Abstract
As coral bleaching events become more frequent and intense, our ability to predict and mitigate future events depends upon our capacity to interpret patterns within previous episodes. Responses to thermal stress vary among coral species; however the diversity of coral assemblages, environmental conditions, assessment protocols, and severity criteria applied in the global effort to document bleaching patterns creates challenges for the development of a systemic metric of taxon-specific response. Here, we describe and validate a novel framework to standardize bleaching response records and estimate their measurement uncertainties. Taxon-specific bleaching and mortality records (2036) of 374 coral taxa (during 1982–2006) at 316 sites were standardized to average percent tissue area affected and a taxon-specific bleaching response index (taxon-BRI) was calculated by averaging taxon-specific response over all sites where a taxon was present. Differential bleaching among corals was widely variable (mean taxon-BRI = 25.06 ± 18.44%, ± SE). Coral response may differ because holobionts are biologically different (intrinsic factors), they were exposed to different environmental conditions (extrinsic factors), or inconsistencies in reporting (measurement uncertainty). We found that both extrinsic and intrinsic factors have comparable influence within a given site and event (60% and 40% of bleaching response variance of all records explained, respectively). However, when responses of individual taxa are averaged across sites to obtain taxon-BRI, differential response was primarily driven by intrinsic differences among taxa (65% of taxon-BRI variance explained), not conditions across sites (6% explained), nor measurement uncertainty (29% explained). Thus, taxon-BRI is a robust metric of intrinsic susceptibility of coral taxa. Taxon-BRI provides a broadly applicable framework for standardization and error estimation for disparate historical records and collection of novel data, allowing for unprecedented accuracy in parameterization of mechanistic and predictive models and conservation plans.
Keywords: biological response to climate change, coral bleaching, response index, Symbiodinium, symbiosis, thermal stress
Introduction
Reef-building corals depend on mutualistic symbioses with photosynthetic dinoflagellates representing the genus Symbiodinium to support their metabolic requirements (Muscatine, 1990). The disruption of these associations (coral bleaching) result in increases in mortality and reductions in resistance to disease, predation, and bioerosion, and reduced capacity for damage repair, competition, growth, and reproduction (Jokiel, 2004; Jones, 2008). As global temperatures increase, corals are experiencing symbiosis-disrupting thermal stress (Hughes et al., 2003; Pandolfi et al., 2003) at increasing frequencies and intensities (Hoegh-Guldberg et al., 2007; Wilkinson, 2008) and are being eliminated at unsustainable rates (19% of coral reefs have been lost and about 35% are severely threatened; Wilkinson, 2008).
Within a bleaching event, coral susceptibility to stress is highly uneven. Coral colonies, inhabiting the same reef and apparently exposed to identical conditions, will bleach and die at different rates (Marshall & Baird, 2000; Loya et al., 2001; Obura, 2001; Done et al., 2003; McClanahan, 2004; Jones, 2008; van Woesik et al., 2011; Guest et al., 2012). Differential bleaching susceptibility among taxa has been attributed to many factors intrinsic to the holobiont (e.g., thermotolerance of Symbiodinium, coral physiological, morphological, and optical characteristics, and the interaction between coral, Symbiodinium, and their microbiota; Coles & Jokiel, 1977; Bhagooli & Hidaka, 2003; Baird et al., 2009; Leggat et al., 2011; van Woesik et al., 2011; Cunning & Baker, 2013; Krediet et al., 2013; Marcelino et al., 2013) or extrinsic (sensu West & Salm, 2003) from the environment (e.g., site-specific environmental conditions, thermal stress, and frequency of thermal anomalies; McClanahan & Maina, 2003; Guest et al., 2012; Pratchett et al., 2013). Knowing which of many potential factors determine differential bleaching susceptibility among taxa will be essential to understanding bleaching mechanisms, and to preserve, manage, or reconstruct coral assemblages.
Although it is clear that bleaching is not uniform, ranking of responses and mechanisms driving them remain partially obscured. There are more than 40 years of coral bleaching data available; however the majority of available records do not include taxon-level bleaching and mortality response information and marshaling the historical data into a systematic summary is complicated due to data nonuniformity. The data are inherently inconsistent due to the diversity of situations encountered, observation periods relative to episode onset, sampling protocols (e.g., assessing the average affected colony, percent of affected colonies, or proportion of affected coral cover), severity criteria (e.g., data binned into categories ranging from increments of 10% change in color to broad definitions of pale, bleached, or dead), and taxonomic uncertainty. Additionally, coral taxa may appear to be differently affected because responses are intrinsically different among coral holobionts, the stresses that corals face are extrinsically different among events and locations, or response is inconsistently measured across reports. Several indices of taxon-specific differential bleaching and mortality (Gleason, 1993; Marshall & Baird, 2000; Done et al., 2003; McClanahan, 2004; McClanahan et al., 2004; Guest et al., 2012; Pratchett et al., 2013) or coral assemblage susceptibility (Manzello et al., 2007; McClanahan et al., 2007a) have been introduced that focus on specific events, taxon sets, or geographic areas; but a consensus of global data remains elusive.
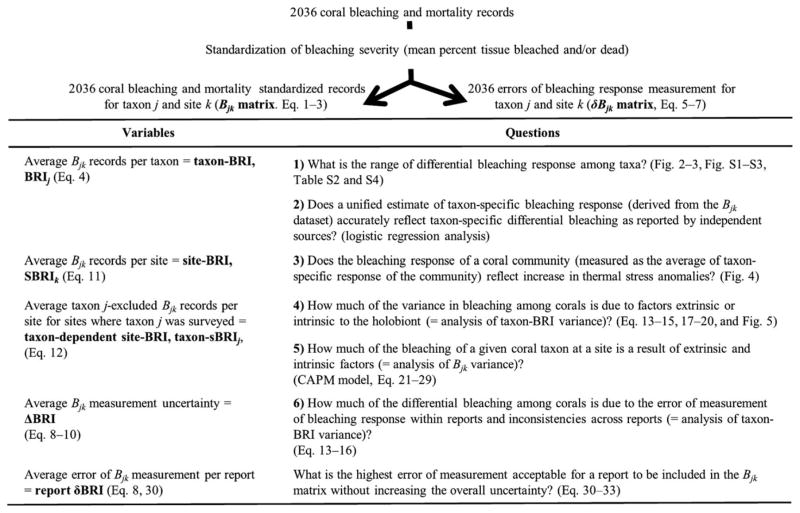
Here, we propose a new framework to compare taxon-specific bleaching and mortality records across diverse reports to create a taxon-specific bleaching response index (taxon-BRI) and estimate uncertainty in their measurement. This new analysis significantly extendes a previous index from 96 (Marcelino et al., 2013) to 374 coral taxa (58 genera, 316 species) using 2036 records from 316 sites across seven biogeographic regions covering the years 1983–2006 (including the pantropical mass bleaching of 1997–1998, and regional events in 2002, GBR, and 2005, Caribbean) (Figs 1 and 2). We created a matrix Bjk of standardized taxon-specific bleaching and mortality records j per site k, allowing us to address the following questions: (i) what is the range of differential bleaching responses among taxa; (ii) does a unified estimate of taxon-specific bleaching response (taxon-BRI or average Bjk for same j over different k) accurately reflect differential bleaching as reported by independent sources; (iii) does bleaching response of a coral assemblage (average Bjk of the assemblage with different j at site k) reflect the intensity and duration of thermal anomalies; (iv) how much of the variance in bleaching response among coral taxa is due to error of measurement within and inconsistencies across reports; (v) how much of the variance in bleaching response among coral taxa is due to factors extrinsic or intrinsic to the holobiont; and (vi) how much of the bleaching response of a given coral taxon at a site is the result of extrinsic and intrinsic factors?
