Abstract
Background
Multiple sclerosis (MS) initiates with a first attack or clinically isolated syndrome (CIS). The importance of an early treatment in MS leads to the search, as soon as possible, for novel biomarkers which can predict conversion from CIS to MS.
Objective
The purpose of this study was to assess the predictive value of the kappa index ( index), using kappa free light light chains (FLCs) in cerebrospinal fluid (CSF), for the conversion of CIS patients to MS, and compare its accuracy with other parameters used in clinical practice.
Methods
FLC levels were analysed in CSF from 176 patients: 70 as control group, 77 CIS, and 29 relapsing–remitting MS. FLC levels were quantified by nephelometry.
Results
Index sensitivity and specificity (93.1%; 95.7%) was higher than those from the immunoglobulin G (IgG) index (75.9%; 94.3%), and lower than those from oligoclonal IgG bands (OCGBs) (96.5%; 98.6%). The optimal cut-off for index was 10.62. Most of the CIS patients with index >10.62 presented OCGBs, IgG index >0.56 and fulfilled magnetic resonance imaging (MRI) criteria.
Conclusion
CIS patients above index cut-off of 10.62 present 7.34-fold risk of conversion to MS than CIS below this value. The index correlated with positive OCGBs, IgG index above 0.56 and MRI criteria.
Keywords: Multiple sclerosis, cerebrospinal fluid, immunoglobulin free light chains, immunoglobulin kappa free light-chains, demyelinating diseases, nephelometry, turbidimetry, clinically isolated syndrome
Introduction
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system (CNS). In most patients, MS initiates with a first attack or a clinically isolated syndrome (CIS),1 which is commonly presented as unilateral optic neuritis, a brainstem syndrome, hemispheric motor or sensitive symptoms or partial myelitis.2 A certain number of CIS patients develop MS over time, while another group of patients never convert to MS. Studies vary widely in the proportion of CIS patients who develop MS. A recent observational study showed that the risk of developing MS was 57% after two years of follow-up, and 75% after four years of follow-up according to the 2001 McDonald criteria.3
The most common age of onset for MS is between 20–40 years, and is more frequent in women.4,5 A study developed over a 20-year period in the Northern Seville District of southern Spain showed that the prevalence of MS as of 31 December 2011 was 90.2 cases per 100,000 people. The incidence was 4.6 per 100,000 and increased in women, but not in men. Furthermore, patients are most likely to be diagnosed in their mid-30 s and females are most likely to present relapsing–remitting MS (RRMS) with a sex ratio female/male of 2.5:1.6
As there is no single clinical feature or diagnostic test sufficient for the diagnosis of this disease, diagnostic criteria have been developed based on the demonstration of lesions disseminated in space (DIS) and disseminated in time (DIT), and after exclusion of alternative causes. In recent years, criteria for the diagnosis of MS have changed, mainly due to the incorporation of new magnetic resonance imaging (MRI) criteria.2 The 2001 McDonald diagnostic criteria defined DIS by the fulfilling of at least three of four Barkhof-Tintoré criteria (BTC) or by the demonstration of at least two MRI lesions7,8 and intrathecal immunoglobulin G (IgG) synthesis. The 2001 McDonald criteria showed high specificity for clinically definite MS (CDMS) but limited sensitivity, which was improved in the 2005 revised McDonald criteria. In these criteria, DIS continued to require the presence of at least three of four BTC, but spinal cord lesions had a greater role than in the previous criteria. MRI criteria for DIT were simplified.9
In 2010, the International Panel on Diagnosis of MS (the ‘Panel') conducted a revision of the McDonald criteria in which it was reaffirmed that positive cerebrospinal fluid (CSF) findings (elevated IgG index or two or more oligoclonal bands (OCBs)) can be important to support the inflammatory demyelinating nature of the underlying condition, to evaluate alternative diagnoses, and to predict CDMS. However, when applying the simplified MRI criteria, the Panel believes that dispensing with MRI requirements in CSF-positive patients is not suitable, as CSF status was not evaluated for its contribution to the MRI criteria.1 Accordingly, when using the 2010 revision of the McDonald criteria, CSF examination is not required for the diagnosis of RRMS, although CSF findings are still part of the criteria for diagnosis of primary progressive MS (PPMS). In some cases, routine CSF outcomes, together with MRI, can help to identify patients with a high probability of developing MS after a first clinical event.10 The study of CSF to analyse intrathecal synthesis, using the IgG Index and OCBs, is employed to support the diagnosis of MS.
