Abstract
Throughout life, the T cell system adapts to shifting resources and demands, resulting in a fundamentally restructured immune system in older individuals. Here we review the cellular and molecular features of an aged immune system and discuss the trade-offs inherent to these adaptive mechanisms. Processes include homeostatic proliferation that maintains compartment size at the expense of partial loss in stemness and incomplete differentiation and the activation of negative regulatory programs, which constrain effector T cell expansion and prevent increasing oligoclonality but also interfere with memory cell generation. We propose that immune failure occurs when adaptive strategies developed by the aging T cell system fail and also discuss how, in some settings, the programs associated with T cell aging culminates in a maladaptive response that directly contributes to chronic inflammatory disease.
The adaptive immune system has the enormous challenge of protecting the host by generating antigen-specific effector mechanisms and building memory of previous encounters to prevent or ameliorate future disease. During the first and second decades of life, before the adaptive immune system has fully matured and before immune memory to a broad range of pathogens has been established, infections are frequent. Once the immune memory repertoire is built, morbidity and mortality from infections declines. However, protective adeptness increasingly deteriorates with age. Evidence of waning adaptive immunity is already apparent in mid adulthood, as early as at the age of 50 years, with increasing clinical relevance in the 7th–10th decade of life (Goronzy and Weyand, 2013; Montecino-Rodriguez et al., 2013; Pinti et al., 2016). Attempts to improve immune competence through vaccination programs of older individuals have been only partially successful; generating immune memory to new antigens and boosting existing memory are less efficient than in childhood (Gross et al., 1995; Hainz et al., 2005; Jefferson et al., 2005; Levin, 2012). What causes this decline? Certainly, sensitivity of basic cellular pathways to aging and cellular senescence contribute (López-Otín et al., 2013). Equally important are adaptive changes in the immune system to changing resources and challenges over lifetime. This is particularly evident for the T cell compartment that needs to maintain a diverse T cell repertoire, preserve a pool of stem-like cells, control chronic or latent infections, and respond to new or recurrent infections and malignancies through clonal expansion and differentiation. Immune aging also is thus a summation of these adaptations, sometimes necessary and beneficial and sometimes harmful to the aging host. Since challenges that drive these adaptations are quite different for rodents, it is uncertain whether and which insights from rodent aging can be translated to human physiology. Here, we will review age-related changes at the system, the cell, and the molecular levels and discuss how these changes enable the maintenance of an effective T cell repertoire capable of protecting from varied immune challenges. We will focus on data from the human system, comparing and contrasting it to findings in the murine system when appropriate. We will examine settings in which these adaptations fail, and the consequences of this failure. Finally, we discuss maladaptations, which actively contribute to a compromised immune state or susceptibility to inflammatory disease.
Maintaining a Naive T Cell Compartment after Thymic Involution
More than any other cellular system, generation and homeostasis of T cells are age sensitive due to the involution of the thymus (Chinn et al., 2012; Palmer, 2013). Thymic involution clearly contributes to the aging-dependent loss of T cell immunity in mice (Hale et al., 2006). Naive murine T cells survive for only 30 days, while cell divisions from homeostatic proliferation occur only every 1 to 2 years (den Braber et al., 2012; Westera et al., 2013). Consequently, the murine naive T cell compartment depends entirely on thymic activity and shrinks with its decline. Age-associated functional changes in naive murine T cells are not a consequence of replication—they at most divide once throughout life—but are a corollary of cellular longevity that even increases after thymectomy or in aged mice (Bains et al., 2009a; Tsukamoto et al., 2009).
T cell homeostasis in humans is fundamentally different. T cell generation during human adult life depends on peripheral proliferation of naive T cells (den Braber et al., 2012; Sauce et al., 2012). Even in young adults, the thymus contributes only 16% of total T cell generation. Thymic contribution declines further to <1% in older individuals who therefore nearly exclusively rely on peripheral proliferation to repopulate T cells (Bains et al., 2009a; Nobile et al., 2004; Westera et al., 2015). Daily turnover rates of naive CD4+ and CD8+ T cells are stable throughout adulthood at about 0.04%, with no apparent need for compensatory increase in homeostatic proliferation with age. Only in later life, naive CD8+ T cells accelerate their turnover (Cicin-Sain et al., 2007; Naylor et al., 2005; Westera et al., 2015). This increased turnover does not derive from truly naive cells, but from CD95+CD45RA+CD28+ T cells that represent reverted memory cells (Pulko et al., 2016), memory stem cells (Fuertes Marraco et al., 2015), or cells that have undergone extensive homeostatic proliferation reminiscent of murine virtual memory cells (Nikolich-Žugich, 2014; Renkema et al., 2014).
Homeostatic proliferation in humans is efficient in maintaining a sizable naive CD4+ compartment. Absolute numbers of naive CD4+ T cells in peripheral blood have been reported to be stable in the elderly. In contrast, circulating naive CD8+ T cells are progressively lost with age (Czesnikiewicz-Guzik et al., 2008; Wertheimer et al., 2014). Assuming that the naive T cell compartment is not biased by antigen stimulation and taking into account the conduit function of circulating blood, the peripheral blood compartment may be considered representative of the overall naive T cell population. However, a recent study found an approximately equal loss of naive T cell subsets in spleen and selected lymphoid tissues, without preference for CD8+ T cells (Thome et al., 2014). This tissue analysis raises the interesting notion that compartmentalization of naive and memory CD4+ and CD8+ T cells in spleen, lymph nodes, and blood changes over life (Thome et al., 2016). Thome et al. (2016) mostly reported relative and not absolute lymphocyte numbers, and thus, the contribution of differences in memory T cell accumulation accounts to these changes is unclear, as is whether naive T cells are variably lost from different compartments.
The production of thymically derived T regulatory (Treg) cells decreases also with thymic involution. Peripheral Treg cell homeostasis in humans has been not sufficiently studied, but peripheral expansion of existing Treg cells and conversion of nonregulatory T cells into Treg cells appear to compensate for thymic involution. Human peripheral blood contains both naive-like CD4+CD45RA+CD25+ as well as memory-like CD4+CD45RO+CD25hi Treg cells, with naive-like Treg cells decreasing and memory-like Treg cells increasing with age (Seddiki et al., 2006; Valmori et al., 2005). Likewise, the population of CD45RA+CCR7+NOX-2+ naive-like CD8+ Treg cells declines with age (Wen et al., 2016). Total CD4+ Treg cell numbers in blood are largely stable or increased (Lages et al., 2008). Treg cells increase in the skin and possibly other compartments, where they contribute to impaired T cell responses in older individuals (Vukmanovic-Stejic et al., 2008).
Thus, human naive CD4+ T cells balance proliferative needs with maintenance of compartment size with considerable success in healthy aging while limiting excessive replicative pressure that could induce cellular senescence. It is undetermined whether naive CD4+ T cell loss is more pronounced in frail older individuals. If this is the case, comparative studies of the factors controlling homeostatic proliferation would provide an opportunity to define an important factor in maintaining immune health. Although it remains to be further explored whether compartmentalization of naive T cells in peripheral secondary lymphoid tissues and blood is uneven, the prevailing notion is that naive CD8+ T cells are less successfully maintained with age than naive CD4+ T cells raising questions on why this is the case and what the implications are. Do cell-intrinsic differences contribute and naive CD8+ T cells are more susceptible to lose stemness or fail to survive? Is the difference related to different local environments in which CD4+ and CD8+ T cells encounter survival signals? What are the implications for vaccination strategies throughout adult age, in particular given that most vaccines are designed to induce a CD4+ T cell and antibody responses?
Age and the Naive T Cell Receptor Repertoire: How Much Diversity Is Needed?
Throughout an individual’s lifetime, T cell generation must maintain not only the size but also the composition of the naive T cell compartment, in particular the diversity of T cell receptor (TCR) sequences enabling the response to all kinds of peptide antigens. Early studies, before the era off next-generation sequencing, concluded that the human TCR repertoire entails approximately 106 different TRB gene nucleotide sequences (Arstila et al., 1999). Similar estimates were derived from the first deep sequencing studies (Robins et al., 2009, 2010). Given that humans have >1011 naive T cells, this number is low and implies naive clonal sizes of at least 10,000 cells. Computer simulations have suggested that postthymic proliferation contributes more than double the number of cells entering the pool each day between the ages of 0 and 20 years than those from the thymus (Bains et al., 2009a). Indeed, substantial T cell proliferation is detected in cord blood of preterm infants when the T cell repertoire is established (Schönland et al., 2003). However, the number of divisions appears to be lower than what is required to generate 10,000 descendants. Frequencies of TCR excision circles (TRECs) that form during TCR-α rearrangement are more consistent with clonal sizes of around 100 cells (Bains et al., 2009b; den Braber et al., 2012).
The difficulties to reliably estimate the number of unique TCRs by next-generation sequencing derive from two major barriers: how can one exclude sequencing artifacts without excluding infrequent sequences, and how can one extrapolate from the analyses of small numbers of circulating cells to the large T cell pool if richness is much higher than the sample size? PCR and sequencing errors can be identified using molecular barcoding during cDNA synthesis (Britanova et al., 2014). Currently, an optimal solution for the latter barrier does not exist (Laydon et al., 2015). The distribution of TCR frequencies in the analyzed sample does not necessarily reflect the distribution in the entire repertoire; moreover, the distribution is likely to change with age, hampering inferential estimates of overall richness (Goronzy et al., 2015; Laydon et al., 2015). Qi et al. (2014) used an incidence-based nonparametric approach analyzing multiple independent blood samples from each individual and the Chao 2 estimator and arrived at minimal estimates of 50 to 100 million different TRB gene sequences for both naive CD4+ and CD8+ T cells in young adults. This estimate is consistent with TREC frequencies as well as with modeling experiments which predict that the number of distinct sequences in humans is by only one order of magnitude lower than the number of total naive cells (Lythe et al., 2016).
