Abstract
Background
The variability of progression of multiple sclerosis (MS) suggests that MS is a heterogeneous entity.
Objective
The objective of this article is to determine whether sickness absence (SA) and disability pension (DP) could be used to identify groups of patients with different progression courses.
Methods
We analyzed mean-annual net months of SA/DP, five years prior to MS diagnosis, until the year of diagnosis, and five years after for 3543 individuals diagnosed 2003–2006, by modeling trajectory subgroups.
Results
Five different groups were identified, revealing substantial heterogeneity among MS patients. Before diagnosis, 74% had a flat trajectory, while the remaining had a sharply increasing degree of SA/DP. After diagnosis, 95% had a flat or marginally increasing trajectory, although at various SA/disability pension (DP) levels, whereas a small group of 5% had decreasing SA/DP. A majority had few or no SA/DP months throughout the 11-year study period. Higher age and a lower educational level were associated with an unfavorable trajectory (p values <0.01).
Conclusions
There’s a considerable heterogeneity of MS progression in terms of SA/DP. Compared with other measures of disability, sickness-absence and disability pension offer a continuous variable that can be assigned to every individual for each time period without missing data. To what extent the SA/DP measure reflects classical MS outcome-measures as well as how correlated it is with co-morbidities and working-conditions needs to be investigated further.
Keywords: Multiple sclerosis, disability pension, sick leave, work incapacity, MS progression
Introduction
Multiple sclerosis (MS) is a chronic disease affecting the central nervous system. It is characterized by a heterogeneous course and generally affects younger individuals in their prime of working life.1,2 Common clinical features are fatigue, pain, depression, physical disability, and cognitive impairment, all of which may be highly disabling.3–7 Although the disease does not dramatically reduce life expectancy, individuals with MS often experience deterioration in functional status and a significant decrease in the ability to remain in the work force.1,8 Also, sickness absence (SA) and disability pension (DP) are highly elevated among MS patients.2,9–11 However, little is known about to what extent the patients were on SA/DP before diagnosis, and if there were different SA/DP patterns. The same goes for possible patterns of how SA/DP develops after diagnosis and whether distinct types of trajectories of SA/DP can be identified and how such patterns before and after diagnosis might be related.
The heterogeneity of MS progression is well known, ranging from rapidly disabling to more benign relapsing–remitting courses (RRMS)7 to insidiously progressing worsening with or without a preceding relapsing phase. The frequency and severity of relapses may vary widely. Negative clinical prognostic factors, in the shorter or longer term, are: short inter-relapse interval, high relapse rate, the shift to the secondary progressive phase, high disability during the first years, and age of onset.12–15 However, these factors have a fairly limited value in providing a reliable prognosis for an individual.16
Most studies on issues associated with the development of MS have, however, employed the Expanded Disability Status Scale (EDSS) as an outcome measure, thus focusing on physical functions, and among them on motor function, which dominates the EDSS.13,17,18 With fewer physical demands in modern work life, EDSS may have a gradually more limited value for predicting the individuals’ work capacity for which other consequences of MS such as fatigue or cognitive disabilities may be more important.18,19
The aims of this study were to: identify and describe different types of trajectories of SA/DP among MS patients over an 11-year period, from five years before MS diagnosis until five years after diagnosis; characterize the trajectory groups with regard to socio-demographics and prior SA/DP; and explore to what extent socio-demographics and prior SA/DP can predict MS patients’ SA/DP trajectories. Thus, we aspired to use information on SA/DP to capture MS patients’ overall disease-induced work incapacity, both as an MS outcome measure and for its own relevance. In the Swedish universal social security system almost all residents of working ages are eligible for SA benefits and all are eligible for DP benefits, making Sweden a particularly suitable setting for studying how work incapacity among MS patients develops and diverges over time.
Materials and methods
Study population
The study cohort consisted of 3543 individuals first diagnosed with MS between 2003 and 2006. Using nationwide register data for inpatient care from 1997 and specialized outpatient care from 2001, year of MS diagnosis was defined as the first time the individuals were identified (2003–2006) with MS as primary or secondary diagnosis (International Statistical Classification of Diseases, 10th revision (ICD-10), G-35), i.e. individuals with a recorded MS diagnosis before 2003 were excluded. The sample was extracted from a database covering all 4,030,986 individuals between 23 and 57 years of age who lived in Sweden as of December 31, 2004, i.e. they were aged 22–59 years at time of diagnosis. Information from other nationwide registers was obtained through data-linkage using the unique personal identity number assigned to all residents in Sweden.
A relative annual time scale was introduced where T0 represents the year of MS diagnosis. T−5 refers to five years prior to diagnosis, while T+5 refers to five years after diagnosis. This 11-year period was then divided into two periods in order to explore how SA/DP trajectories in the pre-diagnosis period (T−5–T0) predicted SA/DP trajectories in the post-diagnosis period (T+1–T+5).