Oligoclonal IgG bands (OCGBs) are a qualitative measure of intrathecal synthesis, present in more than 95% patients with MS,11 and can be detected in the immunoglobulin region by isoelectrofocusing and immunoblotting techniques. Their presence in CSF but not in serum shows that synthesis of immunoglobulins has occurred within the CNS. Furthermore, oligoclonal IgM bands are associated with an aggressive disease course.12 To quantify IgG intrathecal synthesis, almost 20 formulae have been described in the literature, and one of the most commonly analysed is IgG index or the Tibbling-Link index.13 The main limitation of IgG from a CNS local source, is given by the lack of specificity, since intrathecal synthesis occurs in many neurological diseases not just MS, especially those with a central inflammatory involvement.14
So far, OCGBs are the most widely used biochemical marker used to predict MS, but due to the requirement of technicians with considerable methodological experience and the lack of standardisation,15 it is difficult to extend its use within a large number of clinical diagnostic laboratories. Different techniques for OCGB detection have appeared during the last years: two different semiautomated kits have been commercialised (using an anti-IgG antibody labelled with peroxidase) and a high-sensitivity method described (using an anti-IgG antibody labelled with alkaline phosphatase). A previous multicentre study developed in Spain, including Seville, assessed all these techniques. The best results are obtained with the high-sensitivity technique based on IgG detection with antibodies labelled with alkaline phosphatase, which was the one used in our study.16
Recent advances in understanding the aetiology and pathogenesis of MS have contributed to a better diagnosis and a plethora of therapies that substantially affect disease activity and may have a long-term impact on the course and prognosis of MS.17 Therefore, the search for novel biomarkers which can predict the conversion from CIS to MS is of major importance for early treatment in MS because, to date, there is no single diagnostic marker for MS which predicts conversion from CIS to MS.
Several studies indicated that high levels of kappa free light chains (FLCs) and lambda free light chains (λFLCs) in CSF, using either ELISA or nephelometry, may support the diagnosis of MS.18–28 A limitation of these studies can be found in the low comparability of FLC levels due to differences in methods. We used nephelometry providing that it is a validated technique as it is described in the literature.25 More recent studies suggest that high levels of FLC in CSF predict conversion to MS from CIS patients.25,28 In a healthy state, light chains are produced in excess over heavy chains, and the level of unbound light chains, or FLCs, in serum and in CSF is low. The production of FLCs might be abnormally enhanced under pathological conditions such as in certain inflammatory diseases like MS.29
It was the aim of the present investigation to assess the predictive value of the kappa index ( index) for the conversion of CIS patients to MS, and to compare its accuracy with other parameters used in clinical practice: OCGBs, IgG index and MRI criteria.
Materials and methods
In this study samples provided by the Biobank of Virgen Macarena University Hospital, Seville, Spain were used and were stored frozen at −80℃ until analysis. Informed consent was signed by all patients for the use of their specimen for research purposes. The study was approved by the Research Ethical Committee of the aforementioned hospital. CIS patients who had undergone a lumbar puncture for investigation of additional evidence of MS were included.
As a control group samples of patients with non-inflammatory neurologic diseases (NINDs) and with other inflammatory neurologic diseases (INDs) different from MS were used, preferably as homogeneous as possible both in age and diagnosis. Within this control group three subgroups were defined: a more homogeneous group in which all patients had normal pressure hydrocephalus (NPH), a second group in which patients had NINDs different from NPH (ELA (one), dizziness (two), epilepsy (four), facial pain (one), headache (four), miscellaneous (three)), and a third group with INDs different from MS (Sjögren syndrome (one), vasculitis (nine), DEVIC (one), miscellaneous (three)) (characteristics shown in Table 1).
Table 1.