Fig. 1.
Standardization process for bleaching and mortality records with a map of the equations and models used in the calculation of variables used in this study. Rationale: differential coral bleaching is due to an unknown combination of intrinsic (coral-Symbiodinium dependent) factors, extrinsic (environmental and thermal stress factors, approximated as SBRIk) and uncertainty in measuring bleaching response.
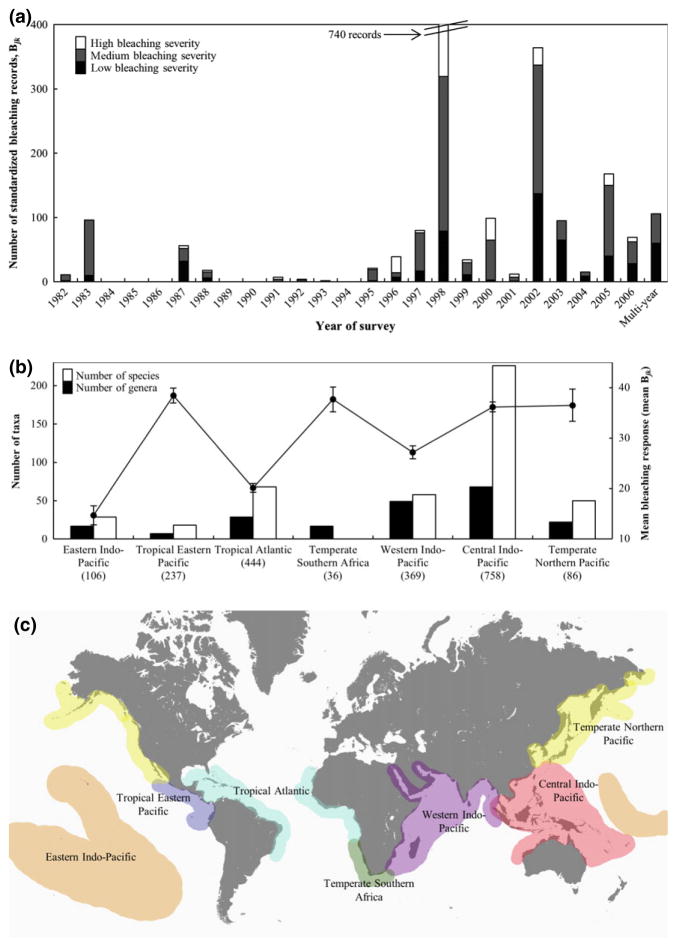
Fig. 2.
Years, locations, and responses of the 2036 standardized bleaching and mortality records (Bjk) used in this study. Number of records per year categorized as high (≥75th percentile; open bars), medium (grey bars), and low (≤25th percentile; black bars) bleaching severity (a). Number of taxa (genera solid and species open bars) and mean (and standard error; line with error bars) response (b) per biogeographic realm after Spalding et al. (2007) (c). Parenthetical values are the total number of records.
Materials and methods
Data selection
We searched the historical record of mass bleaching events documented in peer-reviewed literature, scientific reports, unpublished data (collected by A. Baird), and electronic databases of events occurring from 1983 to 2006. We focused exclusively on taxon-specific (genus or species) records that included explicit severity criteria for both bleaching and bleaching-associated mortality. We selected 42 sources containing 2036 records, which applied a diversity of data collection protocols and bleaching severity criteria (Tables S1 and S2). Estimates of both bleaching and mortality are necessary for an accurate assessment, as neither is fully reflective of the damage caused by a bleaching event (McClanahan, 2004; Obura, 2009; Suggett & Smith, 2011). Similarly, time of observation relative to onset of bleaching is highly variable across reports (ranging from 1–3 to 6–12 months; Table S2) and using both bleaching (greater in early observations) and mortality (greater in later observations) will more accurately reflect the extent of physiological damage caused by an event. See Text S1.1 and Tables S1 and S2 for descriptions of reports used and considerations on bleaching-induced and associated mortality.
Construction of a matrix, Bjk, of standardized taxon-specific bleaching and mortality data per site
A bleaching response matrix, Bjk, was built using 2036 taxon-specific bleaching and mortality records of 374 coral taxa j surveyed at 316 sites k. Reports used different severity criteria, with some using a linear scale proportional to the percent cover affected (e.g., BLAGRRA dataset) while others used weighing coefficients for different levels of response (e.g., McClanahan et al., 2007a; Baird-Palm Island; A. Baird, unpublished data) such that the contribution of the least affected colonies is augmented relative to the most affected (Table S1). Therefore, all records were standardized to be an estimate of the proportion of affected coral tissue (i.e., mean percent tissue bleached and/or dead). There were three types of reporting methodologies encountered, each requiring a unique conversion algorithm for standardization (see Table S1 for details on individual reports).
Methodology A
For reports that specified numbers of colonies in predetermined bleaching severity categories: let c be the vector of bleaching category limits such that ci, i = 1, ···, M + 1. For example, the dataset in Floros et al. (2004) defined bleaching categories as 0, 1–30%, 31–70%, 71–100% of colonies (Table S1). In our standardization, a dead colony is equivalent to 100% bleached; therefore, if we use these categories as an example, c = (0,0.3,0.7,1,1). Furthermore, let b be the vector of the mean of bleaching categories: bi = (ci + ci + 1)/2, i = 1, …, M and Ni, i = 1, …, M the number of taxon-specific coral colonies within each bleaching category (i.e., with bleaching score between ci and ci + 1). Bleaching response B for a given taxon, (fraction of bleached tissue), is then estimated as
| (1) |
where is the probability (or percent of colonies) in bleaching category i.
Methodology B
An example of this is ReefBase (Table S1), where bleaching response for a given taxon is provided as a single score, which we define as s. This score ranged from 0 to 3: s = {0, 1, 2, 3} and is determined by the portion of affected colonies. In this case (see Text S1.2 for derivation), bleaching response can be expressed as
| (2) |
where w = 1/2(ws+1 + ws), if s ≥ 1 or 0 if s = 0 is the portion of affected colonies with w = {0, 0.1, 0.3, 1} corresponding to scores s = {0, 1, 2, 3}, wd = 1/2(wds+1 + wds), if sd ≥1 or 0 if sd = 0 is the portion of dead colonies with wd = {0, 0.1, 0.3, 1} corresponding to the death scores sd = {0,1,2,3}, and b and bd are the expectations of the portion of bleached and dead tissue of these affected colonies. For the ReefBase data, b = bd = 0.55.
Methodology C
These reports only provide the final weighted average of scores n, which we denote here as , with no information on how the bleaching is distributed over different categories. Ba in this case has to be converted into B (see Text S1.2 for derivation):
| (3) |
where constant α ≈ 1 is found as a fitting parameter in equation , (average r2 = 0.98 s vs. b data fit).
Using these three methods, we estimated B for each taxon at each site, thus forming matrix Bjk, where j is the taxon, and k is the site (Table S2).
