Abstract
Purpose
Spinal cord injury (SCI) increases the risk of osteoporosis and low-impact fractures, particularly at the knee. However, during the chronic phase of SCI, the natural history and factors associated with longitudinal change in bone density remain poorly characterized. In this study we prospectively assessed factors associated with change in bone density over a mean of 21 months in 152 men and women with chronic SCI.
Methods
A mixed model procedure with repeated measures was used to assess predictors of change in bone mineral density (PROC MIXED) at the distal femur and proximal tibia. Factors with a p value of <0.10 in the univariate mixed models, as well as factors that were deemed clinically significant (gender, age, and walking status) were assessed in multivariable models. Factors with a p value of ≤0.05 were included in the final model.
Results
We found no association between bone loss and traditional osteoporosis risk factors, including age, gender, body composition, or vitamin D level or status (normal or deficient). In both crude and fully adjusted models, wheelchair users lost bone compared to walkers. Similarly, statin users gained bone compared to nonusers.
Conclusions
The statin finding is supported by reports in the general population where statin use has been associated with a reduction in bone loss and fracture risk. Our results suggest that both walking and statins may be effective osteogenic therapies to mitigate bone loss and prevent osteoporosis in chronic SCI. Our findings also suggest that loss of mechanical loading and/or neuronal factors contribute more to disuse osteoporosis that traditional osteoporosis risk factors.
Keywords: Osteoporosis, Statin, Spinal Cord Injury, Rehabilitation Medicine
INTRODUCTION
An estimated 12,500 new cases of traumatic spinal cord injury (SCI) occur in the US each year. The prevalence has increased from 207,000 cases in 1994 to roughly 276,000 cases in 2014 due to improvements in medical care and greater survival [1]. Long-term follow-up data suggest that, if untreated, more than 30% of these individuals will sustain a low-impact fracture at some point following their injury [2]. Fractures have serious health implications in SCI since they severely reduce independence and mobility and result in significant medical complications and increased mortality [3–5]. Despite this, few SCI-specific risk factors for bone loss have been described and SCI-specific fracture risk prediction tools have yet to be developed.
In the general population, age, gender, body composition, nutritional status, and hormonal status are established or “traditional” risk factors for bone loss. In contrast, lack of mechanical loading of the paralyzed extremities is thought to be the primary contributor to SCI-induced osteoporosis. However, no large, prospective studies have examined the role of ambulatory status on longitudinal bone loss after SCI. Similarly, while it has been speculated that traditional risk factors are important after SCI, this has not been demonstrated in prospective observational studies.
Understanding the natural history and risk factors for chronic bone loss following SCI is essential to designing therapies to reduce bone loss, define fracture risk, and ultimately prevent osteoporotic fractures and their associated morbidity. Therefore, in this study we sought to identify clinical and demographic characteristics associated with longitudinal change in bone density in individuals with SCI. Bisphosphonates are commonly prescribed to treat osteoporosis and inhibit osteoclast activity by blocking the farnesyl diphosphate synthase in the mevalonate pathway. Statins are another widely prescribed class of medications that also inhibit the mevalonate pathway by blocking HMG-CoA reductase. Several recent meta-analyses concluded statins are osteoprotective in the general population [6, 7], however there are no reports examining statins and bone health after SCI. Therefore, we also assessed the association between statin use and bone loss following paralysis. We hypothesized that wheelchair use would be more strongly associated with bone loss after SCI than traditional osteoporosis risk factors, including age and gender. We also hypothesized that, after adjusting for confounders, regular statin users would lose less bone than nonusers.
MATERIALS AND METHODS
Subjects
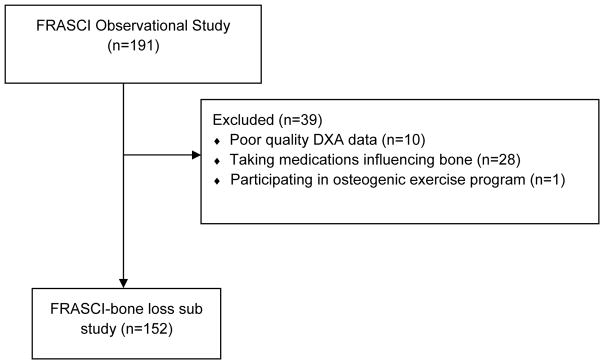
For this bone loss sub study, we assessed participants with chronic SCI who were enrolled in the longitudinal Fracture Risk after SCI (FRASCI) Study. Study inclusion criteria and recruitment methods for the parent cohort study have previously been described [8, 9]. Briefly, participants were eligible if they were 22 years of age or older, one or more years after injury, were not ventilator dependent, did not have a tracheostomy, and had no other neuromuscular disease. 191 participants with SCI were enrolled in this cohort between August 2009 and April 2012 and completed two study visits. We excluded 10 subjects because bone density could not be determined at the distal femur or proximal tibia due to knee replacement, fixation rods, heterotrophic ossification, spasms, or contractures preventing proper scan positioning. We excluded 28 participants actively taking medications known to influence bone metabolism (bisphosphonates (n=18), anti-seizure medications (n=7), or hormones (n=3)). We excluded 1 subject actively participating in an exercise-based clinical trial to improve bone health. The final cohort for this bone loss sub study (FRASCI-bone loss) consisted of 152 men and women with SCI (Figure 1).