Repertoire stability over time requires that the initial clonal size is large enough to avoid complete losses of clonal progenies resulting in repertoire contraction with aging. Britanova et al. (2014, 2016) found a contraction in repertoire diversity with age in total T cells that mostly reflected lower numbers of naive cells in the analyzed samples emphasizing the need to analyze subpopulations. In studies of purified T cell subsets, the number of unique TRB sequences declined by a factor of 2 to 5 between the ages of 30 and 70 years, both for CD4+ and CD8+ naive T cells, nevertheless leaving a very large repertoire (Qi et al., 2014). Thus, the system appears to be robust in this regard, even in case of naive CD8+ T cells that are more compromised in numbers than diversity. This is in contrast to mice, which have a smaller repertoire to start with and functional holes have been described with age (Yager et al., 2008). However, one caveat is that all human studies so far were done in healthy older individuals, known to respond better to vaccination. T cell repertoire studies in frail individuals are lacking. In silico simulation of T cell homeostasis with age predicted severe repertoire contraction to occur in some individuals, under conditions of markedly uneven homeostatic proliferation and irrespective of thymic function (Johnson et al., 2012; Ndifon and Dushoff, 2016). Interestingly, simulations had highly variable outcomes suggesting a large stochastic component in the failure to maintain a diverse repertoire. Whether these massive contractions of the T cell repertoire occur in some individuals, and how they relate to frailty, are important areas requiring investigation.
In addition to the number of unique TCRs, T cell diversity and its functional consequences are determined by the distribution in clonal sizes (Desponds et al., 2016). Increase in clonal sizes is a hallmark of immune memory; moreover, clonal dominance is quite normal for the memory T cell repertoire responding to a single peptide (Obar et al., 2008; van Heijst et al., 2009), presumably due to antigen-driven selective pressures. Uneven homeostatic proliferation, not triggered by exogenous antigen, can also contribute to age-associated changes in clonality in the T cell repertoire. Early studies described clonal populations within naive cells after the age of 60 years (Naylor et al., 2005). More recent studies using deep sequencing reported an age-associated increase in clonal populations more so in the naive CD8+ than in the CD4+ compartment (Qi et al., 2014). While likely including stem-like memory cells, the majority of these clonally expanded, phenotypically naive T cells expressed TCR sequences that did not overlap with those expressed by T cells in the memory compartment and therefore probably represent truly naive cells that have proliferated without encountering exogenous antigens. Such an increasingly uneven clonal size distribution can be biologically relevant. Some clones may be just too infrequent in older individuals to provide a meaningful response to immune challenge, while expanded ones may confer increased responsiveness to specific challenges. Moreover, some of these clones selected during homeostatic proliferation may have a higher affinity to self and therefore promote autoreactive immunity in the elderly (Deshpande et al., 2015; Goronzy and Weyand, 2012; Quinn et al., 2016).
Memory T Cell Homeostasis in the Aging Host
Virus-specific T cell memory is long lived, even in the absence of re-stimulation. Small pox- and yellow fever virus-specific CD8+ T cells persist for several decades after vaccination (Crotty et al., 2003; Hammarlund et al., 2003). Notably, memory T cell homeostasis is more dynamic than that of naive T cells with daily turnover rates of around 0.6%, shows evidence of a higher degree of population heterogeneity in stable isotope-labeling studies (Wallace et al., 2004; Westera et al., 2013), and must cope with many confounding forces over lifetime. Each infectious episode imposes major disturbances due to clonal expansion of antigen-specific T cells. Latent infections with herpes viruses such as varicella zoster virus (VZV), Epstein-Barr virus (EBV), and cytomegalovirus (CMV) are very common in humans and have a major influence on the peripheral blood T cell composition with age. Interestingly, even within the herpes virus family, response patterns vary widely. T cell frequencies to VZV decline with age, presumably exposing aging individuals to VZV reactivation, causing shingles (Levin et al., 2003). By the age of 80 years, almost 50% of individuals have experienced shingles. In contrast, frequencies of CMV-specific and to a lesser extent EBV-specific CD8+ T cells tend to increase, successfully protecting aging individuals from CMV- and EBV-associated morbidities (Khan et al., 2002; Pourgheysari et al., 2007). Indeed, the expansion of memory and in particular terminally differentiated CD8+ effector cell (TEMRA) populations that is seen with age is largely caused by CMV (Wertheimer et al., 2014).
Whether memory cell inflation by CMV compromises the global repertoire and therefore negatively impacts immune health is controversial (Sansoni et al., 2014). Theoretically, CMV-specific effector T cells reside in a different tissue niche than naive or central memory cells and thus should not constrain the repertoire of these cells or their response to vaccination. Moreover, the CD8+ compartment size is plastic and is capable of accommodating the expansion of CMV-specific cells (van Leeuwen et al., 2006). However, in studies examining the impact of latent CMV infection, CD4+ T cell responses to influenza A matrix protein and nucleoprotein were reduced in older CMV carriers (Derhovanessian et al., 2014). Also, frequencies of CD8+ TEMRA cells, many of them reactive to CMV, inversely predicted the antibody response to trivalent influenza vaccination (TIV) (Goronzy et al., 2001; Saurwein-Teissl et al., 2002). In contrast, other studies have not found a relationship between CMV carrier status and reduced influenza vaccine responses with age; on the contrary, CMV infection appeared to even have a positive effect on antibody responses in young individuals, presumably by activating innate cells (Furman et al., 2015). One possible explanation for these diverging observations is that CMV infection does not influence unrelated immune responses but that memory inflation, by itself necessary to contain CMV, is a surrogate marker of immune failure.
Resident memory T cells in lungs, skin, and submucosal sites account for a large fraction of memory cells numbering more than 5 × 1010 cells in an individual. Seeding into tissues occurs in childhood, and the resident population persists throughout adulthood (Farber et al., 2014; Sathaliyawala et al., 2013). In the mouse skin, resident memory T cells persist over long time periods (Biasco et al., 2015). In contrast, in lungs, they appear to more rapidly decline after influenza infection (Wu et al., 2014). Data on the impact of age in humans on frequencies or function of resident memory T cells are lacking. Mathematical modeling has predicted that their decay after infection and the lag-time in generating new resident cells accounts for failed viral control in pulmonary influenza infection (Zarnitsyna et al., 2016). It is therefore an attractive hypothesis that an age-associated depletion or functional change of this population contributes to the increasing morbidity and mortality from influenza infection with age.
In summary, our current understanding of the influence of age on the size of the memory compartment entirely relies on studies of peripheral blood and lacks information on resident memory cells. T cell responses specific for latent herpes viruses are an informative model to understand age-associated changes in T cell memory. Memory cell inflation appears to be beneficial for controlling viral latency while there is currently no convincing evidence that it has adverse effects on overall immunity. Discrepant T cell memory persistence to CMV and VZV could be due to differences in latency and viral load of these herpes virus strains. Alternatively or in addition, dissimilarities in CD4+ and CD8+ T memory cell persistence may contribute because VZV is thought to be mainly controlled by CD4+ T cells which may be less prone to memory cell inflation and differentiation compared to CD8+ TEMRAs. An epigenetic and transcriptome comparison of VZV- and CMV-specific T cells in older individuals will be informative to understand the different response patterns in maintaining T cell memory to these two viruses.
Repertoire Diversity of Memory T Cell Response in the Aging Host
The population of TEMRA T cells that increases with age, in particular in the CD8+ compartment, is highly oligoclonal with single clonotypes reaching frequencies of up to 1% in the peripheral blood compartment (Henson et al., 2012). This is consistent with the interpretation that this population mostly represents an inflated memory T cell response to latent viruses resulting from massive clonal expansion. Outside of the TEMRA populations, the overall number of TCR sequences does not decline with age and the number of clonally expanded memory T cells does not increase, suggesting that the overall diversity is well maintained (Qi et al., 2014). Interestingly, the TCR repertoire of CD4+ memory T cells is much more diverse than that of CD8+ memory T cells in the young as well as in the old adult (Li et al., 2016a; Qi et al., 2014). Whether this is due to a larger pool of antigenic peptides recognized by CD4+ memory T cells or whether the CD4+ TCR repertoire to single peptides is broader remains to be determined.
To definitely address the question of whether changes in the memory T cell repertoire with age compromises adaptive immune responses, studies of memory T cells to single antigenic peptides are needed. Such studies in humans are in their infancy. In mice, the TCR repertoire to an antigenic peptide follows a power law distribution involving a large number of T cell specificities, with only a few of them gaining dominance (Obar et al., 2008; van Heijst et al., 2009). Recall responses in mice did not cause a contraction of the peptide-specific responses (Cukalac et al., 2014). In contrast, human CD8+ T cell responses directed against the HLA-A2-restricted M158-66 peptide of the influenza A virus showed narrowing of the repertoire with age, possibly impacting the ability to recognize antigenically related viruses (Gil et al., 2015). The contraction in the diversity of anti-influenza A CD8+ T cells most likely resulted from repeated stimulation, since the epitope studied is highly conserved between different influenza strains. Repertoire studies of CMV-specific T cells provided evidence that TCR breadth of the peptide-specific response is functionally important (Wang et al., 2012). While memory inflation is sufficient to prevent overt clinical disease from CMV in old adults, a smaller repertoire has been related to more frequent CMV reactivations, which are clinically asymptomatic but may contribute to the inflammatory state in older adults. Likewise, the number of T cell clones involved in the memory response to VZV varies by a factor of ten in individuals older than 50 years (Qi et al., 2016a). Whether this high degree of variability is a function of age and whether it is related to the risk of developing shingles remains to be determined. Importantly, repertoire complexity of antigen-specific memory T cells can be improved by vaccination even in older individuals. Recall immunization with the live VZV vaccine Zostavax resulted in the diversification of the antigen-specific CD4+ TCR repertoire, due to the preferential recruitment of small VZV-specific clones, including those from the naive T cell pool (Qi et al., 2016a). These data suggest that even in older individuals, a reservoir of antigen-specific T cells exists that can be enlisted for broader and more effective immune memory.