Characteristics of patients.
| Patients (n) | Female (%) | Age (years) | FLCs in CSF | index | Lambda index | |
|---|---|---|---|---|---|---|
| Control group | ||||||
| Group 1: NPH | 41 | 11 (26.8) | 75.72 (40–87) | 0.16 (0.10–0.22) | 1.62 (0.99–2.27) | 1.28 (0.93–1.68) |
| Group 2: NIND | 15 | 10 (66.7) | 39.11 (15–71) | 0.12 (0.10–0.20) | 3.37 (1.95–7.05) | 1.35 (0.96–1.90) |
| Group 3: IND | 14 | 9 (64.3) | 44.77 (30–75) | 0.13 (0.04–0.20) | 2.86 (1.47–7.00) | 1.10 (0.81–2.71) |
| Total | 70 | 30 (42.9) | 61.69 (15–87) | 0.15 (0.10–0.20) | 1.96 (1.13–3.56) | 1.27 (0.93–1.69) |
| Case group | ||||||
| Group 4: CIS | 77 | 60 (77.9) | 35.26 (15–62) | 0.73 (0.13–3.2) | 35.61 (4.48–132.73) | 3.81 (1.49–16.95) |
| Group 5: RRMS | 29 | 17 (58.6) | 34.81 (17–57) | 1.45 (0.52–6.67) | 88 (33.96–295.13) | 5.43 (2.01–19.40) |
| Total of patients | 176 | 107 (60.8) | 45.70 (15–87) | |||
The patients were divided in five groups: the control group was classified in three subgroups: normal pressure hydrocephalus (NPH), other non-inflammatory neurologic disease (NIND) different from NPH and other inflammatory neurologic disease (IND) different from multiple sclerosis (MS); case group: Clinically isolated syndrome (CIS) and relapsing–remitting multiple sclerosis (RRMS). Data are shown as the mean and the range for age; kappa free light chains (FLCs) in cerebrospinal fluid (CSF), index and lambda index were found to be non-parametric after applying the Shapiro-Wilk test, so we calculated the median level and the interquartile range (IQR).
As a case group those already diagnosed who had had a first clinical event as CIS and who had had a study of OCGBs to support their diagnosis were included. Subjects who had had at least two years of follow-up after the first clinical presentation were selected. Patients under the age of 14 years were excluded from the study for being atypical cases of early MS. The case group was divided into two subgroups: CIS and RRMS (characteristics shown in Table 1). All diagnoses of patients with CDMS were performed by clinical evaluation and MRI of the patient, considering the McDonald criteria, and supported by laboratory tests (OCGBs).
During diagnosis of patients, levels of FLC and λFLC in the CSF of patients were studied. The use of index was calculated as
that takes into account the blood-CSF barrier function, allows increasing the diagnostic accuracy of FLC analysis and avoids false positives.26 OCGB, IgG index, MRI criteria, BTC, CDMS criteria and conversion to MS, were also registered.
The FLC level was quantified by endpoint nephelometry using the Human Kappa Freelite kit (The Binding Site Group Ltd) on the Siemens BN II analyzer. The measurement principle is based on the detection of the scattered light intensity. This method uses polyclonal antibodies coated onto polystyrene latex for FLC. According to the manufacturer, the lower detection limit is 0.06 mg/l. The sample volume needed for each analysis is 300 μl.
In this study OCGB data which had been determined by isoelectric focusing (IEF) followed by transfer and IgG immunodetection by an alkaline phosphatase-labeled anti-IgG antibody was used, following the protocol described by Villar et al.30 OCGB results were reported as positive or negative.
The data used of IgG index had been provided by the free software Protein Statistic in CSF Analysis with Reibergrams, v.4.17. For these analyses IgG and albumin concentrations were measured in fresh CSF and serum samples by nephelometry on the Siemens BN II analyzer.
Statistical analysis
Statistical analysis was performed using SPSS software v.20.0 (IBM Corp., Armonk, New York, USA, 2011) and MedCalc v.11.4.2.0 (2010). We used the Chi square or Fisher test for categorical variables and t student test for quantitative variables for comparison between groups. Kruskal-Wallis was used to compare the medians between more than two independent groups and Kaplan-Meier analysis for survival curve comparison between defined groups (log-rank test). Hazard ratios (HRs) were calculated using univariate and multivariate Cox regression analysis.
Results
In total, 176 patients were included in the study, of whom 60.8% were females. In the control group (n = 70), 41 presented with NPH, 15 with NIND and 14 with IND. According to previous studies, index showed the highest clinical sensitivity and specificity to detect MS. The λ index calculated as
did not provide greater value for the conversion of CIS patients to MS. As stated in the literature, the results were less informative for λ index than for index.21 As shown in Table 1, index median of 1.96 (95% confidence interval (CI): 1.13–3.56) in the control group, was lower than in CIS (n = 77) and in RRMS (n = 29), whose medians were 35.61 (95% CI: 4.48–132.73) and 88.00 (95% CI: 33.96–295.13) groups respectively, highly increased with regard to control group. We also observed a slight increase in λ index in CIS and RRMS patients (Table 1).