Figure 1.
Motor score
Motor level and completeness of injury were confirmed by physical exam at study entry by a trained rater according to the American Spinal Injury Association Impairment Scale (AIS). Participants were classified as AIS A or B (motor complete, no motor function below the neurological level of injury); AIS C (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level are not strong enough to overcome gravity); or AIS D (motor incomplete, motor function preserved below the neurological level, and more than half the key muscles below the neurological level strong enough to overcome gravity). Injury severity was then classified in 2 categories: motor complete SCI (AIS A/B) or motor incomplete SCI (AIS C or D).
Dual X-ray Absorptiometry (DXA) for Bone Mineral Density (BMD) and Body Composition
We used a 5th generation GE Healthcare iDXA dual x-ray absorptiometry (DXA) scanner with enCore configuration version 12.3 to determine bone density and to assess body composition at baseline and follow-up. Total fat mass (kg) and total lean mass (kg) were calculated by the system software from whole body scans. Fractures are most common at the knee (distal femur or proximal tibia) after SCI. Therefore, bone density was determined at both SCI-specific (distal femur and proximal tibia) and standard (femoral neck and total hip) skeletal sites as previously described [10]. As a standard procedure, a phantom supplied by the manufacturer providing 3 different densities (0.5, 1.0, and 1.5 g/cm2) was measured daily and accuracy was within 0.003 gm/cm2. The RMS-CV was 2.3%, and RMS-SD was 0.012 g/cm2 at the distal femur. At the proximal tibia, the RMS-CV was 2.4%, and the RMS-SD was 0.028 g/cm2.
Biochemical Analyses
Subjects were asked to undergo testing in a fasting state and efforts were made to collect samples in the morning before a meal. For subject safety, individuals were advised to have a light meal or snack if fasting could worsen a medical condition (orthostatic hypotension). In all cases information was collected on time since last meal or snack. Plasma samples were drawn into an EDTA tube and immediately delivered to the core blood research laboratory at our facility. The samples were centrifuged for 15 min at 2600 rpm (1459 × g) at 4°C and stored at −80°C until batch analysis. All biochemical analyses were performed at the Clinical & Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children’s Hospital in Boston, a state-of-the-art reference laboratory that specializes in micro-analysis. Assays were performed in duplicate and any duplicate with >10% CV was repeated. Total osteocalcin was measured as an indicator of bone formation by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) with a detection limit of 0.50 ng/mL. C-telopeptide was measured as an indicator of bone resorption by electrochemiluminescence immunoassay on a 2010 Elecsys autoanalyzer (Roche Diagnostics, Indianapolis, IN) with a detection limit of 0.01 ng/mL. 25 OH vitamin D was quantified by high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) using an API-5000 (AB Sciex, Foster City, CA) with a detection limit of 1.0 ng/ml.
Variable Definition
Information regarding SCI, medical history, medication use, and fracture history was obtained by questionnaire at the time of baseline testing and was updated on follow-up testing. Participants were weighed and supine length measured for the calculation of body mass index (BMI). In subjects with severe joint contractures, length was self-reported (n=8). Age and body mass index (BMI) were considered as continuous variables. For fracture history, information was collected on timing (before SCI, at time of SCI, or after SCI) location, and cause of fracture. Fractures were then categorized as osteoporotic (ie, those occurring from standing height or less or in the absence of trauma) or traumatic fracture as previously described [2, 8, 11]. Fractures occurring prior to baseline testing were considered prevalent and those occurring after baseline testing and before follow-up testing were considered incident fractures. Digit and rib fractures were excluded. When available, medical records were used to confirm self-reported fracture history (35 out of 52 fractures). Osteoporotic fractures that occurred after SCI were considered in the analysis. Usual mobility mode (more than 50% of the time) was considered in the following 2 categories: wheelchair use (motorized wheelchair or hand-propelled wheelchair) or walking (with aid such as crutch, cane or walk without assistance). For post menopausal women and men age 50 or older, T-score was used to classify hip bone density (total hip and femoral neck) according to the World Health Organization (WHO) definitions of normal (T-score ≥−1), osteopenia (T-score <−1 and >−2.5) and osteoporosis (T-score ≤−2.5). For premenopausal women and men under the age of 50, Z-score was used to classify bone density at the hip as normal (Z-score >−2) or as lower than expected for age and sex (Z-score ≤ −2). Statins were categorized as lipophilic (atorvastatin, lovastatin, simvastatin, n=44) or hydrophilic (pravastatin and rosuvastatin, n=12). Because lipophilic statins have greater bioavailability within the bone micro environment, we limited our study to active lipophilic statin users. Therefore, current statin users were defined as those using a lipophilic statin for at least 1 year prior to the follow-up visit.