Construction of the bleaching response index per taxa (taxon-BRI or BRIj)
After construction of matrix Bjk, the bleaching response index per-taxon (taxon-BRI) for species j is found as the average of the bleaching values over all bleaching sites:
| (4) |
where, as above, the sites are indexed with subscript k, and Kj is the total number of sites with bleaching response for taxon j. Taxa were reported at the genus- or species-level in the original reports, which is mirrored here. BRIj values for genera are an average of all Bjk records for the genus and its daughter species at each site; however, if small numbers of species-level records are available within a genus, no genus-level BRIj is calculated (resulting in values for 58 of 90 genera).
Two-approach validation of matrix Bjk
Concordance with independent assessments
We compared taxon-BRI (continuous) to the relative bleaching susceptibility of 181 taxa that match this study from the independent assessment of Carpenter et al. (2008) (categorized as ‘moderately or highly susceptible to bleaching’ or ‘moderately or highly resistant to bleaching’) through logistic regression.
Correlation between site-BRI and thermal anomalies
Bleaching responses of coral assemblages (measured as individual site-BRI) were compared to intensity of thermal anomaly per site × bleaching-month (measured as degree heating weeks, DHW) through regression analysis for a subset of data from the wider Caribbean bioregion (n = 263 Bjk unique-taxon/(site × bleaching-month) records, for 39 taxa, at 35 sites, in years 1998–2006). DHW is a metric of the magnitude and duration of accumulated thermal stress (product of °C above the highest monthly mean sea-surface temperature for a location and its duration in weeks); values above four trigger bleaching and above eight cause mass bleaching and mortality (http://coralreefwatch.noaa.gov). Bleaching-month was selected as the 6-month max DHW prior to bleaching observation in order to capture the peak of stress and accommodate differences in observation period, and were compiled from satellite measurements of the U.S. National Oceanic and Atmospheric Administration (NOAA).
Estimation of error of measurements of individual bleaching response records, δBjk
Bleaching response values Bjk are known only approximately. For example, if a report indicates a taxon has N colonies within the category of 30–70% bleached cover, the exact value is unknown, resulting in an error of measurement for each element, δBjk, calculated through error propagation analysis.
Methodology A, the greatest possible error for a given report and coral taxon is δbi = (ci + 1 − ci)/2, i = 1, …, M because a colony in category i may have bleaching values with the mean bi ± δbi. The error of measurement of a bleaching value for site k (k = 1, …, K with K being the total number of sites in the database) and taxon j (j = 1, …, Jk with Jk being the total number of taxa in site k) is then found using error propagation applied to equation:
| (5) |
| (6) |
where δw = 1/2(ws+1 − ws), if s ≥1 or 0 if s = 0, δwd 1/2(wds+1 + wds), if sd ≥ 1 or 0 if sd = 0, and . The derivation is presented in Text S1.3.
For methodology C (see Text S1.3 for derivation)
| (7) |
Uncertainty of taxon-BRI, ΔBRIj
Since BRIj for a given taxon j is found as the mean of all Bjk over all sites k for which data on taxon j is available, uncertainty of BRIj can be quantified by two error metrics; the error of measurements of BRIj, which is estimated using error propagation applied to BRIj,
| (8) |
and standard error of the mean Bjk,
| (9) |
where stdevk stands for standard deviation of Bjk over site index k.
Although related, these two metrics have distinct uses. The error of measurements quantifies accuracy with which BRIj is calculated as average of Bjk over all sites k where taxon j is surveyed. Standard error of the mean indicates how well this average is likely to approximate the true average in the limit of Kj → ∞. Both types of uncertainty decrease with Kj as . When the number of observations Kj is large, standard error is larger than error of measurements and inherently takes into account variability of observations due to measurement error. However, in certain cases, and especially when the number of observations is small, estimated standard error might be smaller than error of measurements. Therefore, confidence interval (CI) or total uncertainty of BRIj (ΔBjk, i.e., how well the mean of a finite number of Bjk, each having its own error of measurements δBjk, approximates the true mean for bleaching responses that would be observed in the limit of Kj → ∞ and no measurement error), is calculated as the greater of two error metrics:
| (10) |
where standard error is calculated only if the taxon is found in at least Kmin sites; if Kj < Kmin, standard error is not calculated for small Kj due to low accuracy of estimation.
Construction of site-specific bleaching response index (site-BRI or SBRIk)
Reports differentially determined site-specific (coral assemblage-specific) bleaching response by (i) calculating average bleaching response index of taxa at a site in a similar manner to this study (McClanahan et al., 2005), (ii) multiplying taxon-specific bleaching response scores for each taxon by their relative abundance (e.g. McClanahan et al., 2007b, 2015) or (iii) by choosing the most abundant taxa and determining site-bleaching susceptibility as the average product of susceptibility scores and relative abundances of each taxon (Manzello et al., 2007).
Using matrix Bjk, coral assemblage-level response was determined by calculating average taxon-BRI of all taxa j at site k (site-BRI),
| (11) |
Construction of taxon-dependent site-BRI (taxon-sBRIj)
Taxon-dependent site-BRI was calculated as the average bleaching response across communities where taxon j was surveyed,
| (12) |
Inclusion of the target taxon in sBjk artificially amplifies the correlation between BRIj and taxon-sBRIj, particularly at sites with small Jk; therefore, we excluded the target taxon from sBjk. While SBRIk allows comparisons of bleaching response across coral assemblages at different sites, taxon-sBRIj allows comparisons of bleaching response across specific coral assemblages that include the target taxon.
Considering intrinsic and extrinsic factors (sensu West & Salm, 2003)
Intrinsic factors are defined as biological characteristics of the holobiont that affect bleaching resistance (e.g., Symbiodinium thermotolerance; coral morphology, physiology such as early heat shock or oxidative stress response, or skeletal and tissue light-scattering properties that affect internal light amplification; Baird et al., 2009) and were evaluated as a whole (without isolating specific factors). This effect was quantified by analysis of variance of BRIj (see Materials and methods).
Extrinsic factors are defined as environmental characteristics that affect bleaching resistance of the entire coral assemblage at a site (e.g., currents or turbidity, thermal stress, thermal history; West & Salm, 2003; Guest et al., 2012) and were evaluated as a whole (without isolating individual factors). We reasoned that taxon-sBRIj (Eqn 12) is a reasonable approximation of the average effect of extrinsic factors at sites containing taxon j (but see section below, Portion of BRIj variance due to extrinsic factors, for detailed considerations). Text S1.4 examines possible interactions between intrinsic and extrinsic factors.
Effect of uncertainty, extrinsic, and intrinsic factors on differential bleaching among coral (Analysis of BRIj variance)
We consider differential bleaching among taxa to result from a combination of factors intrinsic (taxon-specific) or extrinsic (environmental) to the holobiont and uncertainty of bleaching response measurement (error of measurement within, and inconsistencies across, reports). The effect of each can be assessed through analysis of variance of BRIj for each taxon in the dataset {BRIj}:
| (13) |
where Varint, Varext, and Varδ are variances due to intrinsic factors, extrinsic factors, and uncertainty, respectively. The portion of the BRIj variance explained by each of these factors is expressed as:
| (14) |
When the portion of the BRIj variance related to uncertainty is calculated, the portion of uncertainty-corrected BRIj variance (portion of BRIj variance that would be observed in the absence of error of measurements), explained by intrinsic and extrinsic factors can be estimated as:
| (15) |
Each component of BRIj variance was thus evaluated as discussed below.
Portion of BRIj variance due to measurement uncertainty
The uncertainty component was determined by the total uncertainty ΔBRIj, and Varδ can be estimated as
| (16) |
where T is the total number of taxa in the dataset with ΔBRIj given by equation (10).
