Abstract
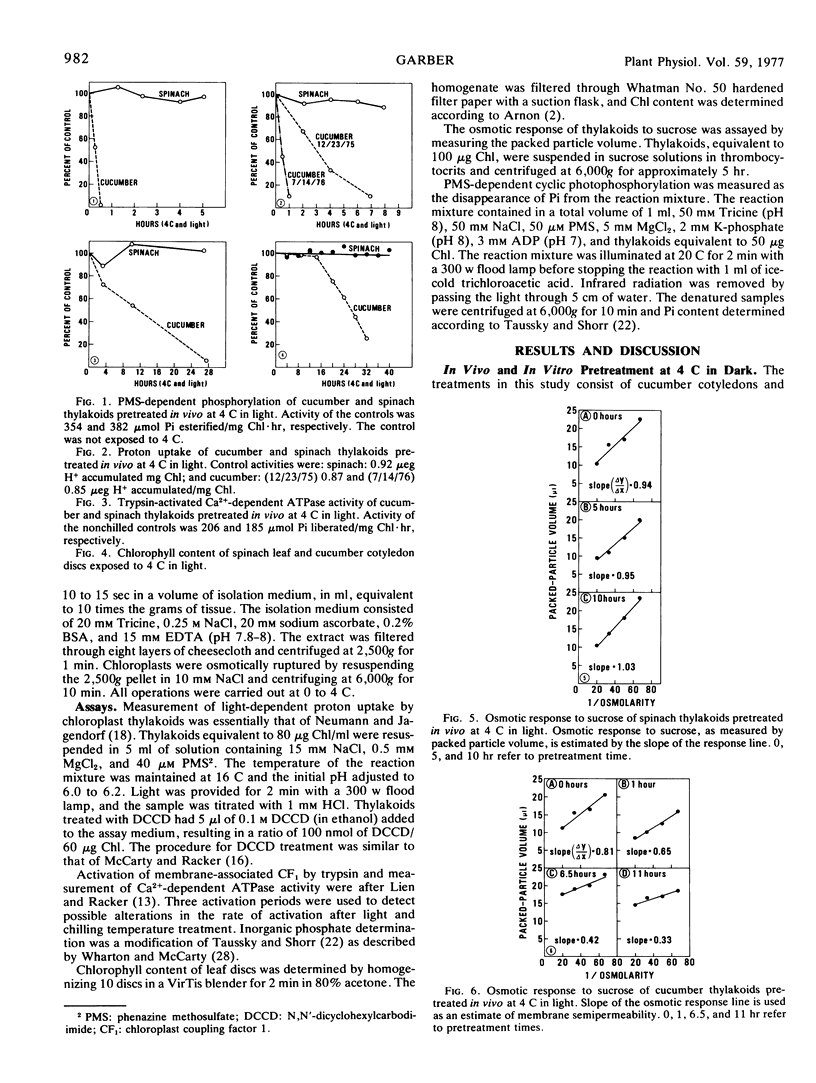
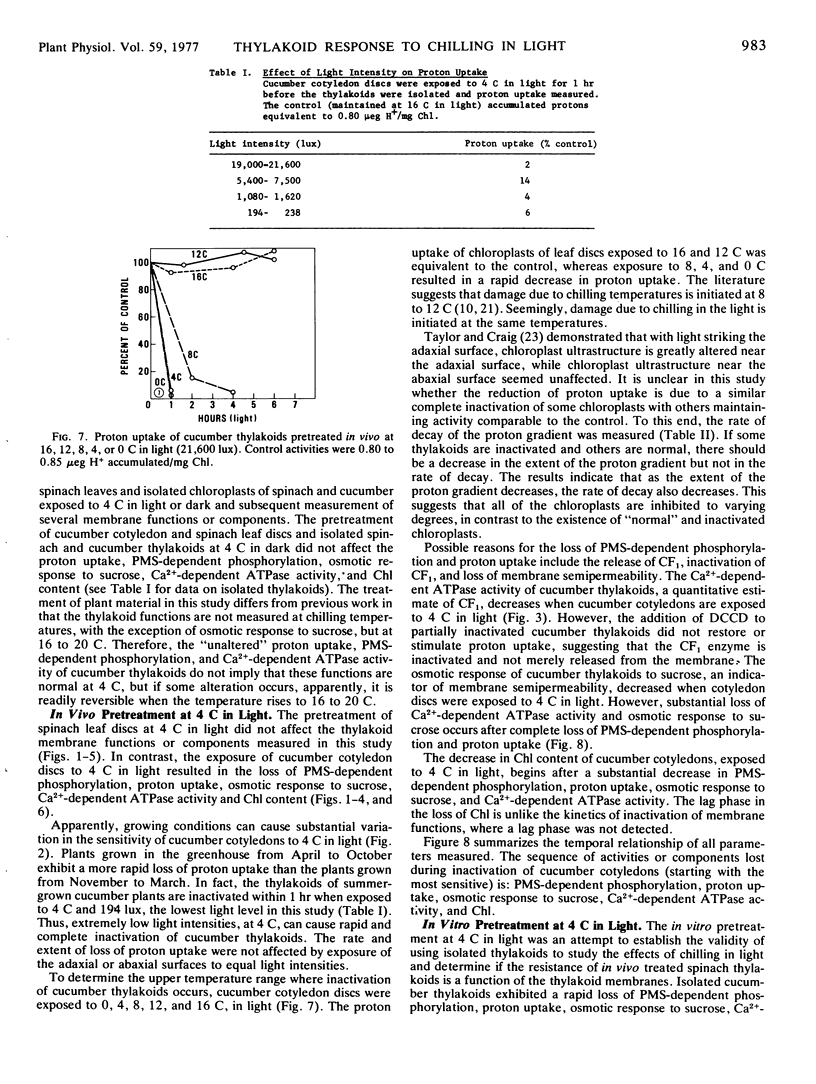
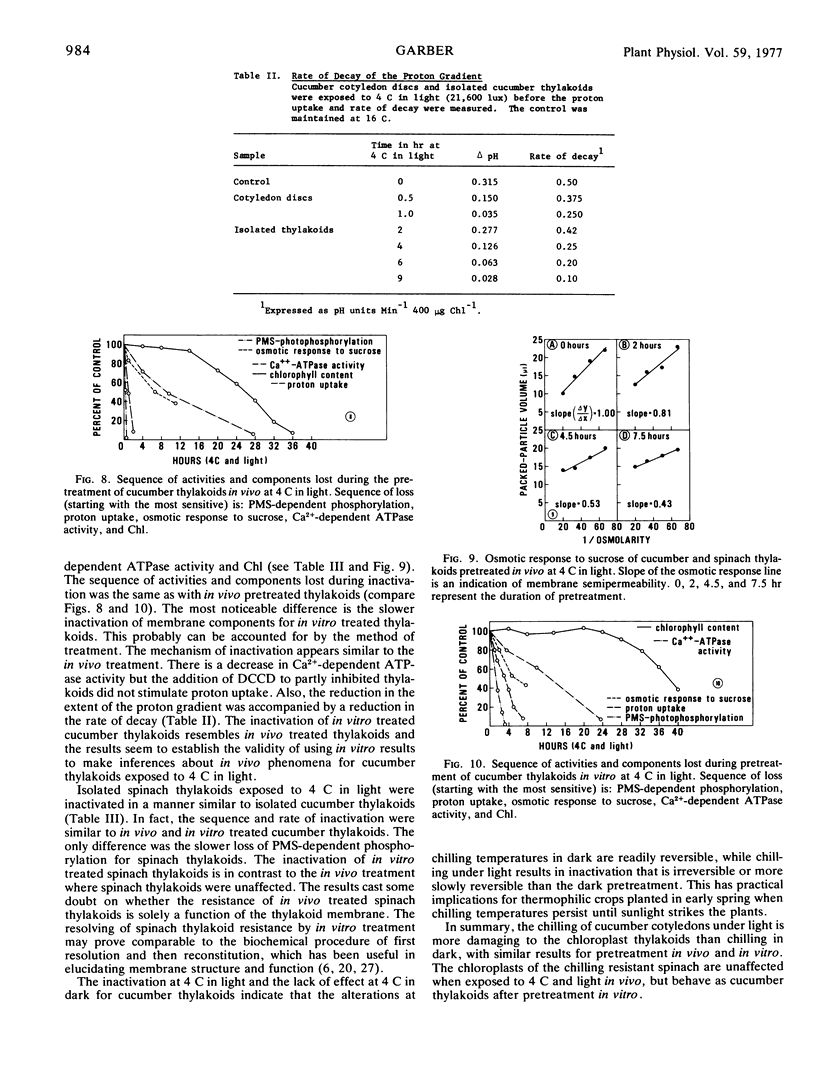
The effects of chilling temperatures, in light or dark, on the isolated thylakoids and leaf discs of cucumber (Cucumis sativa L. “Marketer”) and spinach (Spinacia oleracea L. “Bloomsdale”) were studied. The pretreatment of isolated thylakoids and leaf discs at 4 C in the dark did not affect the phenazine methosulfate-dependent phosphorylation, proton uptake, osmotic response to sucrose, Ca2+-dependent ATPase activity, or chlorophyll content. Exposure of cucumber cotyledon discs and isolated thylakoids of cucumber and spinach to 4 C in light resulted in a rapid inactivation of the thylakoids. The sequence of activities or components lost during inactivation (starting with the most sensitive) are: phenazine methosulfate-dependent cyclic phosphorylation, proton uptake, osmotic response to sucrose, Ca2+-dependent ATPase activity, and chlorophyll. The rate of loss of proton uptake, osmotic response to sucrose, Ca2+-dependent ATPase activity and chlorophyll is similar for isolated cucumber and spinach thylakoids, whereas spinach thylakoids are more resistant to the loss of phenazine methosulfate-dependent phosphorylation. The thylakoids of spinach leaf discs were unaffected by exposure to 4 C in light. The results question whether the extreme resistance of spinach thylakoids treated in vivo is solely a function of the chloroplast thylakoid membranes and establish the validity of using in vitro results to make inferences about cucumber thylakoids treated in vivo at 4 C in light.