Explanatory variables
All socio-demographic variables were measured as of December 31 the year before the start of the respective follow-up periods. The following socio-demographic variables were obtained from Statistics Sweden’s Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA): sex; age groups (22–34, 35–44, 45–59); country of birth (born in Sweden, not born in Sweden); type of place of residence (based on the H-classification scheme20 in the following three categories: larger cities (H1–H2), medium-sized municipalities (H3–H4), or smaller municipalities (H5–H6)); geographical region (categorized in accordance with Eurostat’s Nomenclature of Territorial Units for Statistics (NUTS-level 1) as east, south, north Sweden); living with children (<18 years of age) (yes/no); cohabiting with partner (yes/no); and educational level (high school or less, university education). For the first time period (T−5–T0), days on SA/DP six years prior to diagnosis were used to classify levels of previous SA/DP (0, 1–39 (<median, of those with >0 SA/DP days), >39 days). In the second period (T+1–T+5), we used the variable of estimated trajectory group belonging from the analysis of the first time-period as a covariate. The variable “Year of MS diagnosis” was included as covariate in all models to adjust for potentially influential temporal variations that might have occurred in the Swedish social insurance system or labor market during the study period.
Sickness insurance in Sweden
All residents in Sweden aged 16–64 with income from work or unemployment benefits are covered by the same public sickness insurance, providing sick-leave benefits to people with reduced work capacity due to disease or injury. Sick pay is in most cases among employed individuals paid by the employer during the first 14 sick-leave days. All residents can be granted DP if the work incapacity is predicted to be permanent. Both SA and DP can be granted for part time, that is, if granted partial DP, you can on and off have part-time SA at the same time, which is frequently the case with MS patients.
Outcome
Information on number of days/years with SA and DP with benefit from the Social Insurance Agency was obtained from the LISA-register. Annual number of net days with SA and DP were calculated, e.g. two days of half-time SA or DP constituted one net day. Then these annual net days were transformed into months where 14 to 30 days were rounded to one month and less than 14 was rounded to 0 month. Days exceeding one month was rounded down to the closest full month.
Statistical analyses
The calculated number of months on SA/DP was analyzed during a total time period of 11 years, five years prior to MS diagnosis through five years after diagnosis. First we describe the average number of months on SA/DP at three time points: five years prior to diagnosis, the year of diagnosis, and five years after diagnosis.
Secondly, we used semi-parametric group-based trajectory modeling to estimate developmental trajectories of SA/DP among MS patients (SAS procedure Traj21), separately for the two studied time periods. This procedure models the patterns of change over time in multiple subgroups within a population and estimates a regression model for each discrete group. Group member probabilities are estimated, using a multinomial logit function. We started by identifying the model with the optimal number of subgroups by estimating models with two to six subgroups using the zero-inflated Poisson distribution (ZIP).22 The models for the two time-periods that best fit the data were determined by the Bayesian information criterion (BIC).23 The lowest BIC value (closest to zero) indicates the model that best fits the data.
Thirdly, socio-demographic compositions of the trajectory groups were calculated with descriptive statistics. Thereafter, the effect of each explanatory variable was separately examined with regard to trajectory group belonging, using three complementary statistical procedures: 1) Pearson’s X2 was performed to assess whether the socio-demographic composition and previous SA/DP differed between trajectory groups; 2) to assess whether socio-demographic factors and previous SA/DP were associated with trajectory group, log-likelihood tests were conducted; 3) to gain insight of the socio-demographic factors’ and prior SA/DP’s explanatory power, differences in Nagelkerke-R2 were calculated, i.e. obtained by comparing Nagelkerke R2 (hereafter “diff R2”) between models where the examined factor was included vs. excluded. Two sets of multivariate multinomial regression models were tested; one in which the SA/DP history variable was omitted (Model 1) and one where it was included (Model 2). Diff R2 is used to compare two separately estimated models. Here, one of the models was nested within the other, i.e. we had one full model (including all covariates) and one restricted where the covariate of interest, prior SA/DP, was omitted. The value of diff R2 can be interpreted as a measure of increased explained variances, by adding one variable to the model; the higher the R2, the higher the explained variance.
Ethics approval
The study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Results
In our cohort of 3543 MS patients, 70.1% were female (Table 1). Forty percent were in the oldest age span (45–59) and 36.9% had a university education at the time of diagnosis. It was slightly more common to be cohabiting (53.7%) and living with children (51.9%). Not surprisingly, the average number of months on SA/DP increased with time in all groups and was lowest five years prior to diagnosis. Also, SA/DP was more common among women than among men at all three time points (T−5, T0, T+5). As expected, higher age also showed higher levels of SA/DP at every time point. MS patients living in the north of Sweden had more SA/DP months as did MS patients with a lower educational level. Cohabiting as well as living with children was associated with fewer SA/DP months.
Table 1.