Portion of BRIj variance due to extrinsic factors
The extrinsic factors component was adetermined by performing a correlation analysis between BRIj and taxon-sBRIj. R2-statistic of the correlation between BRIj and taxon-sBRIj is the portion of the variance of BRIj due to extrinsic factors:
| (17) |
Note that this is only valid if sites contain taxa with a wide range of bleaching responses. If, for example, a substantial portion of the sites where taxon j is found has predominantly bleaching-susceptible taxa, this would increase taxon-sBRIj regardless of the effect of extrinsic factors. In this case, R2 provides an overestimation of true Pext. This bias may be lowered by restricting the analysis to sites with a large number of taxa (Jk) to include a wide range of bleaching responses. We addressed this by performing correlation analysis as a function of Jk:
| (18) |
As the number of taxa n increases, the number of sites (N(n)) that satisfy the criterion Jk ≥ n decreases, which may lower the accuracy of correlation analysis. This was assessed by significance of the regression of BRIj vs. taxon − sBRIj|Jk≥ n. We found that although significance decreased with n, the regression remained significant (P < 0.01) for n < 45 taxa (N = 4 sites, see Results). Thus, the asymptomatic behavior of function R2 (n) as n increases can be used as the estimate of Pext:
| (19) |
As discussed above, for any finite n, R2 (n) is an overestimation of the true Pext, and thus can be used as an estimate of the upper bound of Pext.
Portion of BRIj variance due to intrinsic factors
With the uncertainty and extrinsic portions of the BRI variance estimated, variance due to intrinsic biological differences among taxa can be found using the additive variance property:
| (20) |
Effects of intrinsic and extrinsic factors on bleaching response of a given taxon at a site (Bjk)
The effects of intrinsic and extrinsic factors on bleaching response of taxon j at site k, were assessed by performing analysis of variance on elements of matrix Bjk. This leverages principles of the capital asset pricing model (CAPM), used in stock market analysis. In CAPM, ‘extrinsic factors’ are equivalent to global market movements and ‘intrinsic factors’ are equivalent to a stock and how it reacts to market changes; parallel to bleaching susceptibility where intrinsic properties define taxon-specific susceptibility to bleaching given the extrinsic environment. Applying CAPM, we express
| (21) |
where ujk is variability in estimation of Bjk due to error of measurements with Var [ujk] = Var [δBjk], Sk is the measure of extrinsic factors that influenced all taxa at that site, and coefficients βj and αj are taxon-specific, intrinsic parameters. αj quantifies baseline variations in the absence of anomalous stress for both coral (e.g., variation in tissue thickness or colony size/shape; Loya et al., 2001; Stambler & Dubinsky, 2005) and Symbiodinium (e.g., seasonal variation density in the absence of anomalous stress (Fitt et al., 2000; Nir et al., 2014). βj quantifies bleaching response of taxon j compared to other corals in the assemblage at site k; all corals would bleach identically if taxa are not intrinsically different and βj = const.
We estimate Sk as taxon-sBjk. As above (see Eqn 12), when the number of taxa per site is large (as is the case with the number of stocks in a market), Sk can be estimated by averaging all taxa for the site, . Since our data include sites with few taxa, the taxon in question is excluded from the average in order to not confound the relationship between Bjk and Sk. Therefore, we estimate Sk as instead of .
Coefficients βj and αj are found by regression of Bjk on Sk. Accuracy and reliability of this regression depend on the number taxa per site and number of sites a taxon is present. Accuracy of sBjk and Sk estimation increases with number of taxa per site (Jk). Accuracy of βj and αj estimation increases with number of sites reporting a taxon (Kj), which also increases significance of the regression.
Effect of extrinsic and intrinsic factors can be evaluated by two approaches:
Per-taxon diversity analysis: the contribution of extrinsic and intrinsic factors is first determined for each taxon, and then the cumulative contribution for all taxa is determined as the average of all taxa (with each taxon weighted equally). The portion of bleaching response variance explained by both intrinsic and extrinsic factors is:
| (22) |
Pext,int also quantifies the model (Eqn 21) fit to Bjk data. In order to estimate the effect of extrinsic factors alone, we modify the model (Eqn 21) by omitting taxon-specific effects:
| (23) |
Consequently, the portion of response variance explained by extrinsic factors only is
| (24) |
The difference between portions of variance explained by model (Eqn 21) and explained by model (Eqn 23) estimates the influence of intrinsic factors:
| (25) |
Finally, we can find uncertainty-corrected portions of the Bjk variance explained by the extrinsic and intrinsic factors (i.e., ‘true’ effects that would be observed in the absence of Bjk measurement uncertainty):
| (26) |
Per-taxon abundance analysis: the contribution of each factor is calculated for each taxon j in each site (i.e., pair j,k) with the cumulative contribution given by the average over all taxa and sites (with taxa not weighted equally, text S1.5, Eqns 27–29). These two metrics are equivalent if all taxa showed similar abundances, but the per-taxon abundance analysis is predominantly influenced by more abundant taxa.
Evaluation of inclusion of a given report into the meta-analysis based on its uncertainty
Taxon-specific δBjk can be used to evaluate the contribution of each report to overall uncertainty. Reports with large δBjk could potentially be excluded (after considering number and diversity of taxa, region, and year representation). Each additional report may reduce (in Eqn 8, for δBRIj a new report increases the denominator by 1) or increase total variability if the added term dominates (Text S1.6, Eqn 30). A thresh-old of report exclusion based on increase of total uncertainty was determined (Text S1.6, Eqns 31–33), but not applied here.
Results and Discussion
Here, we report a new framework for analysis of the sources of differential bleaching among corals which involves (i) standardizing taxon-specific bleaching and mortality records from disparate surveys, (ii) estimating the uncertainty of bleaching response measurement per-taxon due to error of measurement within- and inconsistencies across-surveys and (iii) calculating the effect of the main sources on bleaching variability: varied exposure to distinct environmental factors (‘extrinsic’), biological differences among taxa (‘intrinsic’) and uncertainty of bleaching response measurement (‘uncertainty’).
What is the range of differential bleaching response among taxa?
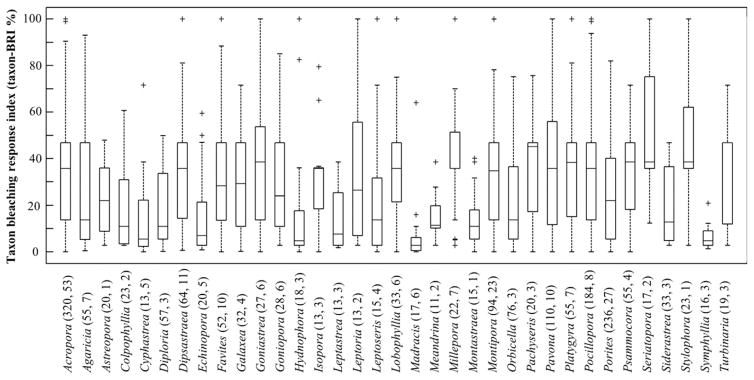
Matrix, Bjk, of standardized (mean % affected tissue) coral taxon-specific bleaching and mortality responses for each taxon j (n = 374) and site k (n = 316) was constructed from 2036 records from 1983 to 2006 (Fig. 1, Tables S1 and S2). Standardization of records was specific to original data collection methods [See Table S3 as example of standardization of records reported in Obura, 2001 using Eqn (1)]. Records included data on mass bleaching events at pantropical (1997–1998), regional (2002 Great Barrier Reef and 2005 Caribbean), and sub-regional scales in seven biogeographic realms and identify the 1997–1998 event as the most severe in the dataset (Fig. 2). Taxon-specific records are either species- (63%) or genus-level (37%). Of the 90 genera surveyed, only six genera had >75 records: Acropora (n = 320), Montipora (n = 94), Orbicella (n = 76), Pavona (n = 110), Pocillopora (n = 184), and Porites (n = 236); these genera are represented by 53, 23, 3, 10, 8, and 27 species respectively (Figs S1 and S2, Table S2). Sites contain 6.5 ± 13.7 (mean ± SD) taxa and range from 1 (60 sites) to 199 (1 combined site) taxa (Fig. S3, Table S2). Average Bjk over all sites k where taxon j was surveyed yields the taxon-Bleaching Response Index (taxon-BRI or BRIj, Eqn 4, Table S4). BRIj is highly variable across the 374 taxa assessed (25.1 ± 18.4, average and standard error), where some genera are highly resistant (e.g., genera Madracis, Montastraea, Symphyllia; BRIj ≤10%) and others highly susceptible (e.g., genera Millepora, Seriatopora, Stylophora; BRIj ≥40%), but most genera are highly variable (mean coefficient of variation = 0.89 for 37 genera with >10 records, Fig. 3). Large intra-genus variability is observed (within the 37 genera that have >10 records; Fig. 3) where some genera have a uniform bleaching response (e.g., Astrea, Cycloseris, Madracis) while others have large inter-species variability (e.g., Leptoria, Pavona, Seriatopora).