The percentage of females compared to males is higher in RRMS and the mean age of RRMS is in the mid-30s, which is consistent with previous studies.6
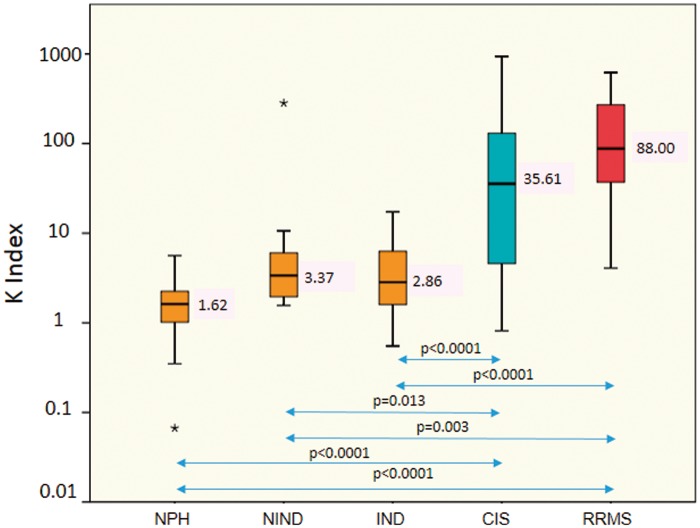
When analysing groups according to the index medians we found statistically significant differences applying the Kruskal-Wallis test between groups (p < 0.0001). After applying post-hoc Bonferroni test, there were no significant differences between control groups: group 1, 2 and 3 (p = 1.000). Therefore, the control groups 1, 2 and 3 were analysed together to increase the sample size of the control group. Nevertheless, there were differences statistically significant between control groups and CIS (p < 0.0001; p = 0.013; p < 0.0001) and between control groups and RRMS (p < 0.0001; p = 0.003; p < 0.0001) as is shown in Figure 1.
Figure 1.
Box and whisker plot index: the median and 25th and 75th percentiles (coloured boxes), the minimum and maximum (error bars), and the outliers (*) are indicated. Patients are divided in five groups: control group classified in three subgroups: normal pressure hydrocephalus (NPH) median 1.62, non-inflammatory neurologic diseases (NINDs) different from NPH median 3.37, other inflammatory neurologic diseases (INDs) different from multiple sclerosis (MS) median 2.86; case group: clinically isolated syndrome (CIS) median: 35.61, and relapsing–remitting multiple sclerosis (RRMS) median: 88.00; significant p values are shown between groups (double-headed arrows).
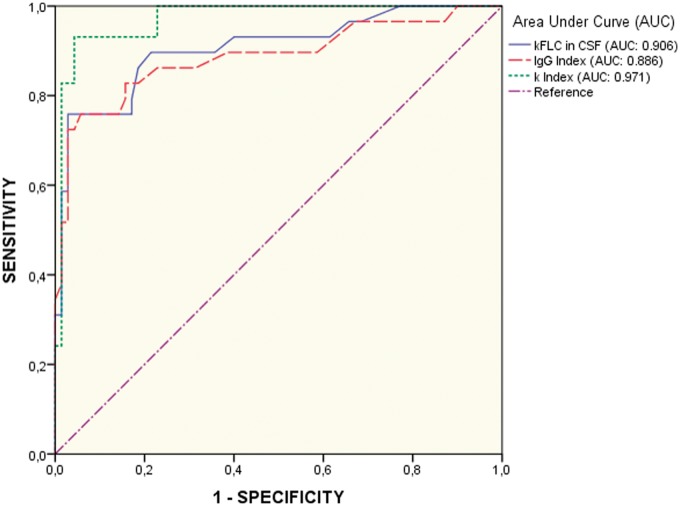
For studying the sensitivity and specificity of index and comparing it with the IgG Index we performed receiver operating characteristic (ROC) curves (Figure 2), analysing values from the control group (n = 70) against the RRMS group (n = 29). The area under the curve (AUC) was higher for index: 0.971, with a sensitivity of 93.10 (95% CI: 77.2–99.2) and a specificity of 95.71 (95% CI: 88.0–99.1) (cut-off value = 10.62), clearly above the results obtained with IgG index, which showed an AUC of 0.869, a sensitivity of 75.9 (95% CI: 56.5–89.7) and a specificity of 94.3 (95% CI: 86–98.4) (cut-off value = 0.56).