Percentage of multiple sclerosis (MS) patients at year of diagnoses (T0) by different covariates and mean annual number of net months on sickness absence/disability pension (SA/DP) five years prior to MS diagnosis (T−5), the year of diagnosis (T0), and five years after diagnosis (T+5), respectively.
| Mean annual net months of SA/DP |
||||
|---|---|---|---|---|
| Percentage (T0) | T−5 | T0 | T+5 | |
| Sex | ||||
| Women | 70.1 | 1.7 | 4.2 | 4.9 |
| Men | 29.9 | 1.1 | 3.5 | 4.4 |
| Age at diagnosis | ||||
| 22–34 years | 26.1 | 0.4 | 2.2 | 2.4 |
| 35–44 | 33.9 | 1.1 | 3.6 | 4.4 |
| 45–59 | 40.1 | 2.6 | 5.4 | 6.7 |
| Country of birth | ||||
| Sweden | 91.3 | 1.5 | 3.9 | 4.7 |
| Other | 8.7 | 1.5 | 4.4 | 5.5 |
| Education | ||||
| ≤12 years | 63.1 | 1.7 | 4.7 | 5.7 |
| >12 years | 36.9 | 1.0 | 2.7 | 3.4 |
| Marital status | ||||
| Married or cohabiting | 53.8 | 1.5 | 3.8 | 4.3 |
| Not married or cohabiting | 46.1 | 1.5 | 4.1 | 5.4 |
| Living with children | ||||
| Yes | 52.3 | 1.6 | 3.7 | 3.8 |
| No | 47.7 | 1.5 | 4.2 | 5.8 |
| Geographical region | ||||
| East Sweden | 39.6 | 1.4 | 3.5 | 4.2 |
| South Sweden | 41.6 | 1.6 | 4.1 | 5.0 |
| North Sweden | 18.7 | 1.6 | 4.6 | 5.5 |
| Type of living area | ||||
| Larger cities | 37.5 | 1.5 | 3.5 | 3.9 |
| Medium-sized municipalities | 35.8 | 1.4 | 4.0 | 4.9 |
| Smaller municipalities | 26.6 | 1.7 | 4.7 | 5.8 |
| Year of MS diagnosis | ||||
| 2003 | 28.8 | 1.5 | 4.1 | 5.3 |
| 2004 | 25.6 | 1.5 | 4.1 | 4.7 |
| 2005 | 24.4 | 1.5 | 4.0 | 4.6 |
| 2006 | 21.2 | 1.6 | 3.6 | 4.3 |
| n | 3539 | 3536 | 3535 | 3470 |
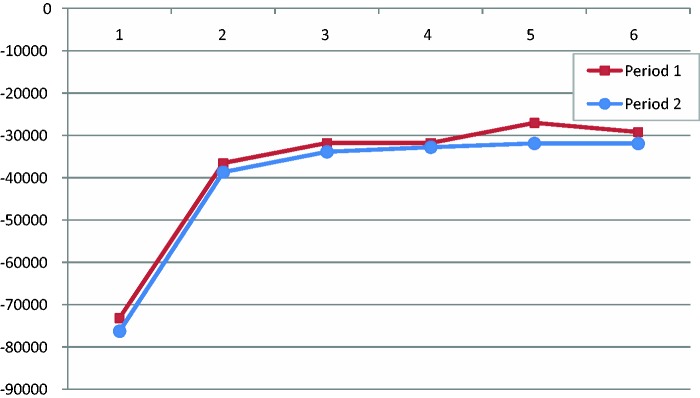
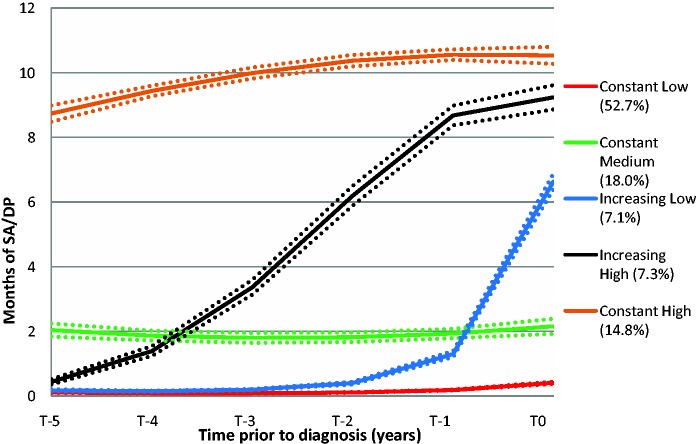
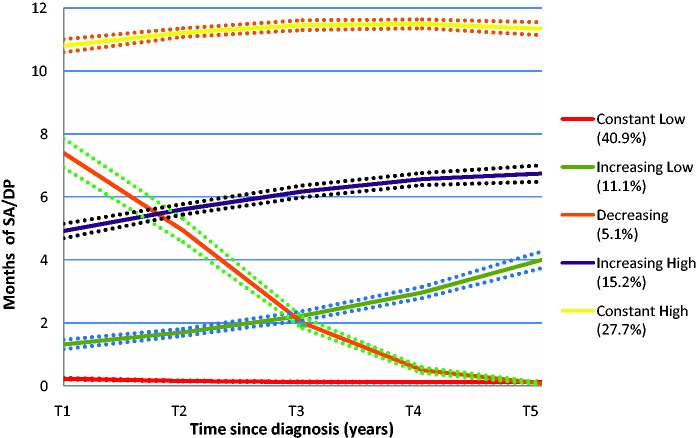
The estimated BIC values suggested five groups in both of the time periods (Figure 1). Proc Traj provides individual fit estimates, i.e. probabilities that each individual belongs to the trajectory groups. Côté et al. recommend that the average probability for members of a trajectory group should be ≥0.70.24 All identified groups had averages above 0.90 during each respective time period, indicating a very good fit. The five trajectory groups clearly display the heterogeneity of MS progression with regard to SA/DP (Figures 2 and 3). Some (14.8%) had a high level of SA/DP already five years prior to MS diagnosis, but the majority had few or no SA/DP months through all 11 years. In the pre-diagnosis period, about 74% of the MS patients had fairly flat trajectories; of these 70% were classified as having few annual net months on SA/DP, while the remaining 26% were classified as having a sharp increase of SA/DP. In the second period (T+1–T+5), 95% were classified as belonging to a group with flat or a marginally increasing trajectory, at various SA/DP levels. The remaining 5% had a decreasing trajectory.