Fig. 3.
Taxon-BRI values representing genera with >10 records and showing the median (centerline), 25–75th percentiles (box), range (whiskers), and outliers beyond 1.5 X the interquartile range (+). Only 35 genera are shown as the variance within Astrea and Cycloseris is minute; 247 species are represented from 1819 records. Parenthetical values are the number of records and species per genus.
Our results demonstrate substantial variation among individual responses. Such variation is typical (Marshall & Baird, 2000; Baird & Marshall, 2002; Oxenford et al., 2008) and is the material upon which natural selection acts. Our results suggest that an adaptive response via natural selection is to be expected in response to changing climate. Furthermore, as individual sites experience serial bleaching episodes, species may acclimate or adapt (McClanahan & Maina, 2003; Guest et al., 2012; Pratchett et al., 2013; Grottoli et al., 2014; Logan et al., 2014). Repeatedly bleached sites could be targeted for evidence of chronological change, as additional datasets are added to the index.
Does a unified estimate of taxon-BRI accurately reflect taxon-specific differential bleaching as reported by independent sources?
Response indices based on meta-analysis of bleaching records could be invalid because of significant disagreement among source reports due to differential severity of events, assemblage structures, environmental conditions, observation periods, data collection protocols, and severity criteria. If BRIj is an accurate reflection of the true taxon-specific bleaching response, then independent assessments should report similar patterns. Comparing BRIj to the conclusions of Carpenter et al. (2008) for the 181 species found in both datasets, there is a significant positive correlation (logistic regression, r2 = 0.03, P < 0.02) indicating congruence between BRIj and an independent assessment. Comparing BRIj to the data of Loya et al. (2001) and the follow-up by van Woesik et al. (2011), we see many examples of similarly categorized species: High-susceptible Seriatopora hystrix (taxon-BRI = 61.46), Stylophora pistillata (56.42), Pocillopora damicornis (42.29), and Porites nigrescens (41.33); medium-susceptible Dipsastraea favus (32.05), Favites pentagona (27.07), Favites halicora (25.66), and Galaxea fascicularis (25.43); and low-susceptible Montipora digitata (18.52), Leptastrea transversa (16.27), Leptastrea purpurea (15.73), and Coelastrea aspera (14.27).
Does the bleaching response of a coral assemblage (site-BRI) reflect the intensity and duration of thermal anomalies?
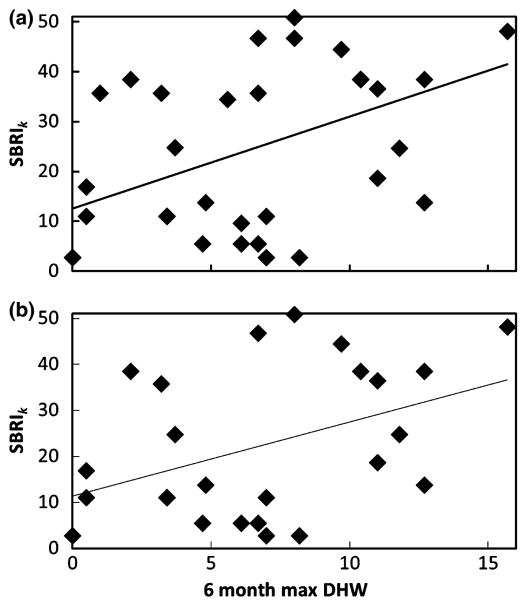
The bleaching response of all taxa surveyed in a site was averaged to calculate the coral assemblage bleaching response (site-BRI or SBRIk, Eqn 11, average 33.76 ± 1.33 over 316 sites), which has been used to compare across sites and identify particular extrinsic factors affecting bleaching response (e.g., water flow, thermal history, acute thermal stress, and temperature variability; Manzello et al., 2007; McClanahan et al., 2007b, 2005). If SBRIk truly reflects assemblage bleaching response at a site, it should account for thermal-stress intensity and thermal-stress history of that assemblage. Comparing SBRIk of 35 Caribbean sites (limited to sites with >2 taxa per site; 39 unique taxa, mean of 7.27 ± 0.7 taxa per site) with the maximum DHW recorded over 6 months prior to the survey, there is a significant positive correlation (linear regression, r2 = 0.21, P = 0.006; Fig. 4) indicating that assemblage response (as measured by SBRIk) is reflective of thermal stress, which is similar to the correlation reported for thermal anomalies and assemblage bleaching responses of 360 Caribbean sites in 2005 (Eakin et al., 2010).
Fig. 4.
Regressions of site-specific BRI (SBRIk, Eqn 11) on 6 month–max degree heating weeks (DHW) for Caribbean sites during 1998–2006. Regressions shown using sites with at least three (35 sites; r2 = 0.21, P = 0.006) (a) or five (25 sites; r2 = 0.17, P = 0.038) (b) taxa per site, which contain a total of 39 or 38 unique taxa and 240 or 209 unique records, respectively.
How much of the variance in bleaching response among coral taxa is due to the error of measurement within reports and inconsistencies across reports?
Bleaching response values Bjk are estimates of the true bleaching response due to biological and experimental variability (e.g., the dynamics of bleaching response may be nonlinear, and variation in observation time relative to onset may yield atypical estimates) and error of measurement (e.g., categorical assessments are variously broad and imprecise). Standard error of the mean includes natural variability in bleaching response, inconsistencies across reports, and error of measurement within reports (Eqn 9) when taxa are frequently surveyed, but standard deviation (and therefore standard error) is not defined for taxa surveyed once (47.5% of taxa) and poorly defined for taxa surveyed twice (16.5% of taxa; Table S4). Error of measurement quantifies accuracy with which BRIj is calculated as average of Bjk (Eqn 8), with measurement error of ± δBjk. Within-survey variability and error are compounded by the number of surveys used to calculate BRIj from individual Bjk records, so error propagation analysis was used to calculate BRIj error of measurement (Table S2 for individual Bjk and Table S4 for individual BRIj). Total uncertainty of taxon-BRI (ΔBRIj) was determined as the greater of two error metrics (standard error and error of measurement, Eqns 8–10, Table S4). The accuracy of determining ΔBRIj is expected to increase with the number of times a taxon is surveyed at different sites. ΔBRIj was determined for taxa surveyed in at least three sites (Kmin = 3) and for taxa surveyed at increasingly higher numbers of sites (Kmin was increased by n + 1 at each analysis; Eqn 10). The ΔBRIj decreased from 11.30 (Kmin = 3), corresponding to 37% of the mean taxon-BRI, to a minimum of 10.44 (Kmin = 8), corresponding to 35% of the mean taxon-BRI. Furthermore, the portion of variance of BRIj due to uncertainty Pδ decreased from 34% (Kmin = 3) to 29% (Kmin = 8 and higher). These results suggest that the bleaching response of a taxon (BRIj) is, on average, at least 2.5 times higher than uncertainty of estimating that response and differential bleaching is due, in part, to uncertainty in estimating bleaching response (about 29%).