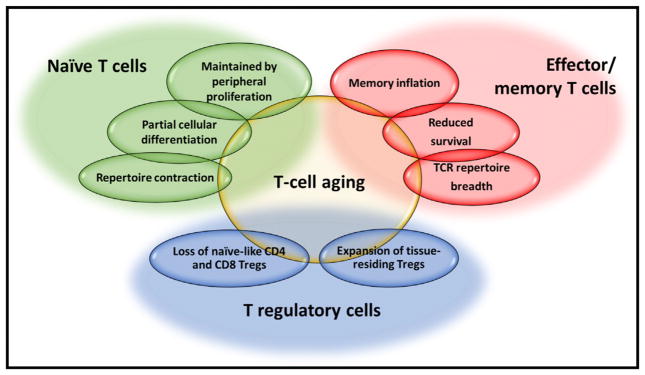
In conclusion, with the likely exception of naive CD8+ T cells, loss of T cell numbers or TCR diversity does not account for a decline in adaptive immune responses in healthy old individuals (Figure 1). Naive and to some extent central memory T cells display features of stemness that allows them to weather the challenges of aging through homeostatic proliferation. Whether and why naive CD8+ T cells are more susceptible to being lost with age remains to be determined. The naive TCR sequence diversity in young adults is high and predicted to exceed the functional diversity so that even a modest loss with age is unlikely to have functional consequences. Repertoire diversity of naive T cells in old individuals is not only important for primary T cell response but continues to be a resource for T cell responses to antigens previously encountered. However, these conclusions are contingent on peripheral homeostatic proliferation being efficient and unbiased, which appears to be the case for healthy older individuals but may not hold up for all individuals. Peripheral selection of proliferating cells could severely reduce compartment size and bias TCR diversity and may correlate with larger loss in immune health. In particular, changes in the cytokine milieu or in the responsiveness of T cells to cytokine stimulation, as reviewed in the following section, could have a major influence on the ability to maintain compartment size and repertoire diversity.
Figure 1. Maintenance of Functional T Cell Compartments with Age.
To be successful in aging, each T cell subset has to cope with unique challenges. In the absence of thymic activity, naive T cells are maintained by peripheral proliferation that with increasing age is associated with partial differentiation and contraction in TCR diversity. In the memory compartment, oligoclonal memory inflation as well as loss of memory T cells can occur. Contraction in TCR repertoire breadth to viral antigens can impair virus control as well as cross-protection. Treg cells change in frequencies, composition, and tissue compartmentalization.
Influence of Inflamm-aging on T Cell Responses
The environmental context, in which T cell responses occur, changes substantially with aging. Notably, circulating levels of pro-inflammatory cytokines, in particular interleukin (IL)-6 and tumor necrosis factor (TNF)-α, increase with age, and these have been correlated with age-associated morbidities such as cardiovascular disease and frailty (Ferrucci et al., 2005). Also, high serum concentrations of IL-6 are predictive of disability and survival in older individuals (Reuben et al., 2002).
To be activated and to differentiate, T cells require dendritic cells (DCs) to present antigen and provide costimulatory signals as well as lineage-determining cytokines, all processes that are sensitive to the inflammatory milieu. Tonic signaling by inflammatory cytokines is expected to attenuate the responsiveness of DCs to stimulation. Studies of human DC functions have not yet led to a unifying concept on how these cells are impacted by age (Wong and Goldstein, 2013). Circulating numbers of myeloid and plasmacytoid DCs, their phenotypes, and their ability to express costimulatory molecules appear to be intact in older individuals (Agrawal et al., 2007; Gupta, 2014). However, myeloid (m)DCs from older individuals have impaired ability to endocytose and present antigen to CD4+ and CD8+ T cells (Gupta, 2014). Most studies describe reduced cytokine production of DCs stimulated with Toll-like receptor ligands in vitro, a defect that has been correlated with reduced responses to vaccination (Panda et al., 2010). Little is known about how aging impacts the function of tissue-resident DCs and the niches in which T cell activation occurs (Su et al., 2013; Vukmanovic-Stejic et al., 2015).
Whether low responsiveness of DCs can be overcome by adjuvants is an area of active research, with some limited success so far. In vivo experience exists mostly for oil-in-water emulsion adjuvants such as MF59, AS01B, or AS03 that enhance the influx of innate immune cells to the site of vaccination and induce their activation. In comparative vaccination trials of older individuals, MF59, widely used in Europe, outperformed the nonadjuvanted vaccine in inducing influenza virus-specific antibodies and improving clinical outcome (Domnich et al., 2017). AS03-adjuvanted TIV vaccine showed a trend toward superior protection that, however, reached significance for only some subtypes (McElhaney et al., 2013). Notably, the AS01B-adjuvanted VZV gE subunit vaccine was highly effective irrespective of age (Cunningham et al., 2016). These adjuvants therefore appear to be efficacious in older individuals, but they cannot fully restore the adaptive immune response to the level of younger adults, possibly with the exception of the VZV vaccine. TLR-activating vaccine adjuvants are of interest given the defects in TLR signaling pathways observed in vitro studies. Experiments in non-human primates suggest that elicited cytokine profiles are dependent on the nature of the TLR ligand (Kwissa et al., 2012); however, in vivo data on the impact of age on the efficacy of these adjuvants are lacking.
TNF-α is known to also directly influence T cell immunity, in part by reducing the expression of CD28 (Bryl et al., 2001, 2005). Inhibition of TNF signaling restores T cell responses in chronic viral infection in mice (Beyer et al., 2016), an observation that may also be relevant for old humans. In addition, the pro-inflammatory environment may be responsible for the blunted response of T cells from older individuals to cytokines, as shown by in vitro studies, possibly due to the induction of negative regulatory pathways (Shen-Orr et al., 2016). Baseline STAT phosphorylation is increased in cells from older individuals, and reduced JAK-STAT signaling to cytokines have been correlated with chronic inflammation and age-associated morbidities.
A distortion of JAK-STAT signaling should have a major impact on T cell maintenance and responses. Different cytokine milieus could bias the functional polarization of T cell immunity. There is no convincing evidence that the induction of a Th1 cell response is compromised in older individuals, consistent with the finding that DCs in the elderly can produce IL-12 and that a pro-inflammatory environment is supportive of Th1 cell responses. However, after vaccination, T follicular helper (Tfh) cells are less frequent in older subjects and appear to be malfunctioning (Barnett et al., 2014; Herati et al., 2014; Linterman, 2014). In murine studies, defective Tfh cell generation can be corrected by supplementing additional IL-6 (Sell et al., 2014), possibly indicating reduced responsiveness to IL-6 due to attenuation of IL-6 receptor signaling in an IL-6-saturated environment.
Systems analyses have identified gene expression signatures related to innate immune activation that correlate with human vaccine responses, giving insights into the response in older individuals. In longitudinal studies of annual influenza vaccinations, inflammatory monocyte transcriptional signatures at baseline consistently correlated with poorer antibody responses, suggesting that an inflammatory state is detrimental to the establishment of protective humoral immunity (Nakaya et al., 2015). Notably, such signatures were more common in older individuals. Also, monocytes in older individuals produce more IL-10 after influenza vaccination, which inversely correlated with the increase in antibody titers (Mohanty et al., 2015). Conversely, early innate responses on the day after influenza vaccination, largely comprised of antiviral and type I IFN-related genes, correlated with superior vaccine responses. This vaccination-induced innate immune signature was impaired in older individuals (Nakaya et al., 2015), consistent with in vitro data that type I IFN production in DCs from older individuals is reduced (Gupta, 2014). Moreover, activated CD4+ T cells from older individuals are also less responsive to type I IFNs, mostly due to increased recruitment of SHP1 to the IFAR signaling complex, potentially amplifying the effect from the lower production of this cytokine (Le Page et al., 2014; Li et al., 2015).
Thus, the inflammatory environment characteristic of the aging individual has to be taken into account when interpreting adaptive immune responses. Frail individuals who have higher concentrations of inflammatory cytokines also have reduced in vitro responses to cytokine stimulation and poorer vaccine responses in vivo, consistent with the model that chronic cytokine exposure is harmful, possibly by attenuating relevant signaling pathways. Anti-inflammatory treatment could be beneficial in restoring adaptive immune competence.
Epigenetic Loss of CD8+ T Cell Stemness
The replicative history of individual T cells from homeostatic proliferation as well as the signals received from the environmental cues present in the aging host should leave an imprint on the organization of the epigenome, and epigenetic signatures should therefore be informative on which processes underlie T cell aging. The recent invention of the assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) has facilitated the ability to interrogate the epigenome using a small number of cells. In a recent study, genome-wide maps of chromatin accessibility were generated for purified CD8+ T cell subsets from age-segregated human individuals and compared to transcriptome analysis (Moskowitz et al., 2017). Changes throughout differentiation were defined by comparing naive, effector, and central memory T cells; these analyses were complemented by examining the impact of age on the chromatin landscapes and identifying the associated regulatory programs. Epigenetic differences were more discriminatory and robust than gene expression differences. Differentiation-associated chromatin accessibility signatures were mostly preserved during aging, indicating the resilience of phenotypically defined T cell subsets. However, naive CD8+ T cells from elderly individuals exhibited an epigenetic profile more similar to that of young central memory T cells, and old central memory CD8+ T cells had features of effector T cell differentiation; both mediated by an increase in sites accessible to bZIP family and a closure of Ets family transcription factor binding sites. In contrast to virtual memory T cells in the mouse, this shift to a more differentiated state was incomplete and not associated with a gain in effector function, but included genes relevant to T cell proliferation. Similar epigenetic studies are lacking for CD4+ T cell subsets. However, phenotypic studies also indicate shifts toward differentiation with age in this compartment, albeit possibly more subtle. The cell surface molecules protein tyrosine kinase 7 and CD31, expressed on a subpopulation of naive cells in children and young adults, are lost with homeostatic proliferation and decline with age (Haines et al., 2009; Kilpatrick et al., 2008; Kimmig et al., 2002). CD31− naive CD4+ T cells have a lower TREC frequency, indicating an increased replicative history. In a cohort of children and adolescents after neonatal thymectomy, CD31− naive T cells had lost the ability to produce IL-8, an effector function attributed to naive cells (van den Broek et al., 2016).