Figure 2.
Comparison of receiver operating characteristic (ROC) curves for immunoglobulin G (IgG) index (dashed line), kappa free light chains (FLCs) in cerebrospinal fluid (CSF) (solid line) and index (dotted line).
The highest clinical sensitivity and specificity (96.5% and 98.6% respectively) to detect MS patients was shown by OCGBs, although the index shows similar sensitivity and specificity (93.1% and 95.7% respectively). The lowest sensitivity and specificity was obtained with the IgG index (75.9% and 94.3% respectively) in agreement with previous reports.26,31
The use of BTC plus index improved specificity and PPV of index alone, however, there is a decrease in the sensitivity and NPV values (Table 2). The same happens using the combination of BTC plus OCGBs in comparison to OCGBs alone. These results are consistent with previous studies, in which the simultaneous use of BTC with either OCGBs or FLCs in CSF also improved the specificity from the clinical point of view.25
Table 2.
Accuracy analysis using receiver operating characteristic (ROC) curves for quantitative variables: immunoglobulin G (IgG) index, kappa () index; and accuracy analysis with crosstabs for categorical variables (oligoclonal IgG bands (OCGBs) and Barkhof-Tintoré criteria (BTC)).
| index | OCGB | IgG index | BT≥3 | BTC+ index | BTC+ OCGB | |
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | 93.1 (77.2–99.2) | 96.5 (82.2–99.9) | 75.9 (56.5–89.7) | 72.4 (52.8–87.3) | 69.0 (49.2–84.7) | 72.4 (52.8–87.3) |
| Specificity (95% CI) | 95.7 (88.0–99.1) | 98.6 (92.3–99.9) | 94.3 (86–98.4) | 100 (94.9–100) | 100 (94.9–100) | 100 (94.9–100) |
| PPV (95% CI) | 90.0 (73.1–98.0) | 96.6 (82.2–99.9) | 84.6 (65.1–95.6) | 100 (83.9–100) | 100 (83.2–100) | 100 (83.9–100) |
| NPV (95% CI) | 97.1 (89.9–99.6) | 98.6 (92.3–99.9) | 90.4 (81.2–96.1) | 89.7 (80.8–95.5) | 88.6 (79.5–94.7) | 89.7 (80.8–95.5) |
| AUC | 0.971 | – | 0.886 | – | – | – |
| Cut-off | 10.62 | – | 0.56 | – | – | – |
AUC: area under the curve; CI: confidence interval. Results for combined test using BTC plus OCGB, and BTC plus index are shown.
The accuracy in predicting conversion from CIS to MS was assessed for high index (>10.62) and high IgG index (>0.56), as well as for OCGB positive, and BTC ≥ 3, considered either independently or in combination (Table 2). Using the optimal cut-off value for index (10.62) obtained in the ROC curve analysis (Figure 2, Table 2), we classified CIS patients in two subgroups: subgroup 1 included 49 CIS patients with index >10.62 ( index median (interquartile range (IQR)): 82.09 (39.73–290.40)), and subgroup 2, 28 patients with index <10.62 ( index median (IQR): 3.68 (2.27–4.87)). Data of CIS patients’ classified according to the cut-off value is shown in Table 3. The optimal IgG index cut-off was also established based on the ROC curve (Figure 2), resulting in 0.56 (Table 2), a cut-off similar to previous studies.14
Table 3.
Clinical and laboratory data of clinically isolated syndrome (CIS) patients using index cut-off of 10.62. Subgroup 1: patients with index > 10.62; subgroup 2: patients with index < 10.62.
| Subgroup 1 | Subgroup 2 | p Value | |
|---|---|---|---|
| Patients (n) | 49 | 28 | – |
| Gender (females %) | 41 (83.7) | 19 (67.9) | – |
| Age (mean (95% CI)) | 34.1 (31.3–36.9) | 37.4 (32.9–41.8) | 0.186 |
| OCGB (+/−) | 44/5 | 4/24 | <0.0001 |
| IgG index (>0.56/<0.56) | 46/3 | 3/25 | <0.0001 |
| ≥3 BCT (%) | 30 (61.2) | 6 (21.4) | 0.008 |
| Fulfilled MRI and OCGB criteria (yes/no) | 29/17 | 3/17 | <0.0001 |
| Conversion to MS (%) | 35 (71.4) | 3 (10.7) | <0.0001 |
BTC: Barkhof-Tintoré criteria; CI: confidence interval; IgG: immunoglobulin G; MRI: magnetic resonance imaging; MS: multiple sclerosis; OGCB: oligoclonal IgG band.