As more surveys of taxon-specific bleaching and mortality become available, accuracy of determining uncertainty ΔBRIj is expected to increase. Taxon-specific δBjk can also be used to evaluate how reports add to overall uncertainty of BRIj against the information provided (e.g., number of new or rare taxa, new bleaching episode or site) so that reports with large δBjk (report average uncertainty, or average-δBjk; Table S1) could be excluded if the information they provide adds little to the matrix (Eqns 30–33, Text S1.6). The threshold at which uncertainty of a report increases total uncertainty can be determined; reports with average-δBjk more than twice the average of all reports for a taxon increase total uncertainty and are candidates for exclusion (if minimizing the uncertainty is the goal, Eqns 30–33, Text S1.6).
How much of the variance in bleaching response among coral taxa is due to factors extrinsic or intrinsic to the holobiont?
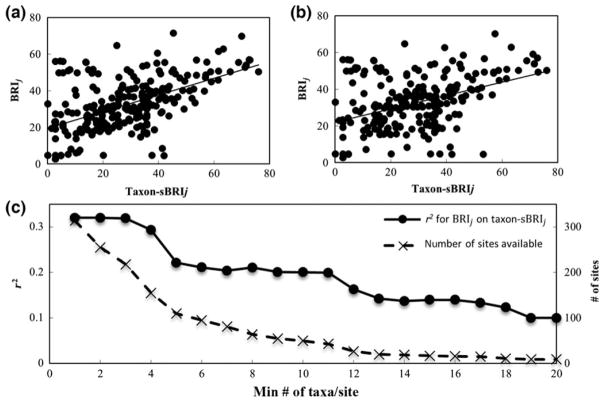
We considered differential bleaching among corals (BRIj variance) to result from taxon-specific intrinsic factors, extrinsic factors, and measurement uncertainty. The effect of each of these factors was assessed by analysis of BRIj variance and estimating the portion of taxon-BRI variance caused by each (Eqns 13–20). Taxon-dependent site-BRI (taxon-sBRIj, Eqn 12) was used as an approximation of the average effect of extrinsic factors experienced by the sites where taxon j is surveyed. The effect of extrinsic factors Pext was found by correlation between BRIj and taxon-sBRIj (Eqn 17). Because we cannot eliminate effects of intrinsic factors on taxon-sBRIj (e.g., a site may contain mostly bleaching-susceptible coral taxa), Pext is the upper bound of the true variance due to extrinsic factors, and it is expected to increase in accuracy for sites with diverse bleaching responses (i.e., in the limit of a high number of taxa per site n, Eqns 17–19). We estimated the effect of extrinsic factors Pext by correlation between BRIj and taxon-sBRIj (Eqn 17) for sites with two (most conservative estimate) and increasingly higher (n up to 44) numbers of taxa. Maximal R2 (0.33) was observed for sites with two taxa, and it monotonically decreased to 0.057 as the number of taxa per site increased to 44 (P < 0.01, Fig. 5 – regression continues to decrease for higher taxa below 0.02, but is no longer significant). Therefore, the effect of extrinsic factors on BRIj is negligibly small, Pext < 6%. Further analysis of distinct sites with well-characterized environmental factors, thermal history, and magnitude of coral bleaching responses could provide a more accurate estimate of the effect of extrinsic factors on differential bleaching among corals.
Fig. 5.
Relationship between the bleaching response of taxa (taxon-BRI or BRIj) and their communities (taxon-dependent site-BRI or taxon-sBRIj, Eqn 12) and its dependence on sample sizes. Regressions of BRIj on taxon-sBRIj for all (314) sites (r2 = 0.33, P < 0.001) (a) and for sites (43) with ≥10 taxa (r2 = 0.20, P < 0.001) (b). As the minimum number of taxa per site increases, r2 for BRIj on taxon-sBRIj regressions decrease (solid line), as well as the number of sites available (containing the minimum number of taxa) for analysis (dashed line).
Effect of intrinsic factors (Pint), estimated as the remainder of BRIj variance after uncertainty Pδ (29%) and extrinsic factors Pext (<6%) are calculated (Eqns 13–20), is 65%. Therefore, BRIj (or taxon-BRI) is a robust measure of innate biological differences among taxa with negligible site-bias and small measurement uncertainty. When averaging coral bleaching responses over several episodes and sites (with diverse environmental conditions and thermal-stress intensities) to determine BRIj, the influence of extrinsic factors becomes minimal and intrinsic biological properties of the taxa themselves drive differences in bleaching response.
How much of the bleaching response of a given coral taxon at a site is a result of extrinsic and intrinsic factors?
It is conceivable that the influence of extrinsic factors (e.g., thermal anomaly) at a site might be the dominant determinant of taxon-specific bleaching in a specific event (Bjk), and simultaneously the global average of bleaching response at multiple events and sites (assessed by BRIj) might be primarily determined by intrinsic biological properties if the taxon is exposed to a diversity of events that average themselves out and thus minimize the effects of extrinsic conditions. In order to test this hypothesis, we assessed the effects of intrinsic and extrinsic factors on bleaching response of taxon j at site k by performing analysis of variance on elements on matrix Bjk using CAPM (where taxon-sBjk is the independent- and Bjk the dependent-variable, Eqns 21–29). Accuracy and reliability of this model (Eqn 21) rely upon the number of taxa per site Jk and sites a taxon is present Kj. Considering only taxa and sites with large Jk (Jk ≥n) and Kj (Kj ≥m) decreases the number of elements available for analysis, therefore a subset of Bjk (897 elements) that maximized n (n = 5) and m (m = 9) while retaining sites (26 sites) was selected. This model (Eqn 21) explained all the uncertainty-corrected variance of , confirming the validity of the approach.
A ‘per-taxon diversity’ analysis (Eqns 22–26), where the portion of variance due to extrinsic and intrinsic factors is identified for each taxon before finding the estimated cumulative contribution of these factors by unweighted averaging of those for each taxon, was able to explain and of the uncertainty-corrected variance of Bjk. A complimentary ‘per-taxon abundance’ analysis (Eqns 27–29, Text S1.6), where the effects of extrinsic and intrinsic factors are determined for all taxa at each site before averaging these values for all elements in matrix Bjk (such that the contribution of each taxon is not weighted equally), returned similar values ( ). If all taxa had the same representation of individuals in the ecosystem (i.e. Kj = const (j)), the two analyses would be equivalent, but since the distribution is unequal variance of Bjk is predominantly affected by the bleaching response of the most common taxa.
These results indicate that both intrinsic and extrinsic factors play a significant role in influencing coral bleaching within a specific bleaching episode at a site (Bjk).