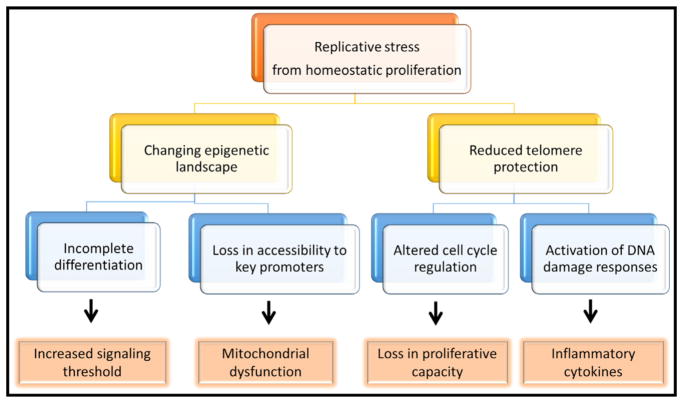
Thus, a major epigenetic change in naive and central memory CD8+ T cells with age is a loss in stemness and a shift toward a more differentiated stage. This shift may be a consequence of the cumulative replicative history of homeostatic proliferation over lifetime (Figure 2). Signatures reminiscent of differential cytokine exposures, such as accessibility to NF-κB and STAT motifs, were not described, but this may be different in frail older individuals who have more evidence of inflamm-aging. Most importantly, it will be of interest to determine whether CD4+ and CD8+ T cells develop the same epigenetic signatures with age and how these signatures relate to the cells’ functional properties and the ability to respond to stimulation.
Figure 2. Functional Consequences Deriving from Cumulative Homeostatic Proliferation.
The decades-long replicative history of naive and memory T cells is associated with reduced telomeric protection and T cell differentiation initiation. Many of the age-associated functional changes including loss of stemness, signal strength calibration, and mitochondrial dysfunction can be traced back to these processes.
Age-Associated Defects in T Cell Activation
Studies of human T cells have associated aging with changes in autophagy, in membrane properties to form rafts, and in reactive oxygen production (Fulop et al., 2014; Phadwal et al., 2012); however, these studies examined total T cell populations, irrespective of naive or memory phenotypes, and are therefore difficult to interpret. Likewise, transcriptome comparisons between young and old adults have been done for total T cell populations (Chen et al., 2013). An analysis of the influence of age on the transcriptome in total CD4+ T cells identified relatively few gene expression differences with no striking pathway enrichment (Reynolds et al., 2015). Changes in CD8+ T cell transcriptomes are largely due to increased frequencies of effector CD8+ T cells (Cao et al., 2010). A separate analysis of CD28+ and CD28− CD8+ T cells in young and old individuals were consistent with the model that old CD28+CD8+ T cells were more differentiated (Lazuardi et al., 2009) as also suggested by the epigenetic studies of CD8+ T cell subsets (Moskowitz et al., 2017). Because a major feature of T cell aging appears to be the initiation of differentiation steps, the analysis of individual T cell subsets in functional studies is important.
Human naive CD4+ T cells maintain reasonable functionality with progressive age, at least in healthy individuals (Gomez et al., 2004). One of the best-defined aging-related signaling defects in CD4+ naive T cells is caused by the reduced expression of miR181a (Li et al., 2012). Expression of miR181a is highest in thymocytes, lower in naive T cells, and further declines with T cell differentiation in both mouse and men (Li et al., 2007). The age-associated decline may therefore represent an example of incomplete differentiation that here impacts signaling cascades (Figure 2). miR181a targets several phosphatases, including the dual-specific phosphatase (DUSP)6 that is overexpressed in older T cells (Li et al., 2012). DUSP6 dephosphorylates ERK, not only attenuating ERK signaling after TCR activation but also interfering with a forward feedback loop in TCR signaling (Stefanová et al., 2003). Forced overexpression of miR181a or DUSP6 silencing largely restores TCR-induced activation in naive CD4+ T cells from older individuals (Li et al., 2012). The DUSP6-mediated signaling defect can also be overcome by increasing stimulation strength. Indeed, recent vaccination studies with TIV have found a benefit from increasing the vaccine dose in older individuals (DiazGranados et al., 2014).
Naive CD8+ T cells appear to be more vulnerable to developing cell-intrinsic defects than CD4+ T cells, matching their higher degree of decline in peripheral blood with age. Briceño et al. (2016) stimulated naive CD8+ T cells with DCs pulsed with Melan/Mart-1 peptide; naive T cells responsive to Melan/Mart-1 peptide are found in relatively high frequencies. In older individuals, clonal expansion of Mart1-specific T cells was reduced, and importantly, these cells failed to differentiate into T-bet-expressing effector T cells. Most interestingly, these in vitro results correlated with in vivo primary responses after vaccination with the tick-born encephalitis virus, supporting the in vivo relevance of these in vitro findings (Briceño et al., 2016). Reduced primary CD8+ T cell responses are also seen in older individuals after vaccination with the live attenuated yellow fever vaccine, which manifested as delayed viral clearance and decreased frequencies of activated CD8+ T cells at peak responses (Schulz et al., 2015). Poor responsiveness to yellow fever vaccine correlated with low numbers of circulating naive CD8+ T cells and DCs, and reduced CD31 expression on CD4+ naive T cells. How CD31 is functionally related to CD8+ T cell responses is unclear, and it is possible that the association may simply reflect a correlation with replicative history. A more direct marker of replicative history is telomere length (Lin et al., 2015; Son et al., 2000). The proliferative responses of influenza M1-specific CD8+ T cells after TIV vaccination inversely correlated to telomere length in peripheral blood mononuclear cells (Najarro et al., 2015), consistent with the notion that cumulative homeostatic proliferation constrains the expansion of antigen-specific CD8+ T cells (Figure 2). The conclusion of these vaccination studies that in vivo CD8+ T cell priming declines with age is not undisputed. Lelic et al. (2012) did not find an effect of age on the frequencies or function of antigen-specific CD8+ memory T cells after West Nile fever infection. However, vaccine studies can be better controlled than population studies, and the evidence for impaired CD8+ T cell priming by age is overall convincing. Since studies on naive CD8+ T cell activation defects so far have been entirely descriptive, more conclusive evidence has to come from mechanistic studies that are lacking for naive CD8+ T cells.
Age-Associated Survival Defects in Memory T Cell Generation
Studies of the T cell memory response after VZV vaccination in a cohort of healthy 50- to 75-year-old individuals found that age was not a major factor determining the expansion of antigen-specific CD4+ T cells in the first 1–2 weeks after vaccination (Qi et al., 2016b). However, age was associated with the increased loss of effector cells after peak responses, compromising the frequency of long-lived memory T cells. Gene expression profiling of in vivo activated CD4+ T cells 1–2 weeks after VZV vaccination of older individuals identified DNA damage response gene modules as the major predictor of a superior vaccine response as determined by the survival of effector T cells and the successful generation of long-lived antigen-specific memory cells (Qi et al., 2016b). The proliferative stage of a T cell response encompasses extreme replicative stress that requires coordination of cell cycle control and DNA repair, and it is therefore not entirely surprising that dysregulation of this process is sensitive to aging and curtails cell survival. Still, the absence of a gene module related to apoptosis is striking.
In vitro studies provide evidence for the notion that, in addition to a defect in cell cycle control and DNA repair, age affects memory T cell survival also through active mechanisms. Apoptosis of activated CD4+ T cells correlated with the expression of the ATPase CD39, which is induced in a subset of CD4+ memory T cells after activation (Fang et al., 2016). CD8+ memory T cells are more prone to express CD39 than CD4+ T cells (Bai et al., 2015), and CD39 is a marker on exhausted CD8+ T cells (Gupta et al., 2015). The propensity to express CD39 after activation increases about 2-fold with age. CD39 ATPase activity is directly responsible for apoptosis susceptibility, in part due to cleaving of secreted ATP, generating adenosine and signaling through the A2AR adenosine receptor. Older activated memory CD4+ T cells and in particular CD39+ effector T cells also express increased concentrations of the nuclear dual-specific phosphatase DUSP4 (Yu et al., 2012). DUSP4 has been implicated in cellular senescence, presumably by terminating nuclear ERK activity (Bignon et al., 2015). The failure of older memory CD4+ T cells to support the proliferation and differentiation of B cells is caused by their increased expression of DUSP4. DUSP4 silencing in old activated CD4+ memory T cells restored helper cell activity (Yu et al., 2012).
Consistent with these in vitro observations, individuals with reduced ability to transcribe CD39 due to a promoter polymorphism (Friedman et al., 2009) have better responses to TIV as well as VZV vaccination (Fang et al., 2016). These data assign a role to purinergic signaling in recall responses and also point to a more prominent role for this pathway in older individuals. While important for vaccine responses, this pathway is also relevant in the inflamed environment of solid tumors that generate elevated extracellular concentrations of ATP (Cekic and Linden, 2016). A2AR signaling has been shown to reduce the cytotoxic activity of tumor-infiltrating CD8+ T cells (Beavis et al., 2013; Mittal et al., 2014). Reducing adenosine production in this setting has been found to activate tumor-associated T cells and to increase the effectiveness of antitumor vaccines and PD1 checkpoint inhibition (Beavis et al., 2015; Mittal et al., 2014; Waickman et al., 2012). The increased CD39 expression on human effector T cells with age may therefore also have consequences for antitumor immune activity.