Figure 1.
Number of trajectory groups (1–6) and their Bayesian information criterion (BIC) values (Y-axis) for period 1 (pre-diagnosis) and period 2 (post-diagnosis).
Figure 2.
The five trajectories of net months of sickness absence/disability pension (SA/DP) during the pre-diagnosis period (T−5–T0), and percentage of patients within each trajectory group (n = 3543). The dotted lines represent 95% confidence intervals.
Figure 3.
The five trajectories of net months of sickness absence/disability pension during post-diagnosis period (T+1–T+5), and percentage of patients within each trajectory group (n = 3543).
Table 2 shows that socio-demographics were fairly similarly distributed between the trajectory groups in both time periods, with the exceptions that older individuals and individuals with a lower educational level were notably overrepresented in the “Constant high” group and underrepresented in the “Constant low” group. Still, all socio-demographic factors, except country of birth (pre-diagnosis period) and cohabiting status (both periods), were in the unadjusted analyses significantly associated with SA/DP trajectory (p < 0.01). However, prior SA/DP was in these unadjusted analyses strongly associated with trajectory-group belonging (i.e. pre-diagnosis period: degree of freedom (df) 8, X2 = 2200.7. Post-diagnosis period: df 16, X2 = 2045.0).
Table 2.
Trajectory-group belonging by socio-demographics and prior sickness absence/disability pension (SA/DP) during pre-diagnosis period (T−5–T0) and post-diagnosis period (T+1–T+5).
| Pre-diagnosis period (n = 3543) |
Post-diagnosis period (n = 3539) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Const. low (%) | Const. medium (%) | Increasing. low (%) | Increasing high (%) | Const. high (%) | Pearson’s X2 (p value) | Const. low (%) | Increasing low (%) | Decreasing (%) | Increasing high (%) | Const. high (%) | Pearson’s X2 (p value) | |
| ALL | 52.7 | 18 | 7.1 | 7.3 | 14.8 | 40.9 | 11.1 | 5.1 | 15.2 | 27.7 | ||
| Sex | ||||||||||||
| Male | 59.6 | 18.1 | 4.6 | 6.5 | 11.2 | 41.7 (<0.01) | 48.8 | 8.1 | 5.2 | 11.6 | 26.3 | 48.5 (<0.01) |
| Female | 49.8 | 18 | 8.2 | 7.7 | 16.3 | 37.6 | 12.3 | 5 | 16.8 | 28.3 | ||
| Age at diagnosis | ||||||||||||
| 22–34 | 70.4 | 16.1 | 5.6 | 5.5 | 2.4 | 344.0 (<0.01) | 58.5 | 12.7 | 7.3 | 10.8 | 10.7 | 382.3 (<0.01) |
| 35–44 | 53.8 | 20.5 | 8.1 | 6.8 | 10.9 | 42.9 | 10.9 | 5.7 | 16.9 | 23.5 | ||
| 45–59 | 40.5 | 17.2 | 7.3 | 9 | 25.9 | 27.7 | 10.1 | 3.2 | 16.7 | 42.2 | ||
| Education | ||||||||||||
| ≤12 years | 48.4 | 19.6 | 6.9 | 8.1 | 17 | 76.2 (<0.01) | 35.1 | 9.3 | 5.4 | 15.6 | 34.7 | 170.2 (<0.01) |
| >12 years | 62.9 | 14.4 | 7.6 | 5.7 | 9.4 | 50.4 | 14.1 | 4.5 | 14.8 | 16.2 | ||
| Country of birth | ||||||||||||
| Sweden | 52.7 | 18.2 | 7.2 | 7.3 | 14.6 | 1.3 (0.87) | 41.5 | 11.1 | 4.8 | 15.5 | 27 | 16.3 (<0.01) |
| Other | 53.2 | 16.1 | 6.8 | 7.7 | 16.1 | 34.8 | 10.3 | 7.7 | 12.3 | 34.8 | ||
| Living with children | ||||||||||||