Limitations and general considerations
Taxon-BRI is an accurate measure of bleaching response if two conditions are satisfied: nonbleaching-related deaths and the probability of corals recovering before observation can be neglected. Given that most surveys are performed soon after bleaching, both conditions are reasonable and the probabilities of violating them are expected to be small (Obura, 2001; Baird & Marshall, 2002; McClanahan et al., 2004).
Elucidating the range of bleaching responses among different taxa, communities, and geographic regions is essential for understanding physiological variability in bleaching within and among taxa (Obura, 2001; van Woesik et al., 2011). To improve estimates of species susceptibility to bleaching, we need much better estimates of bleaching-induced mortality. For example, a survey conducted proximal to a thermal anomaly might overestimate bleaching and underestimate bleaching-induced mortality (with possible increase in Bjk) while a survey conducted distal to a thermal anomaly might be confounded by recovery (decrease in Bjk) or mortality that is bleaching-related but not bleaching-induced (increase in Bjk) (McClanahan et al., 2004; Obura, 2005, 2009; Jones, 2008; Text S1.1). Because the distinction between these types of mortality was often unclear, we included all surveys, which should increase uncertainty of taxon-BRI. Very few studies measure bleaching at the species-level and even fewer follow the fate of individuals from bleaching to either mortality or recovery. This is not surprising as collecting these data requires multiple surveys over many months (e.g. Baird & Marshall, 2002). Consequently, future monitoring efforts should aim to collect data at the species-level and follow individual colonies through time.
Ambiguities in data collection and reporting may potentially underestimate the error of measurement. While some reporting allowed specific quantification of error, others required postulation of quantitative categories from qualitative descriptions (e.g., ‘pale’ corals were assumed to be <10% bleached). Additionally, when cumulative statistics are calculated (e.g., taxon-BRI), uncertainty factors not accounted for by δBjk, should increase standard error of the mean, which is why total uncertainty (ΔBRI) is estimated as the greater of the metrics. However, since accuracy of estimation of standard deviation, and therefore standard error, is decreased for rarely surveyed taxa, total uncertainty for these taxa was not accurately estimated (Fig. 2c, Table S4).
The effect of extrinsic factors on taxon-BRI (Pext) can be estimated through taxon-dependent site-BRI, taxon-sBRIj and the accuracy Pext depends on whether taxon-sBRIj is a good estimate of the severity of environmental stress at a site. This, in turn, is valid only if two conditions are met. First, the site contains high diversity of taxa with a wide range of bleaching responses (rarely the case in practice); to address this limitation, we considered sites containing a large number of taxa (n in Eqns 18 and 19) and evaluated the asymptomatic behavior of Pext at large n. Our results show that Pext is negligibly small indicating that BRI is primarily an intrinsic characteristic. Second, environmental exposure is uniform across the site. While most reports provide bleaching response for specific locations, a few provided bleaching data aggregated from multiple independent locations and bleaching events. In these cases, it cannot be assumed that all locations were exposed to the same conditions, so we excluded from the initial analysis two reports with large aggregates of multisite/multiepisodes (Done et al., 2003; McClanahan et al., 2009). Five other reports provided data on extended sites covering more than 112 kilometers, (about one degree latitude x longitude, labeled with * in Table S1), but were not excluded from the initial analysis since accuracy of estimating Pext and Pint depends on the number of reports. However, we tested the influence of extended-site reports on Pext and Pint and found that exclusion of these five reports did not have a significant effect: Pext did not change for per-taxon abundance calculations (62%) (changed from 58% to 56% for the per-taxon diversity) and Pint did not change for per-taxon abundance calculations (38%) (changed from 42% to 44% for per-taxon diversity). We therefore conclude that the analysis is robust to report selection.
We tested the effect of regional differences on taxon-BRI variance by excluding the Central Indo-Pacific bio-geographic region (758 records, 23% of all surveys) from the analysis. The conclusions of the analysis appeared to be independent of the regional differences among corals: although having more records clearly increases the number of taxa for which the analysis can be performed and improves its accuracy, the main conclusions still hold regardless of the geographical location of the taxa (see Text S1.7 for quantitative analysis of regional differences).
Conclusion
The BRI presented here is the first pantropical assessment of bleaching and mortality, inclusive of measurement uncertainty, that attempts to build a unified comparison of taxon-specific response from the historical record. The index is both expandable to include new records, taxa, and sites, and customizable to target specific locations, events, or times. The quantification of bleaching response provides a tool for assessing traits associated with bleaching, bleaching mechanisms, and management, conservation, or mediation plans. Furthermore, the effect of the uncertainty of bleaching measurement on differential bleaching of corals identified in this study (~29%) suggests that standardization of protocols and reporting would help increase the precision of susceptibility estimates and allow a global repository of standardized bleaching surveys. These standardizations are particularly important as the third pantropical bleaching event, which is expected to affect 38% of all reefs and kill >12 000 km2 of coral (www.globalcoralbleaching.org), is currently unfolding. Estimates of bleaching susceptibility are fundamental to assessing the resilience potential of reef sites, which can then be used to inform management decisions (Maynard et al., 2015). More specifically, accurate estimates of species susceptibility to disturbances, such as bleaching, will allow management to identify and afford protection to susceptible species. In addition, temporal trends of species susceptibility are required to reveal whether or not corals are adapting to climate change (Guest et al., 2012; Pratchett et al., 2013) and this information can assist in deciding whether drastic interventions, such as assisted migration or selective breeding programs are required to conserve susceptible species (van Oppen et al., 2015).
Supplementary Material
Acknowledgments
This research was supported by National Science Foundation (EFRI-1240416, EFRI-0937987 and CBET-1249311) and National Institutes of Health (CA-128641, EB-003682).
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Number of standardized bleaching and mortality records (Bjk) per genus for genera with <50 records and >50 records (in-set) categorized as high (≥75th percentile; open bars), medium (grey bars), and low (≤25th percentile; black bars).
Figure S2. Distribution of species for genera with ≥75 records; showing Pocillopora (a), Montipora (b), Pavona (c), Orbicella (d), Acropora (e), and Porites (f).
Figure S3. Frequency distribution of the number of taxa per site.
Table S1. Source information used in the compilation of the 2068 entries used to determine taxon-specific bleaching response index (BRI).
Table S2. List of 2036 standardized bleaching and mortality records Bjk for 374 taxa j surveyed at 316 sites k used to determine the taxon-BRI (taxon-specific bleaching response index).
Table S3. Standardization of (Obura, 2001) bleaching and mortality records according to Eqn 1.
Table S4. Taxon-specific BRI values for 374 taxa at 316 sites and associated uncertainties (Eqn 10).