In conclusion, survival of effector T cells and transition into memory T cells is a critical step of the recall immune response that is vulnerable to aging. A variety of mechanisms including insufficient DNA repair and modified purinergic signaling due to the expression of CD39 lead to increased cell loss at the peak of the T cell response after antigen encounter. As a consequence, expansion of effector T cells capable of producing Th1 and Th17 cytokines is intact in older individuals, but their inability to provide Tfh cell function and to survive as memory cells compromises the efficacy of vaccination.
Control of T Cell Memory Inflation by Cellular Senescence
Unlike the short-lived effector T cells that are more prone to apoptosis in older individuals, TEMRA T cells are effector T cells that tend to accumulate with age. As discussed above, these cells are mostly specific for latent viruses, such as CMV and EBV (Newell et al., 2012), suggesting that the nature and chronicity of viral stimulation rather than age drives their functional phenotype (Wertheimer et al., 2014). TEMRA T cells have low proliferative potential, short telomeres, and low telomerase activity and are considered to be senescent. Akbar et al. (2016) have proposed a senescence model for TEMRAs that centers on the disproportionate activation of the p38 MAPK pathway (Figure 3). p38 phosphorylation occurs downstream of ATM and ROS through TAB1/AMPK activation rather than TCR signaling (Lanna et al., 2014). Activation of the p38 pathway is directly involved in the loss of telomerase activity and of proliferative capacity, as well as the increased production of inflammatory cytokines similar to the senescence-associated secretory phenotype in fibroblasts (Tchkonia et al., 2013). Unlike cellular senescence in a strict sense, these functional deficits are reversed by inhibiting p38 (Di Mitri et al., 2011; Henson et al., 2015). This model links senescence features directly to DNA damage responses involving ATM and to mitochondrial dysfunction causing ROS production (Henson et al., 2014). In terms of cytokine production and cytotoxic activity, TEMRAs maintain complete competence. They develop features of NK cells leading to the interpretation that adaptive and innate immunity converge with age (Pereira and Akbar, 2016; Warrington et al., 2001). TEMRAs express a host of negative regulatory receptors such as killer immunoglobulin-like receptors and CD85j that interfere with their ability to proliferate but not with cytokine production (Henel et al., 2006; Lamar et al., 2010). Support for the functionality of these cells also comes from the observation that the incidence of clinical disease due to reactivations of CMV and EBV is very low in elderly individuals, in contrast to severely immunosuppressed transplant patients (Rowshani et al., 2005). The induction of this senescence pathway therefore appears to be a successful adaptive mechanism, constraining memory cell inflation while maintaining function. Nevertheless, there are settings when expansion of senescent T cells is desirable, even at the cost of suppressed cytokine production, such as expansion of tumor-specific T cells or antigen-specific T cells after vaccination. It remains to be determined whether this p38-driven senescence signature is also common for T cells outside of the TEMRA population.
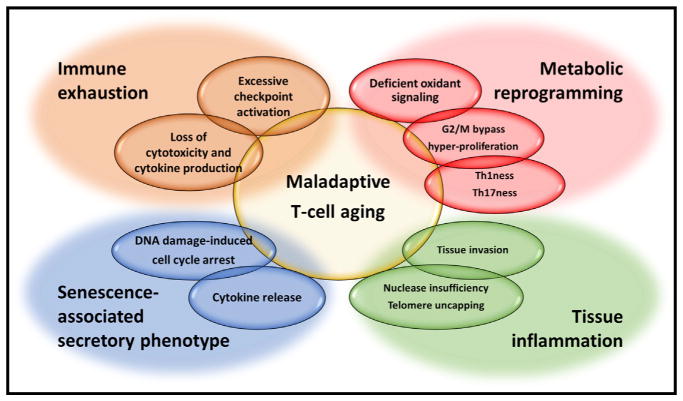
Figure 3. Dimensions of Maladaptive T Cell Aging.
Adaptive processes of T cells to changing demands during aging can lead to maladaptations. Chronically stimulated T cells are exhausted with a decline in effector function and proliferative potential. A phenotype reminiscent of the senescence-associated secretory phenotype in senescent fibroblasts is seen in terminally differentiated CD45RA T cells (TEMRA) that are efficient cytokine producers while having lost proliferative potential due to a DNA damage-induced cell cycle block. Deficient reactive oxygen signaling and metabolic reprogramming in aged naive T cells accelerate proliferative responses and differentiation in Th1/Th17 effector cells. Defective DNA damage responses to telomere uncapping in aged naive T cells promote tissue infiltration and inflammation. Whether these different processes are sequential, co-existing, or interdependent needs to be explored.
Age and T Cell Exhaustion
TEMRAs are different from exhausted cells because they have a selective defect in cell cycle progression, including a defect in telomerase expression, but are otherwise functionally competent (Akbar and Henson, 2011). In contrast, T cells exhausted due to chronic stimulation have entered a differentiation stage where they progressively lose effector functions of cytokine production and cytotoxicity, interfering with their ability to eradicate cancer or control viral infections (Figure 3; Wherry, 2011). The phenotypic hallmark of exhausted cells is the prolonged and high expression of negative regulatory receptors and in particular PD-1. PD-1 expression is low and T-bet expression is high in TEMRAs (Henson et al., 2015). Proliferation of TEMRAs is in part restored by PD-1 blockade, yet without improving telomerase activity (Henson et al., 2015).
While exhausted CD8+ T cells triggered by just age in the absence of infection have been described in the mouse, similar evidence for humans is lacking (Lee et al., 2016). However, some of the pathways that induce T cell exhaustion may be shared with those that are involved in T cell aging and senescence. Epigenetic studies of human naive and central memory CD8+ T cells revealed an age-associated loss of chromatin accessibility at key promoters, in particular at NRF1 binding sites (Moskowitz et al., 2017). The loss in NRF1 binding appears to account for the reduced expression of mitochondrial respiratory chain genes and defective oxidative phosphorylation that could result in poor T cell survival (Figure 2), reminiscent of the mitochondrial dysfunction-associated senescence identified by Wiley et al. (2016) in proliferating cells with mitochondrial defects. Repression of PGC1α, an important cofactor of NRF1 activity, is an early event in T cell exhaustion, indicating a mechanistic overlap between T cell aging and exhaustion (Bengsch et al., 2016). However, CD8+CD45RO+CCR7− effector memory T cells from older individuals show only few differences in terms of chromatin accessibility compared to young effector memory T cells, and they lack the epigenetic signatures of exhausted T cells (Moskowitz et al., 2017; Sen et al., 2016). This observation suggests that T cell aging mainly affects naive and central memory CD8+ T cells, possible employing pathways similar to exhaustion. In contrast, effector T cells in older individuals do not show any evidence of exhaustion.
Recent data in mouse models have suggested the existence of functional subsets of exhausted cells, with only a subset responding to checkpoint inhibition (Im et al., 2016). Moreover, the epigenetic phenotype of exhausted T cells is stable under PD-1 blockade and the restoration of function is therefore only transient in most T cells (Pauken et al., 2016). These observations raise the clinically important question of whether treatment responses to PD-1 blockade decrease with age, either because exhausted T cells age or because the fraction of exhausted T cells that are treatment responsive declines. In a meta-analysis of randomized controlled trials of immune checkpoint inhibitors (ipilimumab, tremelimumab, nivolumab, and pembrolizumab), the response rates of young and old cancer patients were very similar, suggesting that aging does not influence the function of exhausted T cells (Nishijima et al., 2016). However, these data are preliminary and additional work is needed to define the heterogeneity of exhausted T cells in humans and the influence of age on their composition and function.
Maladaptive T Cell Aging: Promoter of Tissue Inflammation
As outlined in previous sections, immune failure occurs when adaptive strategies developed by the aging T cell system fail. More frequently, adaptive mechanisms are successful but come at a price. Pertinent examples are homeostatic proliferation that maintains compartment size at the expense of partial loss in stemness and incomplete differentiation or activation of negative regulatory programs that constrain effector cell expansion and prevent increasing oligoclonality but also interfere with memory cell generation. In some settings, the programs associated with T cell aging culminates in a maladaptive response that directly instigates or at least contributes to disease (Figure 3). As principally studied in the model disease of rheumatoid arthritis (RA), maladaptive T cell aging in response to proliferative pressure and chronic stimulation leads to inflammatory effector functions and sustains chronic, tissue-injurious inflammation. Early studies, utilizing measurements of telomeric length and clonal expansion of CD4+CD28− end-differentiated T cells, estimated that the immune aging process is accelerated by 2–3 decades in patients with RA as compared to age-matched controls (Fujii et al., 2009; Weyand et al., 2014). Molecular analysis of early CD4+ T cell responses in RA patients, modeled by activating CD4+CD45RA+ T cells and studying early effector cells, identified the nuclease MRE11A, the DNA-dependent protein kinase DNA-PKcs, and the cell cycle regulator ATM as molecular mediators of premature T cell aging (Li et al., 2016b; Shao et al., 2009, 2010). During adult life, MRE11A and ATM protein levels in CD4+ T cells gradually decline in healthy individuals. This process is significantly accelerated in RA T cells and affects the naive CD4+ compartment much more than the memory compartment.