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooking I. R., Taylor A. O. Plants under Climatic Stress: V. Chilling and Light Effects on Radiocarbon Exchange between Photosynthetic Intermediates of Sorghum. Plant Physiol. 1973 Aug;52(2):180–182. doi: 10.1104/pp.52.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake B., Raschke K. Prechilling of Xanthium strumarium L. Reduces Net Photosynthesis and, Independently, Stomatal Conductance, While Sensitizing the Stomata to CO(2). Plant Physiol. 1974 Jun;53(6):808–812. doi: 10.1104/pp.53.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaks I. L., Morris L. L. Respiration of Cucumber Fruits Associated with Physiological Injury at Chilling Temperatures. Plant Physiol. 1956 Jul;31(4):308–314. doi: 10.1104/pp.31.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber M. P., Steponkus P. L. Identification of chloroplast coupling factor by freeze-etching and negative-staining techniques. J Cell Biol. 1974 Oct;63(1):24–34. doi: 10.1083/jcb.63.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Tyankova L., Santarius K. A. Effects of freezing on biological membranes in vivo and in vitro. Biochim Biophys Acta. 1973 Jan 2;291(1):23–37. doi: 10.1016/0005-2736(73)90057-6. [DOI] [PubMed] [Google Scholar]
- MARGULIES M. M., JAGENDORF A. T. Effect of cold storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Arch Biochem Biophys. 1960 Oct;90:176–183. doi: 10.1016/0003-9861(60)90565-8. [DOI] [PubMed] [Google Scholar]
- Margulies M. M. Effect of cold-storage of bean leaves on photosynthetic reactions of isolated chloroplasts. Inability to donate electrons to photosystem II and relation to manganese content. Biochim Biophys Acta. 1972 Apr 20;267(1):96–103. doi: 10.1016/0005-2728(72)90141-7. [DOI] [PubMed] [Google Scholar]