References
- Baird AH, Marshall PA. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Marine Ecology Progress Series. 2002;237:133–141. [Google Scholar]
- Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends in Ecology & Evolution. 2009;24:16–20. doi: 10.1016/j.tree.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Bhagooli R, Hidaka M. Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. Journal of Experimental Marine Biology and Ecology. 2003;291:181–197. [Google Scholar]
- Carpenter KE, Abrar M, Aeby G, et al. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321:560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- Coles SL, Jokiel PL. Effects of temperature on photosynthesis and respiration in hermatypic corals. Marine Biology. 1977;43:209–216. [Google Scholar]
- Cunning R, Baker AC. Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nature Climate Change. 2013;3:259–262. [Google Scholar]
- Done TJ, Turak E, Wakeford M, et al. Testing Bleaching Resistance Hypotheses for the 2002 Great Barrier Reef Bleaching Event. Australian Institute of Marine Science; Townsville: 2003. pp. 1–95. [Google Scholar]
- Eakin CM, Morgan JA, Heron SF, et al. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS One. 2010;5:e13969. doi: 10.1371/journal.pone.0013969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitt WK, Mcfarland FK, Warner ME, Chilcoat GC. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnology and Oceanography. 2000;45:677–685. [Google Scholar]
- Floros CD, Samways MJ, Armstrong B. Taxonomic patterns of bleaching within a South African coral assemblage. Biodiversity and Conservation. 2004;13:1175–1194. [Google Scholar]
- Gleason MG. Effects of disturbance on coral communities – bleaching in Moorea, French-Polynesia. Coral Reefs. 1993;12:193–201. [Google Scholar]
- Grottoli AG, Warner ME, Levas SJ, et al. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology. 2014;20:3823–3833. doi: 10.1111/gcb.12658. [DOI] [PubMed] [Google Scholar]
- Guest JR, Baird AH, Maynard JA, et al. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One. 2012;7:e33353. doi: 10.1371/journal.pone.0033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Baird AH, Bellwood DR, et al. Climate change, human impacts, and the resilience of coral reefs. Science. 2003;301:929–933. doi: 10.1126/science.1085046. [DOI] [PubMed] [Google Scholar]
- Jokiel PL. Temperature stress and coral bleaching. In: Rosenberg E, Loya Y, editors. Coral Health and Disease. Heidelberg, Springer-Verlag; Berlin: 2004. pp. 401–425. [Google Scholar]
- Jones RJ. Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Marine Biology. 2008;154:65–80. [Google Scholar]
- Krediet CJ, Ritchie KB, Paul VJ, Teplitski M. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20122328. doi: 10.1098/rspb.2012.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggat W, Seneca F, Wasmund K, Ukani L, Yellowlees D, Ainsworth TD. Differential responses of the coral host and their algal symbiont to thermal stress. PLoS One. 2011;6:e26687. doi: 10.1371/journal.pone.0026687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CA, Dunne JP, Eakin CM, Donner SD. Incorporating adaptive responses into future projections of coral bleaching. Global Change Biology. 2014;20:125–139. doi: 10.1111/gcb.12390. [DOI] [PubMed] [Google Scholar]
- Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, Van Woesik R. Coral bleaching: the winners and the losers. Ecology Letters. 2001;4:122–131. [Google Scholar]
- Manzello DP, Berkelmans R, Hendee JC. Coral bleaching indices and thresholds for the Florida reef tract, Bahamas, and St. Croix, US Virgin Islands. Marine Pollution Bulletin. 2007;54:1923–1931. doi: 10.1016/j.marpolbul.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Marcelino LA, Westneat MW, Stoyneva V, et al. Modulation of light-enhancement to symbiotic algae by light-scattering in corals and evolutionary trends in bleaching. PLoS One. 2013;8:e61492. doi: 10.1371/journal.pone.0061492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PA, Baird AH. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs. 2000;19:155–163. [Google Scholar]
- Maynard JA, McKagan S, Raymundo L, et al. Assessing relative resilience potential of coral reefs to inform management. Biological Conservation. 2015;192:109–119. [Google Scholar]
- McClanahan TR. The relationship between bleaching and mortality of common corals. Marine Biology. 2004;144:1239–1245. [Google Scholar]
- McClanahan TR, Maina J. Response of coral assemblages to the interaction between natural temperature variation and rare warm-water events. Ecosystems. 2003;6:551–563. [Google Scholar]
- McClanahan TR, Baird AH, Marshall PA, Toscano MA. Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Marine Pollution Bulletin. 2004;48:327–335. doi: 10.1016/j.marpolbul.2003.08.024. [DOI] [PubMed] [Google Scholar]
- McClanahan TR, Maina J, Moothien-Pillay R, Baker AC. Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Marine Ecology Progress Series. 2005;298:131–142. [Google Scholar]
- McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Sebastian CR, Guillaume MMM, Bruggemann JH. Western Indian Ocean coral communities: bleaching responses and susceptibility to extinction. Marine Ecology Progress Series. 2007a;337:1–13. [Google Scholar]
- McClanahan TR, Ateweberhan M, Sebastián CR, Graham NAJ, Wilson SK, Bruggemann JH, Guillaume MMM. Predictability of coral bleaching from synoptic satellite and in situ temperature observations. Coral Reefs. 2007b;26:695–701. [Google Scholar]
- McClanahan TR, Weil EJ, Cortes J, Baird AH, Ateweberhan M. Consequences of coral bleaching for sessile reef organisms. In: Van Oppen MJH, Lough JM, editors. Ecological Studies 205. Coral Bleaching: Patterns, Processes, Causes and Consequences. Springer-Verlag; Berlin, Heidelberg: 2009. pp. 121–138. [Google Scholar]
- McClanahan TR, Maina J, Ateweberhan M. Regional coral responses to climate disturbances and warming is predicted by multivariate stress model and not temperature threshold metrics. Climatic Change. 2015;131:607–620. [Google Scholar]
- Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z, editor. Ecosystems of the World, 25. Coral Reefs. Amsterdam: Elsevier; 1990. pp. 75–87. [Google Scholar]
- Nir O, Gruber DF, Shemesh E, Glasser E, Tchernov D. Seasonal mesophotic coral cleaching of Stylophora pistillata in the northern Red Sea. PLoS One. 2014;9:e84968. doi: 10.1371/journal.pone.0084968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obura DO. Can differential bleaching and mortality among coral species offer useful indicators for assessment and management of reefs under stress? Bulletin of Marine Science. 2001;69:421–442. [Google Scholar]
- Obura DO. Resilience and climate change: lessons from coral reefs and bleaching in the Western Indian Ocean. Estuarine, Coastal and Shelf Science. 2005;63:353–372. [Google Scholar]
- Obura DO. Reef corals bleach to resist stress. Marine Pollution Bulletin. 2009;58:206–212. doi: 10.1016/j.marpolbul.2008.10.002. [DOI] [PubMed] [Google Scholar]
- van Oppen MJH, Oliver JK, Putnam HM, Gates RD. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2307–2313. doi: 10.1073/pnas.1422301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenford H, Roach R, Brathwaite A, et al. Quantitative observations of a major coral bleaching event in Barbados, Southeastern Caribbean. Climatic Change. 2008;87:435–449. [Google Scholar]
- Pandolfi JM, Bradbury RH, Sala E, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- Pratchett MS, Mccowan D, Maynard JA, Heron SF. Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS One. 2013;8:e70443. doi: 10.1371/journal.pone.0070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding MD, Fox HE, Halpern BS, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience. 2007;57:573–583. [Google Scholar]
- Stambler N, Dubinsky Z. Corals as light collectors: an integrating sphere approach. Coral Reefs. 2005;24:1–9. [Google Scholar]
- Suggett DJ, Smith DJ. Interpreting the sign of coral bleaching as friend vs. foe. Global Change Biology. 2011;17:45–55. [Google Scholar]
- West JM, Salm RV. Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conservation Biology. 2003;17:956–967. [Google Scholar]
- Wilkinson C. Status of the Coral Reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre; Townsville, Australia: 2008. pp. 1–298. [Google Scholar]
- van Woesik R, Sakai K, Ganase A, Loya Y. Revisiting the winners and the losers a decade after coral bleaching. Marine Ecology Progress Series. 2011;434:67–76. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.