MRE11A is a nuclease and a component of the MRN complex critically involved in DNA damage sensing and repair. The kinase ATM is recruited to damaged DNA sites, acts as a sensor and amplifier of the DNA repair machinery, and regulates the cell cycle checkpoint and the cell’s life-death decisions. Loss-of-function mutations in DNA repair molecules have been identified as the underlying defect in progeria syndromes. Patients with the progeria Nijmegen breakage syndrome, who have a mutated NBN protein that forms the MRN complex with MRE11, display many of the immune aging findings in circulating T cells (Meijers et al., 2017). A similar observation has been made for patients with mutated ATM (Carney et al., 2012).
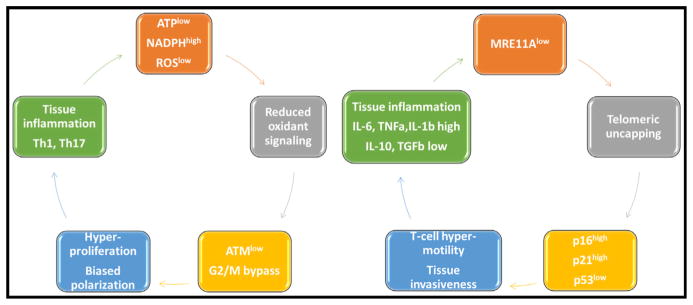
In RA CD4+ T cells, the lack of MRE11A nucleolytic activity is relevant for telomeric maintenance (Li et al., 2016b). MRE11Alo telomeres in RA T cells are coated by damage proteins and resemble uncapped, structurally abnormal telomeres. Impairment of the nuclease induces the senescence markers p16 and p21 but fails to upregulate p53, thus avoiding the classical hallmark of senescence, irreversible cell cycle arrest. With maintaining proliferative capacity, MRE11A RA T cells become highly mobile, capable of effective pro-inflammatory functions in the tissue environment (Figure 4). T cells rendered MRE11A-deficient infiltrate into synovial tissue where they promote a strong inflammatory response, inducing the hallmark RA cytokine signature of excessive IL-6, IL-1β, and TNF-α production. Precise mechanisms of how MRE11Alo T cells foster tissue inflammation need to be clarified.
Figure 4. Inflammation Resulting from Maladaptive DNA Damage Responses in T Cell Aging.
Defects in DNA damage responses mediated by ATM (left) and MRE11 loss (right) in naive CD4+ T cells have been linked to increased tissue inflammation in patients with rheumatoid arthritis, who present with premature immune aging.
ATMlo T cells from RA patients are highly capable of pro-inflammatory effector functions as well (Figure 4). They utilize a different pathway to induce and sustain inflammation in synovial tissue. ATM loss biases T cells to shift their differentiation pattern to a preferential commitment for the Th1 and Th17 cell lineages (Yang et al., 2016). T cells from healthy individuals treated with an ATM inhibitor invade into synovial tissue and acquire arthritogenic effector functions in vivo, suggesting a molecular link between DNA damage repair, cell cycle regulation, and functional behavior of T cells.
Mechanisms leading to loss in ATM activity in RA T cells have been explored. Besides its role in DNA damage and cell cycle checkpoint control, ATM functions as a redox sensor. RA T cells fail to appropriately phosphorylate ATM and repress transcription of the gene, a defect correctable by increasing cellular ROS levels. RA naive CD4+ T cells are constitutively low in intracellular ROS due to metabolic reprogramming and a fundamental shift in glucose utilization (Yang et al., 2016). CD4+ naive T cells from healthy individuals undergoing activation preferentially channel glucose to glycolytic breakdown and ATP generation. Due to reduced production of the glycolytic enzyme PFKFB3 and the upregulation of G6PD, RA T cells shunt glucose toward the pentose phosphate pathway, switching from a catabolic to a synthetic metabolism (Yang et al., 2013, 2016). Excess production of NADPH depletes the cell’s oxidative elements and impairs oxidative signaling. The functional outcome is an ATMlo T cell favoring pro-inflammatory effector functions.
Essentially, shared molecular features in the inherited progeria syndromes and in the chronic inflammatory disease rheumatoid arthritis emphasize the role of DNA repair mechanisms and cell cycle control in determining T cell aging and T cell functional behavior. Emerging data suggest that the failure of capping telomeres and repairing broken DNA may be mechanistically linked to fundamental metabolic processes.
Concluding Remarks
As long-lived cells that are able to survive for decades, T cells are exposed to abundant environmental encounters, while tackling the need to constantly regenerate. Continuous low-rate proliferation of naive T cells in the periphery is the major adaptation to prevent lymphopenia and to preserve the resource of a diverse repertoire of naive T cells. In healthy aging, humans utilize this mechanism of cell generation quite successfully, in particular for CD4+ T cells, yet pay the price of imposing replicative stress on somatic cells that inherently limits their replicative potential and also triggers cell differentiation. Many of the unwanted functional changes occurring with age are related to a partial loss of stemness and incomplete acquisition of features characteristic of memory or effector T cells. Although most of the shifts in molecular pathways identified so far are relatively subtle, data are mostly based on examination of healthy individuals, and studies are needed to investigate how these pathways change in case of less successful aging and in disease states. Unlike naive T cells, for which those circulating in peripheral blood embodies a reasonably representative population, aging studies of memory T cells have to appreciate the impact of compartmentalization, as exemplified by resident memory T cells. Generally, the aging memory T cell system has to find a balance between maintaining and expanding memory cells, while avoiding oligoclonality and clonal dominance. Memory inflation can be successful to keep latent infections in check as exemplified by immune responses to CMV. However, the aging immune system commits considerable resources to a multitude of negative regulatory pathways to control clonal expansion in acute T cell responses as well as preventing memory inflation to chronic stimulation. While these pathways function to balance the memory cell compartment, their disproportionate activation contributes to the failure of generating immune memory in the aging host. A clinically dreaded complication of maladaptive T cell aging stems from the propensity of such aged T cells to rapidly invade into tissue sites and promote tissue inflammation. Understanding mechanisms driving healthy and maladaptive T cell aging is beginning to provide a series of molecular targets and opens the door toward therapeutic corrections, extending the life and functionality of T cells and preventing aging-related inflammation.
Acknowledgments
This work was supported by the NIH (R01 AR042527, R01 HL117913, R01 AI108906, and P01 HL129941 to C.M.W. and R01 AI108891, R01 AG045779, U19 AI057266, and I01 BX001669 to J.J.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. doi: 10.1016/j.it.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, Robson SC. NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun. 2015;6:8819. doi: 10.1038/ncomms9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains I, Antia R, Callard R, Yates AJ. Quantifying the development of the peripheral naive CD4+ T-cell pool in humans. Blood. 2009a;113:5480–5487. doi: 10.1182/blood-2008-10-184184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains I, Thiébaut R, Yates AJ, Callard R. Quantifying thymic export: combining models of naive T cell proliferation and TCR excision circle dynamics gives an explicit measure of thymic output. J Immunol. 2009b;183:4329–4336. doi: 10.4049/jimmunol.0900743. [DOI] [PubMed] [Google Scholar]
- Barnett LG, Simkins HM, Barnett BE, Korn LL, Johnson AL, Wherry EJ, Wu GF, Laufer TM. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol. 2014;192:3607–3617. doi: 10.4049/jimmunol.1301284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci USA. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res. 2015;3:506–517. doi: 10.1158/2326-6066.CIR-14-0211. [DOI] [PubMed] [Google Scholar]
- Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA, Delgoffe GM, Wherry EJ. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer M, Abdullah Z, Chemnitz JM, Maisel D, Sander J, Lehmann C, Thabet Y, Shinde PV, Schmidleithner L, Köhne M, et al. Tumor-necrosis factor impairs CD4(+) T cell-mediated immunological control in chronic viral infection. Nat Immunol. 2016;17:593–603. doi: 10.1038/ni.3399. [DOI] [PubMed] [Google Scholar]
- Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, et al. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med. 2015;7:273ra13. doi: 10.1126/scitranslmed.3010314. [DOI] [PubMed] [Google Scholar]
- Bignon A, Régent A, Klipfel L, Desnoyer A, de la Grange P, Martinez V, Lortholary O, Dalloul A, Mouthon L, Balabanian K. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood. 2015;125:2507–2518. doi: 10.1182/blood-2014-08-598565. [DOI] [PubMed] [Google Scholar]
- Briceño O, Lissina A, Wanke K, Afonso G, von Braun A, Ragon K, Miquel T, Gostick E, Papagno L, Stiasny K, et al. Reduced naïve CD8(+) T-cell priming efficacy in elderly adults. Aging Cell. 2016;15:14–21. doi: 10.1111/acel.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, Bolotin DA, Lukyanov S, Bogdanova EA, Mamedov IZ, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- Britanova OV, Shugay M, Merzlyak EM, Staroverov DB, Putintseva EV, Turchaninova MA, Mamedov IZ, Pogorelyy MV, Bolotin DA, Izraelson M, et al. Dynamics of individual T cell repertoires: from cord blood to centenarians. J Immunol. 2016;196:5005–5013. doi: 10.4049/jimmunol.1600005. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Weyand CM, Goronzy JJ. Down-regulation of CD28 expression by TNF-alpha. J Immunol. 2001;167:3231–3238. doi: 10.4049/jimmunol.167.6.3231. [DOI] [PubMed] [Google Scholar]
- Bryl E, Vallejo AN, Matteson EL, Witkowski JM, Weyand CM, Goronzy JJ. Modulation of CD28 expression with anti-tumor necrosis factor alpha therapy in rheumatoid arthritis. Arthritis Rheum. 2005;52:2996–3003. doi: 10.1002/art.21353. [DOI] [PubMed] [Google Scholar]
- Cao JN, Gollapudi S, Sharman EH, Jia Z, Gupta S. Age-related alterations of gene expression patterns in human CD8+ T cells. Aging Cell. 2010;9:19–31. doi: 10.1111/j.1474-9726.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- Carney EF, Srinivasan V, Moss PA, Taylor AM. Classical ataxia telangiectasia patients have a congenitally aged immune system with high expression of CD95. J Immunol. 2012;189:261–268. doi: 10.4049/jimmunol.1101909. [DOI] [PubMed] [Google Scholar]
- Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- Chen G, Lustig A, Weng NP. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci USA. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Cukalac T, Chadderton J, Handel A, Doherty PC, Turner SJ, Thomas PG, La Gruta NL. Reproducible selection of high avidity CD8+ T-cell clones following secondary acute virus infection. Proc Natl Acad Sci USA. 2014;111:1485–1490. doi: 10.1073/pnas.1323736111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mögling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Derhovanessian E, Maier AB, Hähnel K, McElhaney JE, Slagboom EP, Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol. 2014;193:3624–3631. doi: 10.4049/jimmunol.1303361. [DOI] [PubMed] [Google Scholar]
- Deshpande NR, Parrish HL, Kuhns MS. Self-recognition drives the preferential accumulation of promiscuous CD4(+) T-cells in aged mice. eLife. 2015;4:e05949. doi: 10.7554/eLife.05949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desponds J, Mora T, Walczak AM. Fluctuating fitness shapes the clone-size distribution of immune repertoires. Proc Natl Acad Sci USA. 2016;113:274–279. doi: 10.1073/pnas.1512977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+CD27− memory T cells. J Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]
- DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, Pollak R, Christoff J, Earl J, Landolfi V, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- Domnich A, Arata L, Amicizia D, Puig-Barberà J, Gasparini R, Panatto D. Effectiveness of MF59-adjuvanted seasonal influenza vaccine in the elderly: A systematic review and meta-analysis. Vaccine. 2017;35:513–520. doi: 10.1016/j.vaccine.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Fang F, Yu M, Cavanagh MM, Hutter Saunders J, Qi Q, Ye Z, Le Saux S, Sultan W, Turgano E, Dekker CL, et al. Expression of CD39 on activated T cells impairs their survival in older individuals. Cell Rep. 2016;14:1218–1231. doi: 10.1016/j.celrep.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes Marraco SA, Soneson C, Cagnon L, Gannon PO, Allard M, Abed Maillard S, Montandon N, Rufer N, Waldvogel S, Delorenzi M, Speiser DE. Long-lasting stem cell-like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci Transl Med. 2015;7:282ra48. doi: 10.1126/scitranslmed.aaa3700. [DOI] [PubMed] [Google Scholar]
- Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci USA. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Le Page A, Fortin C, Witkowski JM, Dupuis G, Larbi A. Cellular signaling in the aging immune system. Curr Opin Immunol. 2014;29:105–111. doi: 10.1016/j.coi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJ, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med. 2015;7:281ra43. doi: 10.1126/scitranslmed.aaa2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Yassai MB, Naumov YN, Selin LK. Narrowing of human influenza A virus-specific T cell receptor α and β repertoires with increasing age. J Virol. 2015;89:4102–4116. doi: 10.1128/JVI.03020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez I, Marx F, Gould EA, Grubeck-Loebenstein B. T cells from elderly persons respond to neoantigenic stimulation with an unimpaired IL-2 production and an enhanced differentiation into effector cells. Exp Gerontol. 2004;39:597–605. doi: 10.1016/j.exger.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Qi Q, Olshen RA, Weyand CM. High-throughput sequencing insights into T-cell receptor repertoire diversity in aging. Genome Med. 2015;7:117. doi: 10.1186/s13073-015-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Gupta S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol. 2014;54:47–52. doi: 10.1016/j.exger.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 2015;11:e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232–3235. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci USA. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Henel G, Singh K, Cui D, Pryshchep S, Lee WW, Weyand CM, Goronzy JJ. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 2006;107:4449–4457. doi: 10.1182/blood-2005-06-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012;24:476–481. doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Henson SM, Lanna A, Riddell NE, Franzese O, Macaulay R, Griffiths SJ, Puleston DJ, Watson AS, Simon AK, Tooze SA, Akbar AN. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J Clin Invest. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson SM, Macaulay R, Riddell NE, Nunn CJ, Akbar AN. Blockade of PD-1 or p38 MAP kinase signaling enhances senescent human CD8(+) T-cell proliferation by distinct pathways. Eur J Immunol. 2015;45:1441–1451. doi: 10.1002/eji.201445312. [DOI] [PubMed] [Google Scholar]
- Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, Kurupati RK, Kannan S, Ertl H, Schmader KE, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol. 2014;193:3528–3537. doi: 10.4049/jimmunol.1302503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Yates AJ, Goronzy JJ, Antia R. Peripheral selection rather than thymic involution explains sudden contraction in naive CD4 T-cell diversity with age. Proc Natl Acad Sci USA. 2012;109:21432–21437. doi: 10.1073/pnas.1209283110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, Phair J, Jamieson BD. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig S, Przybylski GK, Schmidt CA, Laurisch K, Möwes B, Radbruch A, Thiel A. Two subsets of naive T helper cells with distinct T cell receptor excision circle content in human adult peripheral blood. J Exp Med. 2002;195:789–794. doi: 10.1084/jem.20011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwissa M, Nakaya HI, Oluoch H, Pulendran B. Distinct TLR adjuvants differentially stimulate systemic and local innate immune responses in nonhuman primates. Blood. 2012;119:2044–2055. doi: 10.1182/blood-2011-10-388579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar DL, Weyand CM, Goronzy JJ. Promoter choice and translational repression determine cell type-specific cell surface density of the inhibitory receptor CD85j expressed on different hematopoietic lineages. Blood. 2010;115:3278–3286. doi: 10.1182/blood-2009-09-243493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanna A, Henson SM, Escors D, Akbar AN. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol. 2014;15:965–972. doi: 10.1038/ni.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laydon DJ, Bangham CR, Asquith B. Estimating T-cell repertoire diversity: limitations of classical estimators and a new approach. Philos Trans R Soc Lond B Biol Sci. 2015;370:370. doi: 10.1098/rstb.2014.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuardi L, Herndler-Brandstetter D, Brunner S, Laschober GT, Lepperdinger G, Grubeck-Loebenstein B. Microarray analysis reveals similarity between CD8+CD28− T cells from young and elderly persons, but not of CD8+CD28+ T cells. Biogerontology. 2009;10:191–202. doi: 10.1007/s10522-008-9167-1. [DOI] [PubMed] [Google Scholar]
- Le Page A, Fortin C, Garneau H, Allard N, Tsvetkova K, Tan CT, Larbi A, Dupuis G, Fülöp T. Downregulation of inhibitory SRC homology 2 domain-containing phosphatase-1 (SHP-1) leads to recovery of T cell responses in elderly. Cell Commun Signal. 2014;12:2. doi: 10.1186/1478-811X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Shin KS, Kim GY, Song YC, Bae EA, Kim IK, Koh CH, Kang CY. Characterization of age-associated exhausted CD8+ T cells defined by increased expression of Tim-3 and PD-1. Aging Cell. 2016;15:291–300. doi: 10.1111/acel.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelic A, Verschoor CP, Ventresca M, Parsons R, Evelegh C, Bowdish D, Betts MR, Loeb MB, Bramson JL. The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog. 2012;8:e1003076. doi: 10.1371/journal.ppat.1003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24:494–500. doi: 10.1016/j.coi.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Smith JG, Kaufhold RM, Barber D, Hayward AR, Chan CY, Chan IS, Li DJ, Wang W, Keller PM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ju J, Weyand CM, Goronzy JJ. Age-associated failure to adjust type I IFN receptor signaling thresholds after T cell activation. J Immunol. 2015;195:865–874. doi: 10.4049/jimmunol.1402389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HM, Hiroi T, Zhang Y, Shi A, Chen G, De S, Metter EJ, Wood WH, 3rd, Sharov A, Milner JD, et al. TCRβ repertoire of CD4+ and CD8+ T cells is distinct in richness, distribution, and CDR3 amino acid composition. J Leukoc Biol. 2016a;99:505–513. doi: 10.1189/jlb.6A0215-071RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen Y, Hohensinner P, Ju J, Wen Z, Goodman SB, Zhang H, Goronzy JJ, Weyand CM. Deficient activity of the nuclease MRE11A induces T cell aging and promotes arthritogenic effector functions in patients with rheumatoid arthritis. Immunity. 2016b;45:903–916. doi: 10.1016/j.immuni.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, Morris C, Longo DL, Zhan M, Ferrucci L, et al. Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clin Sci. 2015;128:367–377. doi: 10.1042/CS20140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA. How T follicular helper cells and the germinal centre response change with age. Immunol Cell Biol. 2014;92:72–79. doi: 10.1038/icb.2013.77. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lythe G, Callard RE, Hoare RL, Molina-París C. How many TCR clonotypes does a body maintain? J Theor Biol. 2016;389:214–224. doi: 10.1016/j.jtbi.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM, van Essen GA, Caplanusi A, Claeys C, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13:485–496. doi: 10.1016/S1473-3099(13)70046-X. [DOI] [PubMed] [Google Scholar]
- Meijers RW, Dzierzanowska-Fangrat K, Zborowska M, Solarska I, Tielemans D, van Turnhout BA, Driessen G, van der Burg M, van Dongen JJ, Chrzanowska KH, Langerak AW. Circulating T cells of patients with Nijmegen breakage syndrome show signs of senescence. J Clin Immunol. 2017;37:133–142. doi: 10.1007/s10875-016-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal D, Young A, Stannard K, Yong M, Teng MW, Allard B, Stagg J, Smyth MJ. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 2014;74:3652–3658. doi: 10.1158/0008-5472.CAN-14-0957. [DOI] [PubMed] [Google Scholar]
