Abstract
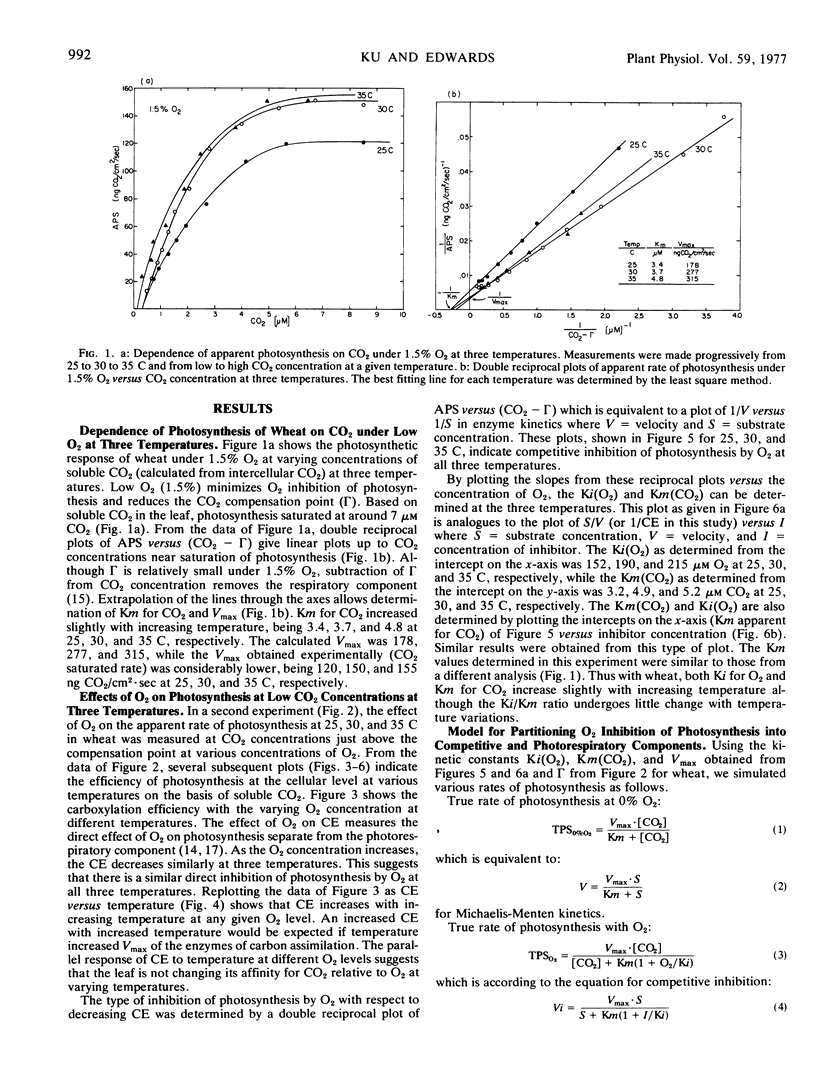
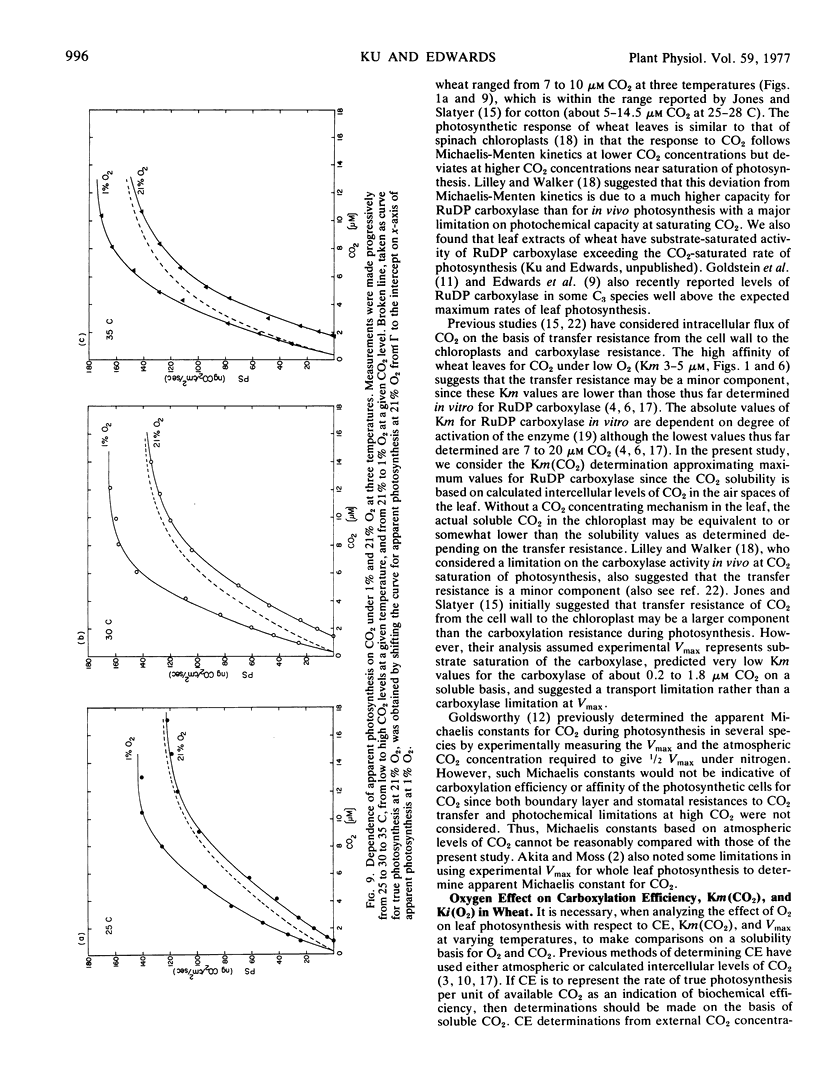
The response of whole leaf photosynthesis of wheat (Triticum aestivum L.) in relation to soluble CO2 available to the mesophyll cells, under low (1.5%) O2 at 25, 30, and 35 C, followed Michaelis-Menten kinetics up to saturating CO2 but deviated at high CO2 levels where the experimental Vmax is considerably less than the calculated Vmax. The affinity of the leaves for CO2 during photosynthesis was similar from 25 to 35 C with Km (CO2) values of approximately 3.5 to 5 μM.
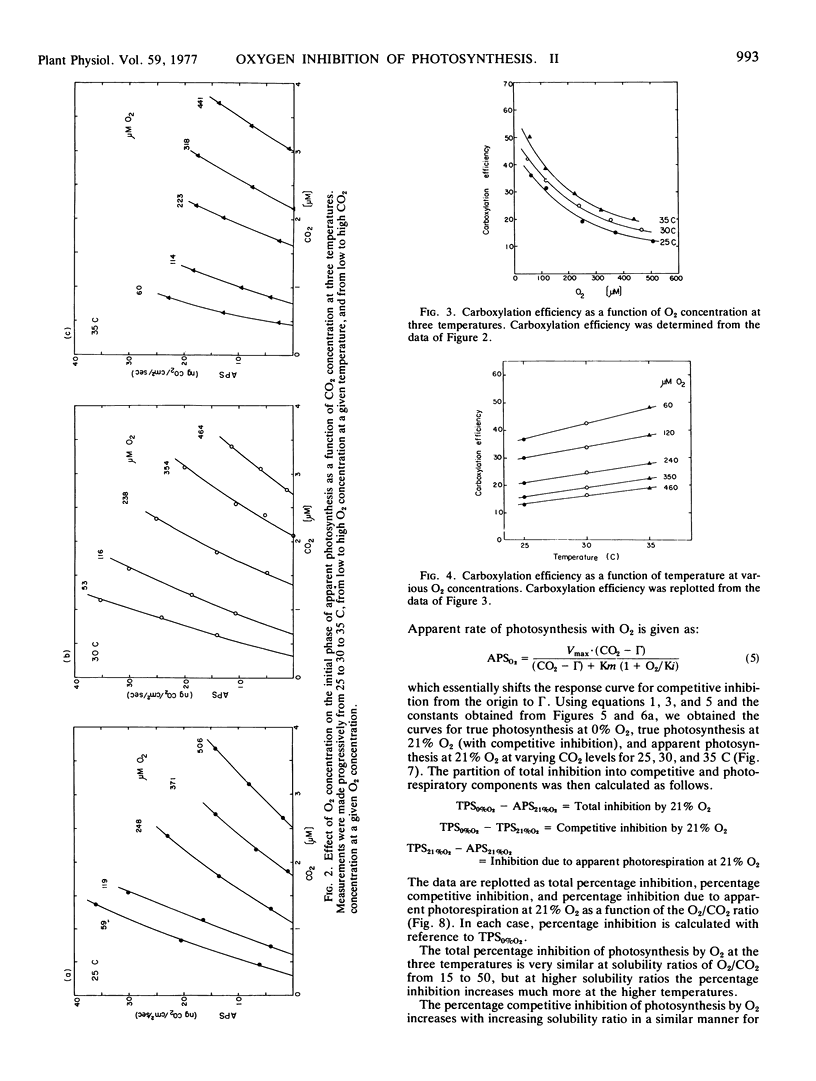
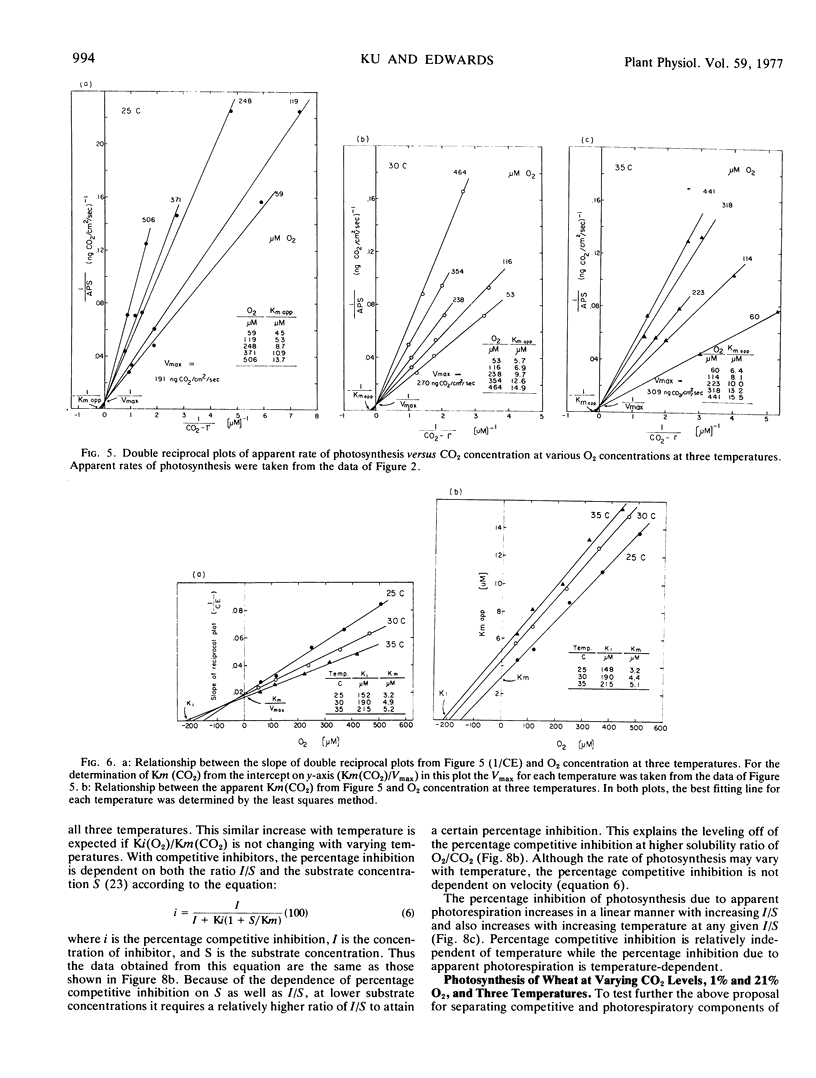
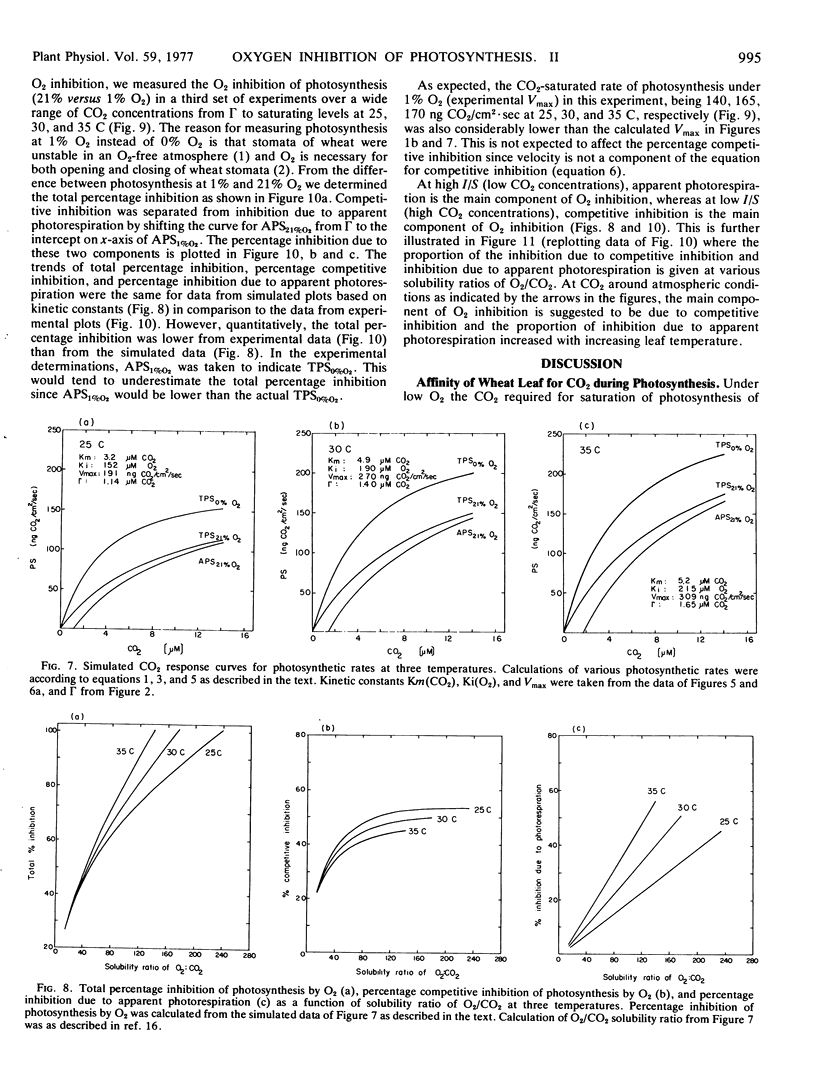
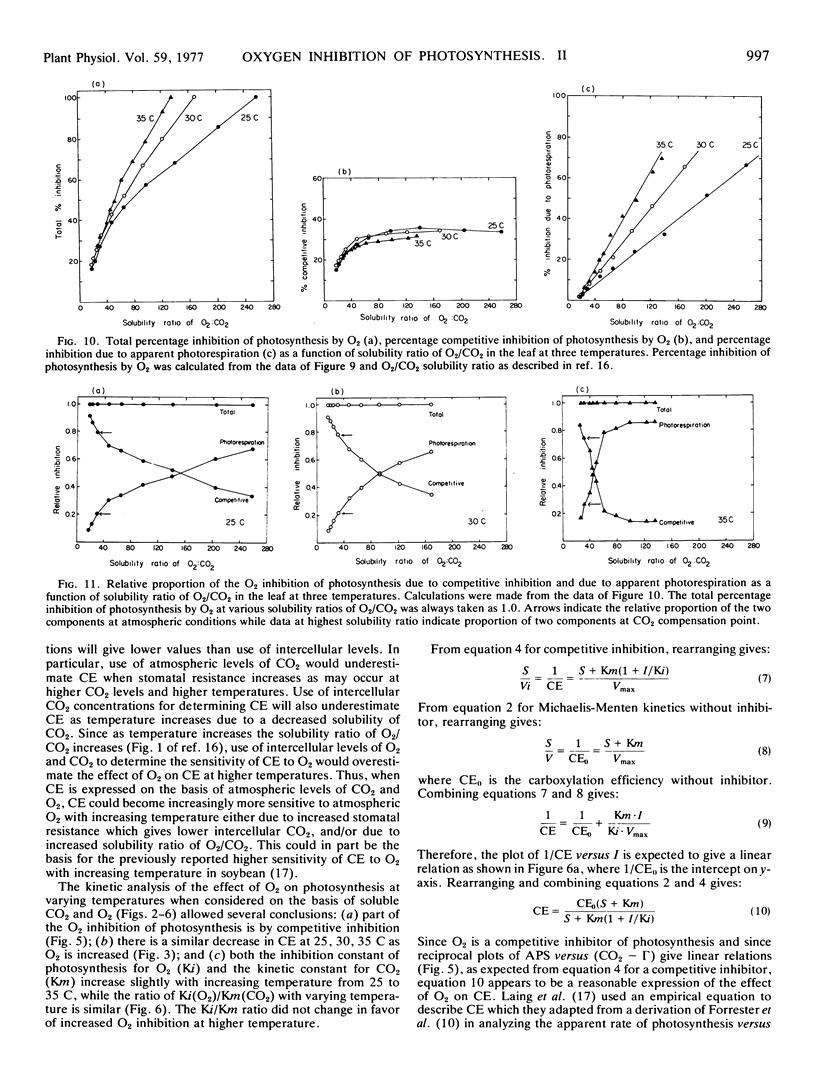