| Yes | 48.5 | 19.4 | 8.2 | 8.3 | 15.6 | 29.5 (<0.01) | 41.3 | 12.4 | 5.5 | 15.9 | 24.9 | 20.3 (<0.01) |
| No | 57.2 | 16.6 | 6 | 6.3 | 13.8 | 40.3 | 9.6 | 4.6 | 14.6 | 30.9 | ||
| Living with partner | ||||||||||||
| Yes | 50.1 | 18.5 | 7.9 | 8.3 | 15.2 | 12.2 (0.02) | 41 | 12.1 | 4.6 | 15.4 | 26.8 | 7.1 (0.13) |
| No | 55.2 | 17.6 | 6.4 | 6.4 | 14.3 | 40.7 | 9.8 | 5.6 | 15.1 | 28.8 | ||
| Size of living area | ||||||||||||
| Large | 57.7 | 15.9 | 6.2 | 6.1 | 14.1 | 40.0 (<0.01) | 48.6 | 10.1 | 5.1 | 12.8 | 23.4 | 73.0 (<0.01) |
| Medium | 53.5 | 17.5 | 7.4 | 8.1 | 13.5 | 39.3 | 11.7 | 5.4 | 16.1 | 27.5 | ||
| Small | 45.1 | 21.5 | 8.1 | 8 | 17.4 | 32.1 | 11.6 | 4.6 | 17.6 | 34.1 | ||
| Geographical region | ||||||||||||
| East Sweden | 57.5 | 16.3 | 6.2 | 6.7 | 13.3 | 36.2 (<0.01) | 45.7 | 11.8 | 4.8 | 13.2 | 24.4 | 43.8 (<0.01) |
| South Sweden | 52.1 | 17.8 | 7.1 | 7.3 | 15.8 | 39.7 | 10 | 5.3 | 15.1 | 29.9 | ||
| North Sweden | 44.5 | 22.2 | 9.2 | 8.8 | 15.4 | 33.1 | 11.8 | 5.2 | 20 | 29.9 | ||
| Year of MS diagnosis | ||||||||||||
| 2003 | 50.3 | 19.4 | 7.4 | 7 | 15.9 | 20.9 (0.05) | 37.2 | 11.9 | 4.7 | 15.3 | 31 | 22.5 (0.03) |
| 2004 | 52.4 | 18 | 5.5 | 8.9 | 15.1 | 41.3 | 9.2 | 6.1 | 15.9 | 27.6 | ||
| 2005 | 53.7 | 18.2 | 6.9 | 7.8 | 13.4 | 41 | 12.5 | 4.6 | 15.4 | 26.5 | ||
| 2006 | 55.2 | 16.1 | 8.9 | 5.5 | 14.4 | 45.5 | 10.6 | 4.9 | 14.2 | 24.7 | ||
| SA/DP at T−6 | ||||||||||||
| 0 days | 64.5 | 20.1 | 5.1 | 6.9 | 2.6 | 2200.7 (<0.01) | x | x | x | x | x | |
| 1–39 days | 36.8 | 22.6 | 17 | 15.6 | 8 | x | x | x | x | x | ||
| >39 days | 5.3 | 3.7 | 10.2 | 5.4 | 75.5 | x | x | x | x | x | ||
| SA/DP Pre-diagnosis period | ||||||||||||
| Constant low | x | x | x | x | x | 67.6 | 14.5 | 3.5 | 9.6 | 4.9 | 2045.0 (<0.01) | |
| Constant medium | x | x | x | x | x | 14.4 | 9.7 | 10 | 26.9 | 39 | ||
| Increasing low | x | x | x | x | x | 30.8 | 20.6 | 4 | 23.3 | 21.3 | ||
| Increasing high | x | x | x | x | x | 3.8 | 2.3 | 7.7 | 23.5 | 62.7 | ||
| Constant high | x | x | x | x | x | 1.3 | 0.4 | 4 | 13.2 | 81.1 | ||
MS: multiple sclerosis.
In Table 3, the multivariate analyses for the pre- and post-diagnosis periods are shown. When omitting earlier SA/DP as a covariate in the pre-diagnosis period (Model 1(a)), only age markedly improved the explanatory power of the model (diff R2 = 0.11). This explanatory power diminished substantially when entering previous SA/DP as a covariate. Previous SA/DP (Model 2(a)) improved the fit of the model extensively (diff R2 = 0.31). Also, sex and education remained significantly associated with group belonging in Model 2(a), but the diff R2 were marginal. For the post-diagnosis period the difference in pseudo R2 for previous SA/DP trajectory was as high as 0.35 (Model 2(b)). For the socio-demographic factors, the diff R2 measure (Model 2(b)) once again suggested a marginal effect. Still, sex, age, educational level, and country of birth significantly predicted type of SA/DP trajectory (p < 0.01).
Table 3.