The mean age for both CIS subgroups studied according index cut-off was quite similar, remaining in their mid-30s (p = 0.186). There were significant differences between subgroups 1 and 2 (Table 3). Most of the patients in subgroup 1 presented as OCGB positive (89.8%), IgG index >0.56 (93.9%) and fulfilled ≥3 BTC (61.2%). However, in subgroup 2, most of them presented as OCGB negative (85.7%), IgG index <0.56 (89.3%) and not fulfilled ≥3 BTC (78.6%). Only three patients of 28 from subgroup 2 fulfilled both, MRI and OCGB criteria whereas 29 patients of 49 from subgroup 1 fulfilled both criteria. Furthermore, in subgroup 1, 71.4% of patients converted to MS while 89.3% of patients of subgroup 2 remain as CIS.
Index is well correlated with IgG index, 93.9% of patients with index above 10.62 (subgroup 1) presented with IgG index >0.56 and in subgroup 2 ( index < 10.62) only 10.7% of them showed IgG index >0.56 (Table 3). The same happens with OCGB, 89.8% of patients with index above 10.62 presented as OCGB positive, whereas only 14.3% of patients with index below 10.62 presented as OCGB positive.
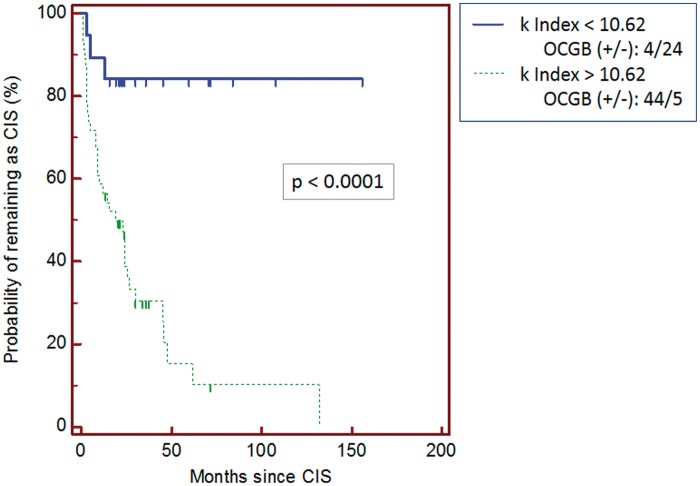
To study the probability of conversion to MS, we performed a Kaplan-Meier analysis (Figure 3) in CIS patients (p < 0.0001) according to a index cut-off value of 10.62. The median time for conversion for subgroup 1 ( index > 10.62) was 19 months with a standard error of 0.0741 (HR of 7.34 (95% CI: 3.82–14.11)).
Figure 3.
Kaplan-Meier analysis between clinically isolated syndrome (CIS) patients in which is shown the probability of remaining as CIS according to cut-off value for index: above 10.62 (dotted line) and below 10.62 (solid line). The time is represented in months since CIS. OCGB: oligoclonal immunoglobulin G band.
As is shown in the Kaplan-Meier analysis (Figure 3), the probability of conversion to MS is higher in CIS patients with index above 10.62 than in those with low index (below 10.62).
In the multivariate Cox regression analysis we studied index together with age, sex and BTC. We observed that age and sex do not have an influence in survival curves (p > 0.05). The HR adjusted for BTC was 5.30 (95% CI: 1.59–17.63)
Discussion
According to previous studies, the index showed higher clinical sensitivity and specificity to detect MS compared to FLC in CSF. In our pilot study we came to the same conclusions, so that, we have focused the present study on index. In our study, we found a median and IQR for CIS patients with index above the 10.62 cut-off (82.09 (39.73–290.40)) similar to that in RRMS patients (88 (33.96–295.13)). This finding further supports the cut-off of 10.62 obtained for index by ROC curve, as a valuable biomarker for conversion to MS in CIS patients.