- Mohanty S, Joshi SR, Ueda I, Wilson J, Blevins TP, Siconolfi B, Meng H, Devine L, Raddassi K, Tsang S, et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis. 2015;211:1174–1184. doi: 10.1093/infdis/jiu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz DM, Zhang DW, Hu B, Le Saux S, Yanes RE, Ye Z, Buenrostro JD, Weyand CM, Greenleaf WJ, Goronzy JJ. Epigenomics of human CD8 T cell differentiation and aging. Sci Immunol. 2017;2:1–14. doi: 10.1126/sciimmunol.aag0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarro K, Nguyen H, Chen G, Xu M, Alcorta S, Yao X, Zukley L, Metter EJ, Truong T, Lin Y, et al. Telomere length as an indicator of the robustness of B- and T-cell response to influenza in older adults. J Infect Dis. 2015;212:1261–1269. doi: 10.1093/infdis/jiv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Hagan T, Duraisingham SS, Lee EK, Kwissa M, Rouphael N, Frasca D, Gersten M, Mehta AK, Gaujoux R, et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015;43:1186–1198. doi: 10.1016/j.immuni.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Ndifon W, Dushoff J. The Hayflick limit may determine the effective clonal diversity of naive T cells. J Immunol. 2016;196:4999–5004. doi: 10.4049/jimmunol.1502343. [DOI] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Nobile M, Correa R, Borghans JA, D’Agostino C, Schneider P, De Boer RJ, Pantaleo G Swiss HIV Cohort Study. De novo T-cell generation in patients at different ages and stages of HIV-1 disease. Blood. 2004;104:470–477. doi: 10.1182/blood-2003-12-4265. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BI, Akbar AN. Convergence of innate and adaptive immunity during human aging. Front Immunol. 2016;7:445. doi: 10.3389/fimmu.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadwal K, Alegre-Abarrategui J, Watson AS, Pike L, Anbalagan S, Hammond EM, Wade-Martins R, McMichael A, Klenerman P, Simon AK. A novel method for autophagy detection in primary cells: impaired levels of macroautophagy in immunosenescent T cells. Autophagy. 2012;8:677–689. doi: 10.4161/auto.18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, Larbi A, Weinberger B, Cossarizza A. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, Knox K, Bush EC, Sims PA, Sinari S, et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. 2016;17:966–975. doi: 10.1038/ni.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, Goronzy JJ. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci USA. 2014;111:13139–13144. doi: 10.1073/pnas.1409155111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Cavanagh MM, Le Saux S, NamKoong H, Kim C, Turgano E, Liu Y, Wang C, Mackey S, Swan GE, et al. Diversification of the antigen-specific T cell receptor repertoire after varicella zoster vaccination. Sci Transl Med. 2016a;8:332ra46. doi: 10.1126/scitranslmed.aaf1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Cavanagh MM, Le Saux S, Wagar LE, Mackey S, Hu J, Maecker H, Swan GE, Davis MM, Dekker CL, et al. Defective T memory cell differentiation after varicella zoster vaccination in older individuals. PLoS Pathog. 2016b;12:e1005892. doi: 10.1371/journal.ppat.1005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KM, Zaloumis SG, Cukalac T, Kan WT, Sng XY, Mirams M, Watson KA, McCaw JM, Doherty PC, Thomas PG, et al. Heightened self-reactivity associated with selective survival, but not expansion, of naïve virus-specific CD8+ T cells in aged mice. Proc Natl Acad Sci USA. 2016;113:1333–1338. doi: 10.1073/pnas.1525167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Žugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, Seeman TE. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Ding J, Taylor JR, Lohman K, Soranzo N, de la Fuente A, Liu TF, Johnson C, Barr RG, Register TC, et al. Transcriptomic profiles of aging in purified human immune cells. BMC Genomics. 2015;16:333. doi: 10.1186/s12864-015-1522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins HS, Srivastava SK, Campregher PV, Turtle CJ, Andriesen J, Riddell SR, Carlson CS, Warren EH. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. 2010;2:47ra64. doi: 10.1126/scitranslmed.3001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ. Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2005;79:381–386. doi: 10.1097/01.tp.0000148239.00384.f0. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni FF, Akbar A, Arens R, Chiu YL, Cičin-Šain L, Dechanet-Merville J, Derhovanessian E, Ferrando-Martinez S, et al. New advances in CMV and immunosenescence. Exp Gerontol. 2014;55:54–62. doi: 10.1016/j.exger.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauce D, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, Sidi D, Sibony-Prat J, Appay V. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J Immunol. 2012;189:5541–5548. doi: 10.4049/jimmunol.1201235. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Schönland SO, Zimmer JK, Lopez-Benitez CM, Widmann T, Ramin KD, Goronzy JJ, Weyand CM. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–1434. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- Schulz AR, Mälzer JN, Domingo C, Jürchott K, Grützkau A, Babel N, Nienen M, Jelinek T, Niedrig M, Thiel A. Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J Immunol. 2015;195:4699–4711. doi: 10.4049/jimmunol.1500598. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Fazekas de Saint Groth B. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- Sell S, Guest I, McKinstry KK, Strutt TM, Kohlmeier JE, Brincks E, Tighe M, Blackman MA, Woodland DL, Dutton RW, Swain SL. Intraepithelial T-cell cytotoxicity, induced bronchus-associated lymphoid tissue, and proliferation of pneumocytes in experimental mouse models of influenza. Viral Immunol. 2014;27:484–496. doi: 10.1089/vim.2014.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354:1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Orr SS, Furman D, Kidd BA, Hadad F, Lovelace P, Huang YW, Rosenberg-Hasson Y, Mackey S, Grisar FA, Pickman Y, et al. Defective signaling in the JAK-STAT pathway tracks with chronic inflammation and cardiovascular risk in aging humans. Cell Syst. 2016;3:374–384. e4. doi: 10.1016/j.cels.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with age. J Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- Stefanová I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- Su DM, Aw D, Palmer DB. Immunosenescence: a product of the environment? Curr Opin Immunol. 2013;25:498–503. doi: 10.1016/j.coi.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJC, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, Farber DL. Long-term maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol. 2016;1:1–11. doi: 10.1126/sciimmunol.aah6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, Haynes L, Swain SL. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci USA. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek T, Delemarre EM, Janssen WJ, Nievelstein RA, Broen JC, Tesselaar K, Borghans JA, Nieuwenhuis EE, Prakken BJ, Mokry M, et al. Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J Clin Invest. 2016;126:1126–1136. doi: 10.1172/JCI84997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijst JW, Gerlach C, Swart E, Sie D, Nunes-Alves C, Kerkhoven RM, Arens R, Correia-Neves M, Schepers K, Schumacher TN. Recruitment of antigen-specific CD8+ T cells in response to infection is markedly efficient. Science. 2009;325:1265–1269. doi: 10.1126/science.1175455. [DOI] [PubMed] [Google Scholar]
- van Leeuwen EM, Koning JJ, Remmerswaal EB, van Baarle D, van Lier RA, ten Berge IJ. Differential usage of cellular niches by cytomegalovirus versus EBV- and influenza virus-specific CD8+ T cells. J Immunol. 2006;177:4998–5005. doi: 10.4049/jimmunol.177.8.4998. [DOI] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Agius E, Booth N, Dunne PJ, Lacy KE, Reed JR, Sobande TO, Kissane S, Salmon M, Rustin MH, Akbar AN. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–3650. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, Jackson SE, Fuentes-Duculan J, Suárez-Fariñas M, Mabbott NA, et al. The characterization of varicella zoster virus-specific T cells in skin and blood during aging. J Invest Dermatol. 2015;135:1752–1762. doi: 10.1038/jid.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waickman AT, Alme A, Senaldi L, Zarek PE, Horton M, Powell JD. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol Immunother. 2012;61:917–926. doi: 10.1007/s00262-011-1155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Zhang Y, Ghattas H, Worth A, Irvine A, Bennett AR, Griffin GE, Beverley PC, Tough DF, Macallan DC. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173:1787–1794. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor αβ diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med. 2012;4:128ra42. doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wen Z, Shimojima Y, Shirai T, Li Y, Ju J, Yang Z, Tian L, Goronzy JJ, Weyand CM. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126:1953–1967. doi: 10.1172/JCI84181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Žugich D, Kaye J, Nikolich-Žugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westera L, Drylewicz J, den Braber I, Mugwagwa T, van der Maas I, Kwast L, Volman T, van de Weg-Schrijver EH, Bartha I, Spierenburg G, et al. Closing the gap between T-cell life span estimates from stable isotope-labeling studies in mice and humans. Blood. 2013;122:2205–2212. doi: 10.1182/blood-2013-03-488411. [DOI] [PubMed] [Google Scholar]
- Westera L, van Hoeven V, Drylewicz J, Spierenburg G, van Velzen JF, de Boer RJ, Tesselaar K, Borghans JA. Lymphocyte maintenance during healthy aging requires no substantial alterations in cellular turnover. Aging Cell. 2015;14:219–227. doi: 10.1111/acel.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26:93–100. doi: 10.1097/BOR.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Goldstein DR. Impact of aging on antigen presentation cell function of dendritic cells. Curr Opin Immunol. 2013;25:535–541. doi: 10.1016/j.coi.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Shen Y, Oishi H, Matteson EL, Tian L, Goronzy JJ, Weyand CM. Restoring oxidant signaling suppresses proarthritogenic T cell effector functions in rheumatoid arthritis. Sci Transl Med. 2016;8:331ra38. doi: 10.1126/scitranslmed.aad7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Li G, Lee WW, Yuan M, Cui D, Weyand CM, Goronzy JJ. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci USA. 2012;109:E879–E888. doi: 10.1073/pnas.1109797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnitsyna VI, Handel A, McMaster SR, Hayward SL, Kohlmeier JE, Antia R. Mathematical model reveals the role of memory CD8 T cell populations in recall responses to influenza. Front Immunol. 2016;7:165. doi: 10.3389/fimmu.2016.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]