In considering the effect of O2 on photosynthesis at 25, 30, and 35 C where O2 and CO2 are expressed on a solubility basis: (a) the effect of O2 on carboxylation efficiency was similar at the three temperature; (b) increasing temperature caused only a slight increase in kinetic constants Ki(O2) and Km(CO2), while the ratio of Ki(O2)/Km(CO2) was similar at the three temperatures; and (c) the reciprocal plots of apparent rate of photosynthesis versus (CO2 - Γ) at various O2 levels showed O2 to be a competitive inhibitor of photosynthesis.
A model for separating O2 inhibition of photosynthesis into two components, direct competitive inhibition and inhibition due to photorespiration, was presented from both simulated and experimental data of photosynthetic response curves to varying CO2 concentrations at low O2versus 21% O2. The photorespiratory part of O2 inhibition is considered as a major component at Γ and increases with increasing temperature and with increase in O2/CO2 solubility ratio. The competitive component of O2 inhibition is considered as a major component of O2 inhibition under atmospheric CO2 levels and is relatively independent of temperature at a given O2/CO2 ratio.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita S., Moss D. N. The Effect of an Oxygen-free Atmosphere on Net Photosynthesis and Transpiration of Barley (Hordeum vulgare L.) and Wheat (Triticum aestivum L.) Leaves. Plant Physiol. 1973 Dec;52(6):601–603. doi: 10.1104/pp.52.