Socio-demographics and prior sickness absence/disability pension’s (SA/DP) association and explanatory power of SA/DP trajectory-group belonging among multiple sclerosis (MS) patients before (T−5–T0) and after (T+1–T+5) diagnosis.
| Pre-diagnosis period |
Post-diagnosis period |
|||||||
|---|---|---|---|---|---|---|---|---|
| Model 1a, without prior SA/DP |
Model 2a, with prior SA/DP |
Model 1b, without prior SA/DP |
Model 2b, with prior SA/DP |
|||||
| X2 (p value) | Diff in R2 | X2 (p value) | Diff in R2 | X2 (p value) | Diff in R2 | X2 (p value) | Diff in R2 | |
| Sex | 61.7 (<0.01) | 0.02 | 19.8 (<0.01) | <0.01 | 62.5 (<0.01) | 0.01 | 32.8 (<0.01) | <0.01 |
| Age at diagnosis | 421.5 (<0.01) | 0.11 | 149.8 (<0.01) | 0.03 | 377.0 (<0.01) | 0.11 | 124.3 (<0.01) | 0.02 |
| Education | 97.8 (<0.01) | 0.03 | 69.3 (<0.01) | 0.01 | 134.5 (<0.01) | 0.03 | 29.3 (<0.01) | 0.01 |
| Country of birth | 1.4 (0.76) | 0 | 4.4 (0.38) | <0.01 | 21.0 (<0.01) | <0.01 | 23.9 (<0.01) | <0.01 |
| Living with children | 8.0 (0.09) | <0.01 | 2.7 (0.60) | <0.01 | 11.5 (<0.01) | <0.01 | 4.8 (0.30) | <0.01 |
| Living with partner | 13.7 (<0.01) | <0.01 | 5.3 (0.26) | <0.01 | 15.8 (<0.01) | <0.01 | 4.6 (0.32) | <0.01 |
| Size of living area | 7.3 (0.25) | <0.01 | 8.0 (0.44) | <0.01 | 22.5 (<0.01) | <0.01 | 15.2 (0.04) | <0.01 |
| Geographical region | 18.6 (0.02) | <0.01 | 16.8 (0.03) | <0.01 | 16.8 (0.03) | <0.01 | 9.5 (0.30) | <0.01 |
| Year of MS diagnosis | 23.0 (0.03) | <0.01 | 35.8 (<0.01) | <0.01 | 24.5 (0.02) | <0.01 | 16.0 (0.19) | <0.01 |
| Previous SA/DPa | – | – | 1480.5(<0.01) | 0.31 | – | – | 1799.0 (<0.01) | 0.35 |
Diff R2 was calculated as the difference in Nagelkerke R2 between two separately estimated models of which one is nested within the other, i.e. one including all covariates (full model) vs. a restricted in which the covariate of interest has been omitted. Nagelkerke R2 in pre-diagnosis period: full model 1 0.18, full model 2 0.49. Corresponding Nagelkerke R2 for post-diagnosis period: 0.20 and 0.55.
For period 1: SA/DP at T−6 (0, 1–39, and >39 days). For period 2: Estimated trajectory-group belonging in period 1 (Constant low, Constant medium, Increasing low, Increasing high and Constant high.
Discussion
Studying a sizable population-based cohort of patients diagnosed with MS from five years before to five years after the year of diagnosis, we have identified risk factors for SA/DP as well as found evidence for a heterogeneity in the progression of MS, potentially indicating meaningful subgroups within the MS entity. Thus, some patients already had a high level of SA/DP five years prior to MS diagnosis, whereas the majority had few or no SA/DP months through all 11 years studied. Although the trajectory groups had a fairly similar socio-demographic profile, individuals with higher education were underrepresented in the trajectory group with most SA/DP months whereas older MS patients were more likely to have a constant high level of SA/DP. In fact, most of the included socio-demographic factors were statistically associated with trajectory group belonging in both time periods, i.e. both before and after MS diagnosis. Nonetheless, we found that socio-demographic factors themselves poorly predicted MS patients’ type of SA/DP trajectory, with the possible exception of age, which had some predictive power. Previous SA/DP was, however, strongly associated with SA/DP trajectories. Hence, SA/DP trajectories before diagnosis can be a valuable indicator of disease progression, in terms of work incapacity, following MS diagnosis. Previous SA/DP is also known to be a predictor of later SA/DP in general.25 Thus we wanted to examine if that was also the case in this setting. We chose, however, to present one model with and one model without previous SA/DP.
To the best of our knowledge, this is the first study of trajectories of SA/DP in MS patients, and possibly one of the first studies of any types of trajectory groups in MS patients. The difference in SA/DP trajectories found in this study are well in line with the established fact that the spectrum of MS severities is very broad, especially early in the disease course.26,27 For the majority of MS patients, the disease begins with a relapsing course (RRMS), later followed by a progressive phase (secondary progressive, SPMS). In some patients, the relapsing phase is missed and the disease is progressive from the onset (primary progressive, PPMS).26 As the data sources of our study did not include clinical data, it was not possible to test to which extent the disease trajectories correlated with the SA/DP trajectories. It is tempting to speculate that PPMS patients were enriched in the rather small trajectory group “Increasing high” (7.4%) in the pre-diagnosis period, where a steep increase was observed from almost no SA/DP months at T−5 to more than nine months at T0. The relatively higher age of patients in this group would support that notion.
