calibrations. Clinical Imaging. 2014;38(3):226–230. doi: 10.1016/j.clinimag.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Dupont S. A., Lanzino G., Wijdicks E. F. M., Rabinstein A. A. The use of clinical and routine imaging data to differentiate between aneurysmal and nonaneurysmal subarachnoid hemorrhage prior to angiography: clinical article. Journal of Neurosurgery. 2010;113(4):790–794. doi: 10.3171/2010.4.JNS091932. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H., Shiba M., Nakatsuka Y., Nakano F., Nishikawa H. Higher cerebrospinal fluid pH may contribute to the development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Translational Stroke Research. 2017;8(2):165–173. doi: 10.1007/s12975-016-0500-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y. W., Lawson M. F., Hoh B. L. Nonaneurysmal subarachnoid hemorrhage: an update. Current Atherosclerosis Reports. 2012;14(4):328–334. doi: 10.1007/s11883-012-0256-x. [DOI] [PubMed] [Google Scholar]
- 16.Kang P., Raya A., Zipfel G. J., Dhar R. Factors associated with acute and chronic hydrocephalus in nonaneurysmal subarachnoid hemorrhage. Neurocritical Care. 2016;24(1):104–109. doi: 10.1007/s12028-015-0152-7. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe A., Hirano K., Kamada M., et al. Perimesencephalic nonaneurysmal subarachnoid haemorrhage and variations in the veins. Neuroradiology. 2002;44(4):319–325. doi: 10.1007/s00234-001-0741-3. [DOI] [PubMed] [Google Scholar]
- 18.Alén J. F., Lagares A., Campollo J., et al. Idiopathic subarachnoid hemorrhage and venous drainage: are they related? Neurosurgery. 2008;63(6):1106–1112. doi: 10.1227/01.NEU.0000335777.14055.71. [DOI] [PubMed] [Google Scholar]
- 19.van der Schaaf I. C., Velthuis B. K., Gouw A., Rinkel G. J. E. Venous drainage in perimesencephalic hemorrhage. Stroke. 2004;35(7):1614–1618. doi: 10.1161/01.str.0000131657.08655.ce. [DOI] [PubMed] [Google Scholar]
- 20.Yamakawa H., Ohe N., Yano H., Yoshimura S., Iwama T. Venous drainage patterns in perimesencephalic nonaneurysmal subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2008;110(6):587–591. doi: 10.1016/j.clineuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Daenekindt T., Wilms G., Thijs V., Demaerel P., Van Calenbergh F. Variants of the basal vein of Rosenthal and perimesencephalic nonaneurysmal hemorrhage. Surgical Neurology. 2008;69(5):526–529. doi: 10.1016/j.surneu.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Song J. H., Yeon J. Y., Kim K. H., Jeon P., Kim J. S., Hong S.-C. Angiographic analysis of venous drainage and a variant basal vein of Rosenthal in spontaneous idiopathic subarachnoid hemorrhage. Journal of Clinical Neuroscience. 2010;17(11):1386–1390. doi: 10.1016/j.jocn.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura Y., Narumi O., Chin M., Yamagata S. Variant deep cerebral venous drainage in idiopathic subarachnoid hemorrhage. Neurologia Medico-Chirurgica. 2011;51(2):97–100. doi: 10.2176/nmc.51.97. [DOI] [PubMed] [Google Scholar]
- 24.Sabatino G., Della Pepa G. M., Scerrati A., et al. Anatomical variants of the basal vein of Rosenthal: prevalence in idiopathic subarachnoid hemorrhage. Acta Neurochirurgica. 2014;156(1):45–51. doi: 10.1007/s00701-013-1907-6. [DOI] [PubMed] [Google Scholar]
- 25.Jung J. Y., Kim Y. B., Lee J. W., Huh S. K., Lee K. C. Spontaneous subarachnoid haemorrhage with negative initial angiography: a review of 143 cases. Journal of Clinical Neuroscience. 2006;13(10):1011–1017. doi: 10.1016/j.jocn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Little A. S., Garrett M., Germain R., et al. Evaluation of patients with spontaneous subarachnoid hemorrhage and negative angiography. Neurosurgery. 2007;61(6):1139–1151. doi: 10.1227/01.neu.0000306091.30517.e7. [DOI] [PubMed] [Google Scholar]
- 27.Carvi y Nievas M. N., Archavlis E. Atypical causes of nontraumatic intracranial subarachnoid hemorrhage. Clinical Neurology and Neurosurgery. 2009;111(4):354–358. doi: 10.1016/j.clineuro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Fontanella M., Rainero I., Panciani P. P., et al. Subarachnoid hemorrhage and negative angiography: clinical course and long-term follow-up. Neurosurgical Review. 2011;34(4):477–484. doi: 10.1007/s10143-011-0323-8. [DOI] [PubMed] [Google Scholar]