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine J. J., Stevens M. A. Genotypic variation in carboxylation of tomatoes. Plant Physiol. 1976 Feb;57(2):325–333. doi: 10.1104/pp.57.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Bowes G. pH Dependence of the Km(CO(2)) of Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Nov;56(5):630–633. doi: 10.1104/pp.56.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R., Anderson L. L. Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch Biochem Biophys. 1976 Sep;176(1):344–351. doi: 10.1016/0003-9861(76)90173-9. [DOI] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. G., Slatyer R. O. Estimation of the transport and carboxylation components of the intracellular limitation to leaf photosynthesis. Plant Physiol. 1972 Aug;50(2):283–288. doi: 10.1104/pp.50.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E. Oxygen Inhibition of Photosynthesis: I. Temperature Dependence and Relation to O(2)/CO(2) Solubility Ratio. Plant Physiol. 1977 May;59(5):986–990. doi: 10.1104/pp.59.5.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Ludwig L. J., Canvin D. T. The Rate of Photorespiration during Photosynthesis and the Relationship of the Substrate of Light Respiration to the Products of Photosynthesis in Sunflower Leaves. Plant Physiol. 1971 Dec;48(6):712–719. doi: 10.1104/pp.48.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B., MASSEY V. Newer knowledge of succinic dehydrogenase. Adv Enzymol Relat Subj Biochem. 1957;18:65–111. doi: 10.1002/9780470122631.ch2. [DOI] [PubMed] [Google Scholar]