Our results further indicate that prediction of MS patients’ belonging to specific SA/DP trajectories would be possible by using information about previous SA/DP, regardless of which diagnoses these benefits were granted for. When taking previous SA/DP into account, noteworthy results were that both age and educational level had a very marginal influence on how SA/DP develops over time. This can be interpreted as the influence of age and education on SA/DP trajectory mainly is attributable to the fact that both these factors are highly correlated with health and functional status. Comorbidities have, furthermore, been reported to be highly prevalent among MS patients.11 In particular mental disorders, which together with musculoskeletal disorders are the most common reasons for SA or DP in the general population,28 are elevated among MS patients. In addition, it has been shown that MS patients with musculoskeletal or mental disorders have a higher risk for DP.11 Comorbidity among MS patients may either be a risk factor for a worse trajectory in itself, or may interact synergistically with MS in producing the same consequences. Comorbidity may thus contribute to why older individuals and those with a lower educational level often have a less-favorable SA/DP trajectory.
It could also be argued that the weak association between education and SA/DP trajectory that, nonetheless, still remained after adjusting for previous SA/DP is a consequence of the fact that individuals with a lower educational level more often have more physically demanding and/or inflexible work environments that may be particularly hard to sustain with a disease like MS.
Our present study adds to this knowledge in analyzing the diversity of SA/DP during the years before and after diagnosis.
The strengths of this study are the population-based cohort design, based on the total population aged 22–59 years in the entire country of Sweden, equaling 3543 MS patients. Another strength is that data on MS diagnosis, SA/DP, and all covariates were obtained from nationwide registers, which represent highly reliable sources.29 Thereby, there were no loss to follow-up, data are not subjected to self-reporting bias, and misclassification is very uncommon. All probabilities of belonging to the selected trajectory groups were above 0.9, indicating a very good fit,24 i.e. that we could identify reliable trajectories.
However, this study also has limitations. We assumed that the year of MS diagnosis was the same as the year when the individual first was identified with an MS diagnosis in the in- or outpatient registers. Individuals who have not been hospitalized or received specialized care with a recorded MS diagnosis for a number of preceding years are thus misclassified with regard to year of diagnosis. However, such misclassifications are likely to be few given that most MS patients visit their neurologist on a regular annual basis. It should also be emphasized that MS onset usually precedes MS diagnosis by a couple of years and that analyses performed during year of onset may render different results.
Residual confounding might also be a problem. As previous research has shown disability status (EDSS) to be highly relevant for SA and DP, such associations should be included in future studies.30
Previous studies have shown high rates of SA/DP among MS patients.11,25 This study deepens the analysis; it is the first study to estimate developmental trajectories of SA/DP around the year of diagnosis indicating a considerable heterogeneous pattern of SA/DP trajectories among MS patients. On a positive note, our results show that a majority of MS patients have no, or a low level, of SA/DP, even five years after diagnosis. Further, prediction of SA/DP in MS patients seems to some extent possible even before MS diagnosis. This highlights the value of using additional non-MS-specific health-related data to improve prediction of the development of MS patients’ SA/DP trajectories. Further studies are needed to investigate to what extent the observed heterogeneity of SA/DP trajectories is attributable to (and/or can be predicted by) working conditions, comorbidity, MS treatment, and clinical MS-specific factors. Maybe even more important, it will be very interesting to investigate to what extent the trajectory groups differ in clinical terms and how they relate to similar groups obtained using EDSS in identifying clinically and even patho-genetically relevant subgroups within the MS entity.
Acknowledgment
The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Funding
This work was supported by unrestricted grants from Biogen Idec, by the Swedish Research Council for Health, Working Life and Welfare (grant number 2007-1762), and the Swedish Research Council (Project numbers: K2009-61P-21304-04-4; K2009-61X-21305-01-1).
Conflicts of interest.
JH has over the years received honoraria for serving on advisory boards for Biogen Idec and Novartis and speaker’s fees from Biogen Idec, Merck-Serono, Bayer-Schering, Teva Novartis, and Sanofi-Aventis. He has served as principal investigator for projects or received unrestricted research support from Biogen Idec, Merck-Serono, Teva, Sanofi-Aventis, and Bayer-Schering, and is in the process of negotiating a research grant from Novartis.
CB, KA, MW and PT have nothing to declare.