- 29.Maslehaty H., Petridis A. K., Barth H., Mehdorn H. M. Diagnostic value of magnetic resonance imaging in perimesencephalic and nonperimesencephalic subarachnoid hemorrhage of unknown origin: clinical article. Journal of Neurosurgery. 2011;114(4):1003–1007. doi: 10.3171/2010.6.JNS10310. [DOI] [PubMed] [Google Scholar]
- 30.Kelliny M., Maeder P., Binaghi S., Levivier M., Regli L., Meuli R. Cerebral aneurysm exclusion by CT angiography based on subarachnoid hemorrhage pattern: a retrospective study. BMC Neurology. 2011;11, article 8 doi: 10.1186/1471-2377-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado Almandoz J. E., Jagadeesan B. D., Refai D., et al. Diagnostic yield of repeat catheter angiography in patients with catheter and computed tomography angiography negative subarachnoid hemorrhage. Neurosurgery. 2012;70(5):1135–1142. doi: 10.1227/neu.0b013e318242575e. [DOI] [PubMed] [Google Scholar]
- 32.Lin N., Zenonos G., Kim A. H., et al. Angiogram-negative subarachnoid hemorrhage: relationship between bleeding pattern and clinical outcome. Neurocritical Care. 2012;16(3):389–398. doi: 10.1007/s12028-012-9680-6. [DOI] [PubMed] [Google Scholar]
- 33.Brown C. M., Bushnell C. D., Samsa G. P., Goldstein L. B., Colton C. A. Chronic systemic immune dysfunction in african-americans with small vessel-type ischemic stroke. Translational Stroke Research. 2015;6(6):430–436. doi: 10.1007/s12975-015-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapadia A., Schweizer T. A., Spears J., Cusimano M., Macdonald R. L. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: diagnosis, pathophysiology, clinical characteristics, and long-term outcome. World Neurosurgery. 2014;82(6):1131–1143. doi: 10.1016/j.wneu.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 35.van Gijn J., van Dongen K. J., Vermeulen M., Hijdra A. Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology. 1985;35(4):493–497. doi: 10.1212/wnl.35.4.493. [DOI] [PubMed] [Google Scholar]
- 36.Schlunk F., Greenberg S. M. The pathophysiology of intracerebral hemorrhage formation and expansion. Translational Stroke Research. 2015;6(4):257–263. doi: 10.1007/s12975-015-0410-1. [DOI] [PubMed] [Google Scholar]
- 37.Lansberg M. G. Concurrent presentation of perimesencephalic subarachnoid hemorrhage and ischemic stroke. Journal of Stroke and Cerebrovascular Diseases. 2008;17(4):248–250. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurosu A., Suzukawa K., Amo M., Horinaka N., Arai H. Perimesencephalic non-aneurysmal subarachnoid hemorrhage caused by cavernous sinus thrombosis—case report. Neurologia Medico-Chirurgica. 2007;47(6):258–260. doi: 10.2176/nmc.47.258. [DOI] [PubMed] [Google Scholar]
- 39.Cruz J. P., Sarma D., Noel de Tilly L. Perimesencephalic subarachnoid hemorrhage: when to stop imaging? Emergency Radiology. 2011;18(3):197–202. doi: 10.1007/s10140-011-0939-6. [DOI] [PubMed] [Google Scholar]
- 40.Ruigrok Y. M., Rinkel G. J. E., Buskens E., Velthuis B. K., Van Gijn J. Perimesencephalic hemorrhage and CT angiography: a decision analysis. Stroke. 2000;31(12):2976–2983. doi: 10.1161/01.STR.31.12.2976. [DOI] [PubMed] [Google Scholar]
- 41.Karamessini M. T., Kagadis G. C., Petsas T., et al. CT angiography with three-dimensional techniques for the early diagnosis of intracranial aneurysms. Comparison with intra-arterial DSA and the surgical findings. European Journal of Radiology. 2004;49(3):212–223. doi: 10.1016/S0720-048X(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 42.Muir K. W., Macrae I. M. Neuroimaging as a selection tool and endpoint in clinical and pre-clinical trials. Translational Stroke Research. 2016;7(5):368–377. doi: 10.1007/s12975-016-0487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hochmuth A., Spetzger U., Schumacher M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR American Journal of Neuroradiology. 2002;23(7):1199–1205. [PMC free article] [PubMed] [Google Scholar]
- 44.Ishihara H., Kato S., Akimura T., Suehiro E., Oku T., Suzuki M. Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. Journal of Clinical Neuroscience. 2007;14(3):252–255. doi: 10.1016/j.jocn.2006.01.011. [DOI] [PubMed] [Google Scholar]