- Shneyour A., Raison J. K., Smillie R. M. The effect of temperature of the rate of photosynthetic electron transfer in chloroplasts of chilling-sensitive and chilling-resistant plants. Biochim Biophys Acta. 1973 Jan 18;292(1):152–161. doi: 10.1016/0005-2728(73)90259-4. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Taylor A. O., Craig A. S. Plants under Climatic Stress: II. Low Temperature, High Light Effects on Chloroplast Ultrastructure. Plant Physiol. 1971 May;47(5):719–725. doi: 10.1104/pp.47.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Jepsen N. M., Christeller J. T. Plants under Climatic Stress: III. Low Temperature, High Light Effects on Photosynthetic Products. Plant Physiol. 1972 May;49(5):798–802. doi: 10.1104/pp.49.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Rowley J. A. Plants under Climatic Stress: I. Low Temperature, High Light Effects on Photosynthesis. Plant Physiol. 1971 May;47(5):713–718. doi: 10.1104/pp.47.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. O., Slack C. R., McPherson H. G. Plants under Climatic Stress: VI. Chilling and Light Effects on Photosynthetic Enzymes of Sorghum and Maize. Plant Physiol. 1974 Nov;54(5):696–701. doi: 10.1104/pp.54.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J. N., Racker E. A method for increasing contrast of mitochondrial inner membrane spheres in thin sections of epon-araldite embedded tissue. J Cell Biol. 1973 May;57(2):580–586. doi: 10.1083/jcb.57.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]