References
- 1.Amato MP, Battaglia MA, Caputo D, et al. The costs of multiple sclerosis: A cross-sectional, multicenter cost-of-illness study in Italy. J Neurol 2002; 249: 152–163. [DOI] [PubMed] [Google Scholar]
- 2.Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life in multiple sclerosis in Europe: Method of assessment and analysis. Eur J Health Econ 2006; 7(Suppl 2): S5–S13. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez M, Siva A, Ward J, et al. Impairment, disability, and handicap in multiple sclerosis: A population-based study in Olmsted County, Minnesota. Neurology 1994; 44: 28–33. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi R. Fatigue associated with multiple sclerosis: Diagnosis, impact and management. Mult Scler 2003; 9: 219–227. [DOI] [PubMed] [Google Scholar]
- 5.Beiske AG, Pedersen ED, Czujko B, et al. Pain and sensory complaints in multiple sclerosis. Eur J Neurol 2004; 11: 479–482. [DOI] [PubMed] [Google Scholar]
- 6.Patten SB, Beck CA, Williams JV, et al. Major depression in multiple sclerosis: A population-based perspective. Neurology 2003; 61: 1524–1527. [DOI] [PubMed] [Google Scholar]
- 7.Ruggieri RM, Palermo R, Vitello G, et al. Cognitive impairment in patients suffering from relapsing–remitting multiple sclerosis with EDSS < or = 3.5. Acta Neurol Scand 2003; 108: 323–326. [DOI] [PubMed] [Google Scholar]
- 8.Shahrbanian S, Auais M, Duquette P, et al. Does pain in individuals with multiple sclerosis affect employment? A systematic review and meta-analysis. Pain Res Manag 2013; 18: e94–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg J, Lindgren P, Fredrikson S, et al. Costs and quality of life of multiple sclerosis in Sweden. Eur J Health Econ 2006; 7(Suppl 2): S75–S85. [DOI] [PubMed] [Google Scholar]
- 10.Kobelt G, Berg J, Lindgren P, et al. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry 2006; 77: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinghög P, Hillert J, Kjeldgård L, et al. High prevalence of sickness absence and disability pension among multiple sclerosis patients: A nationwide population-based study. Mult Scler 2013; 19: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 12.Boiko A, Vorobeychik G, Paty D, et al. Early onset multiple sclerosis: A longitudinal study. Neurology 2002; 59: 1006–1010. [DOI] [PubMed] [Google Scholar]
- 13.Ghezzi A, Pozzilli C, Liguori M, et al. Prospective study of multiple sclerosis with early onset. Mult Scler 2002; 8: 115–118. [DOI] [PubMed] [Google Scholar]
- 14.Simone IL, Carrara D, Tortorella C, et al. Course and prognosis in early-onset MS: Comparison with adult-onset forms. Neurology 2002; 59: 1922–1928. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SR, McLeod JG, Macaskill P, et al. Multiple sclerosis in Australia: Prognostic factors. J Clin Neurosci 2000; 7: 16–19. [DOI] [PubMed] [Google Scholar]
- 16.Degenhardt A, Ramagopalan SV, Scalfari A, et al. Clinical prognostic factors in multiple sclerosis: A natural history review. Nat Rev Neurol 2009; 5: 672–682. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 18.Nortvedt MW, Riise T, Myhr KM, et al. Quality of life in multiple sclerosis: Measuring the disease effects more broadly. Neurology 1999; 53: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell AJ, Benito-Leόn J, González JM, et al. Quality of life and its assessment in multiple sclerosis: Integrating physical and psychological components of wellbeing. Lancet Neurol 2005; 4: 556–566. [DOI] [PubMed] [Google Scholar]
- 20.Sweden S. Rikets indelningar: årsbok över regionala indelningar med koder, postadresser, telefonnummer m m. 2003 [Country classifications: Yearbook of regional classifications with codes, postal addresses, phone numbers, etc. 2003] Statistics Sweden, 2003.
- 21.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001; 29: 374–393. [Google Scholar]
- 22.Jones B. Proc traj, http://www.andrew.cmu.edu/user/bjones/documentation.htm (accessed January 2014).
- 23.Nagin D, Tremblay RE. Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Dev 1999; 70: 1181–1196. [DOI] [PubMed] [Google Scholar]
- 24.Côté S, Tremblay RE, Nagin D, et al. The development of impulsivity, fearfulness, and helpfulness during childhood: Patterns of consistency and change in the trajectories of boys and girls. J Child Psychol Psychiatry 2002; 43: 609–618. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson NE, Carstensen JM, Gjesdal S, et al. Risk factors for disability pension in a population-based cohort of men and women on long-term sick leave in Sweden. Eur J Public Health 2008; 18: 224–231. [DOI] [PubMed] [Google Scholar]
- 26.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: An overview. Brain Pathol 2007; 17: 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature 1999; 399(6738 Suppl): A40–A47. [DOI] [PubMed] [Google Scholar]
- 28.Alexanderson K, Norlund A. Swedish Council on Technology Assessment in Health Care (SBU). Chapter 1. Aim, background, key concepts, regulations, and current statistics. Scand J Public Health Suppl 2004; 63: 12–30. [DOI] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundström P, Nyström L, Svenningsson A, et al. Sick leave and professional assistance for multiple sclerosis individuals in Vasterbotten County, northern Sweden. Mult Scler 2003; 9: 515–520. [DOI] [PubMed] [Google Scholar]