The use of index to detect intrathecal immunoglobulin synthesis was widely reported previously by other groups.21,22,26,32 In our cohort, high index above 10.62 increased the risk of conversion to MS. This index is higher than the cut-off published by Presslauer et al. in 200822 with a index cut-off of 5.9 (96% sensitivity; 86% specificity). Our cut-off ( index = 10.62), showed higher specificity (95.7%) and lower sensitivity (93.1%). Despite index sensitivity and specificity, OCGBs had better accuracy (96.5% sensitivity; 98.6% specificity), which was consistent with previous studies in which the methodology employed for OCGB analysis was the same as the one used in our study.25
However, by determining the index, the drawbacks involved in OCGB analysis, such as the requirement for skilled technicians to perform the technique as well as the experience required to interpret the gels and the lack of standardisation (low interlaboratory reproducibility), can be avoided. Therefore, the index is the most interesting alternative to OCGB analysis in screening for MS.
Moreover, CIS patients with a index above 10.62 were found to be at greater risk of conversion to MS (HR = 7.34) than patients with index below this limit (p < 0.0001). When using Cox regression analysis corrected using BTC we found an HR of 5.30, slightly lower than in the univariate analysis, which is consistent with previous studies.25
This predictive value of the index of 10.62 is further strengthened by the fact that both CIS subgroups show statistical significant differences when comparing BTC for dissemination of lesions in space, MRI and OCGB criteria. High index is also a strong predictor of intrathecal IgG synthesis (positive OCGB and IgG index > 0.56), which supports observations from other studies.23,27
The fact that 71.4% of patients with index above 10.62 convert to MS, while 28.6% remained as CIS, and this is in line with previous publications correlating high index with conversion to MS.25,33
Our data showed that the risk of conversion to MS in CIS patients is increased with high values of index. The importance of the study is highlighted by the fact that a high number of subjects were selected and by the strict inclusion criteria, which made it possible to confirm findings of previous studies. Our study supports the increased value of index as a possible biomarker to predict conversion to MS with good accuracy. This suggests that the index was appropriate to help in the diagnose of MS in our population, which could be extrapolated to other areas.
Due to advances in technology, the index can be measured quickly using an automated system so this test can be easily incorporated in the daily routine of immunology laboratories. The search for a suitable biomarker which predicts conversion from CIS to MS is vital for providing early treatment in these patients enhancing their quality of life.
Acknowledgments
The authors wish to thank the Nodo Biobanco Hospitalario Virgen Macarena (Biobanco del Sistema Sanitario Público de Andalucía) for its help and support in the donation of clinical samples used in this work. This Biobank is integrated in the Spanish Biobanks Network (www.redbiobancos.es) and supported by Instituto de Salud Carlos III-FEDER, Spanish Health Ministry (grant PT13/0010/0041) (“Plan estatal 2013-2016”).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of conflicting interests.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Moreno M, Diaz-Sanchez M, Ramos-Gonzalez A. Application of the 2010 McDonald criteria for the diagnosis of multiple sclerosis in a Spanish cohort of patients with clinically isolated syndromes. Mult Scler 2012; 18: 39–44. [DOI] [PubMed] [Google Scholar]
- 3.D’Alessandro R, Vignatelli L, Lugaresi A, et al. Risk of multiple sclerosis following clinically isolated syndrome: A 4-year prospective study. J Neurol 2013; 260: 1583–1593. [DOI] [PubMed] [Google Scholar]
- 4.Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359: 1221–1231. [DOI] [PubMed] [Google Scholar]
- 5.Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo G, Venegas A, Sanabria C, et al. Long-term epidemiology of multiple sclerosis in the Northern Seville District. Acta Neurol Scand 2015; 132: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkhof F, Filippi M, Miller DH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 1997; 120: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 8.Tintore M, Rovira A, Martinez MJ, et al. Isolated demyelinating syndromes: Comparison of different MR imaging criteria to predict conversion to clinically definite multiple sclerosis. AJNR Am J Neuroradiol 2000; 21: 702–706. [PMC free article] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 Revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 10.Stangel M, Fredrikson S, Meinl E, et al. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev 2013; 9: 267–276. [DOI] [PubMed] [Google Scholar]
- 11.Dobson R, Topping J, Davis A, et al. Cerebrospinal fluid and urinary biomarkers in multiple sclerosis. Acta Neurol Scand 2013; 128: 321–327. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Cermeno JC, Villar LM. Multiple sclerosis: Oligoclonal bands–a useful tool to avoid MS misdiagnosis. Nat Rev 2013; 9: 303–304. [DOI] [PubMed] [Google Scholar]
- 13.Fitzner B, Hecker M, Zettl UK. Molecular biomarkers in cerebrospinal fluid of multiple sclerosis patients. Autoimmun Rev 2015; 14: 903–913. [DOI] [PubMed] [Google Scholar]
- 14.Cordero M, Vinuela F, Angulo S, et al. Sensitivity and efficiency of intrathecal IgG secretion in multiple sclerosis. Comparison of several indices and formulas using pre-established values of specificity. Neurologia 1997; 12: 277–280. [PubMed] [Google Scholar]
- 15.Franciotta D, Lolli F. Interlaboratory reproducibility of isoelectric focusing in oligoclonal band detection. Clin Chem 2007; 53: 1557–1558. [DOI] [PubMed] [Google Scholar]
- 16.Abraira V, Alvarez-Cermeno JC, Arroyo R, et al. Utility of oligoclonal IgG band detection for MS diagnosis in daily clinical practice. J Immunol Methods 2011; 371: 170–173. [DOI] [PubMed] [Google Scholar]
- 17.Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev 2014; 13: 518–524. [DOI] [PubMed] [Google Scholar]
- 18.Rudick RA, Medendorp S V, Namey M, et al. Multiple sclerosis progression in a natural history study: Predictive value of cerebrospinal fluid free kappa light chains. Mult Scler 1995; 1: 150–155. [DOI] [PubMed] [Google Scholar]
- 19.Krakauer M, Schaldemose Nielsen H, Jensen J, et al. Intrathecal synthesis of free immunoglobulin light chains in multiple sclerosis. Acta Neurol Scand 1998; 98: 161–165. [DOI] [PubMed] [Google Scholar]
- 20.Fischer C, Arneth B, Koehler J, et al. Kappa free light chains in cerebrospinal fluid as markers of intrathecal immunoglobulin synthesis. Clin Chem 2004; 50: 1809–1813. [DOI] [PubMed] [Google Scholar]
- 21.Desplat-Jego S, Feuillet L, Pelletier J, et al. Quantification of immunoglobulin free light chains in cerebrospinal fluid by nephelometry. J Clin Immunol 2005; 25: 338–345. [DOI] [PubMed] [Google Scholar]
- 22.Presslauer S, Milosavljevic D, Brucke T, et al. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J Neurol 2008; 255: 1508–1514. [DOI] [PubMed] [Google Scholar]
- 23.Arneth B, Birklein F. High sensitivity of free lambda and free kappa light chains for detection of intrathecal immunoglobulin synthesis in cerebrospinal fluid. Acta Neurol Scand 2009; 119: 39–44. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan B, Aizenbud BM, Golderman S, et al. Free light chain monomers in the diagnosis of multiple sclerosis. J Neuroimmunol 2010; 229: 263–271. [DOI] [PubMed] [Google Scholar]
- 25.Villar LM, Espino M, Costa-Frossard L, et al. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin Chim Acta 2012; 413: 1813–1816. [DOI] [PubMed] [Google Scholar]
- 26.Duranti F, Pieri M, Centonze D, et al. Determination of kappa FLC and kappa index in cerebrospinal fluid: A valid alternative to assess intrathecal immunoglobulin synthesis. J Neuroimmunol 2013; 263: 116–120. [DOI] [PubMed] [Google Scholar]
- 27.Senel M, Tumani H, Lauda F, et al. Cerebrospinal fluid immunoglobulin kappa light chain in clinically isolated syndrome and multiple sclerosis. PLoS One 2014; 9: e88680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan-Smith G, Durant L, Tsentemeidou A, et al. High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis. J Neuroimmunol 2014; 276: 175–179. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan B, Livneh A, Sela BA. Immunoglobulin free light chain dimers in human diseases. Scientific World Journal 2011; 11: 726–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villar LM, Masjuan J, Sadaba MC, et al. Early differential diagnosis of multiple sclerosis using a new oligoclonal band test. Arch Neurol 2005; 62: 574–577. [DOI] [PubMed] [Google Scholar]
- 31.Mares J, Herzig R, Urbanek K, et al. Correlation of the IgG index and oligoclonal bands in the cerebrospinal fluid of patients with multiple sclerosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008; 152: 247–249. [DOI] [PubMed] [Google Scholar]
- 32.Presslauer S, Milosavljevic D, Huebl W, et al. Kappa free light chains: Diagnostic and prognostic relevance in MS and CIS. PLoS One 2014; 9: e89945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinker JR, 2nd, Trinkaus K, Cross AH. Elevated CSF free kappa light chains correlate with disability prognosis in multiple sclerosis. Neurology 2006; 67: 1288–1290. [DOI] [PubMed] [Google Scholar]