Statistical Analysis
All analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC). The mean of triplicate BMD determinations at the proximal tibia and distal femur was calculated, and change in BMD at each site was calculated by subtracting mean BMD at baseline from BMD at follow-up and dividing by time between scans. To account for the multiple BMD determinations measured within the same person, a mixed model procedure with repeated measures with unstructured correlation was used to assess predictors of change in BMD (PROC MIXED) at SCI-specific skeletal sites (distal femur and proximal tibia). Factors with a p value of <0.10 in the univariate mixed models, as well as factors that were deemed clinically significant (gender, age, and walking status) were assessed in multivariable models. We included baseline BMD to assess the impact of adjusting for regression to the mean. Factors with a p value of ≤0.05 were included in the final model.
RESULTS
Subject Characteristics
Subject characteristics are presented in Table 1. Participants were aged 55.1 ± 14.4 (SD) years (ranged from 24.7 – 87.1) years, and were 17.9 ± 13.3 (1.0 – 58.0) years post-injury. The majority was male (87.5%) and white (86.8%). 60% of participants used a wheelchair as their primary mode of mobility and 48% had a motor complete injury. The mean BMI was 27.7±5.7 kg/m2 and 72.5% of participants had normal vitamin D levels (>20 ng/mL). 41.4% of participants had osteoporosis. 20.3% of all participants and 77% with motor complete SCI reported a prevalent post-SCI osteoporotic fracture (n=48 fractures) that occurred prior to baseline testing. Prevalent fractures were most common at the knee (distal femur, proximal tibia, or proximal fibula, n=20, 42%), followed by ankle (n=16, 33%), hip (n=9, 19%), humerus (n=2, 4%), and pelvis (n=1, 2%). Fall was the most common cause, followed by physiologic ranging/loading of the joint (23%), rolling over in bed (4%), twisting the leg during a transfer (2%), or of unknown cause (2%). 2 (1.3%) participants reported a fall-related post-SCI osteoporotic incident fracture (one wrist fracture and one proximal tibia fracture). 44 (29%) participants were active statin users (taking a lipophilic statin for at least 1 year prior to the follow-up visit). Simvastatin was the most commonly used statin (Table 3, 84.1%), followed by atorvastatin and lovastatin (9.1% and 6.8%, respectively). The mean duration of simvastatin use was 7.1 years and ranged from 1.7 to 21.5 years. There was no difference in change in either total body (−0.19±2.1 kg statin users vs −0.22±2.5 kg non users, p=0.96) or lower extremity lean mass (−0.01±1.6 kg statin users vs −0.05±1.4 kg non users, p=0.89) based on statin use, suggesting statin use was not associated with loss of lean mass. 55% of statin users ambulated independently versus 45% of statin nonusers.
Table 1.
FRASCI-bone loss cohort participant characteristics
| Variable | n=152 |
|---|---|
|
| |
| Age (years) [Mean ± SD] | 55.1 ± 14.4 |
|
| |
| Years since injury [Mean ± SD] | 17.9 ± 13.3 |
|
| |
| Male, n(%) | 133 (87.5) |
|
| |
| White, n(%) | 132 (86.8) |
|
| |
| ASIA level | |
| Motor complete: | |
| • A/B, n(%) | 74 (48.7) |
| Motor incomplete: | |
| • C, n(%) | 13 (8.5) |
| • D, n(%) | 65 (42.8) |
|
| |
| Wheelchair users, n(%) | 91 (59.9) |
|
| |
| BMI (kg/m2) [Mean ± SD] | 27.7 ± 5.7 |
|
| |
| Total body mass (kg) [Mean ± SD] | 86.4 ± 19.4 |
|
| |
| Percent total fat [Mean ± SD] | 37.4 ± 8.3 |
|
| |
| Total lean mass (kg) [Mean ± SD] | 51.6 ± 10.0 |
|
| |
| Change in total body mass (kg) [Mean ± SD] | −0.67 ± 5.6 |
|
| |
| Change in total lean mass (kg) [Mean ± SD] | −0.21 ± 2.4 |
|
| |
| 25 OH Vitamin D (ng/mL)1 [Mean ± SD] | 23.9 ± 7.8 |
| • Deficient (<20 ng/mL) n(%) | 41 (27.5) |
| • Normal (>20 ng/mL) n(%) | 108 (72.5) |
|
| |
| Baseline BMD (g/cm2) [Mean ± SD] | |
| SCI-specific skeletal sites | |
| • Distal femur | 0.783 ± 0.218 |
| • Proximal tibia | 0.844 ± 0.291 |
| Traditional sites2 | |
| • Femoral neck | 0.863 ± 0.196 |
| • Total hip | 0.884 ± 0.235 |
|
| |
| Baseline hip bone density classification2, n(%) | |
| • Normal/Osteopenia | 82 (58.6) |
| • Osteoporosis/BMD lower than expected | 58 (41.4) |
| • Hip BMD not available | 12 |
|
| |
| Post SCI osteoporotic prevalent fracture history, n(%) | |
| • Yes | 31 (20.3) |
| • No | 121 (79.7) |
| Post SCI osteoporotic incident fracture, n(%) | 2 (1.3) |
|
| |
| Markers of bone turnover (ng/mL)3 [Mean ± SD] | |
| • C-telopeptide | 0.33 ± 0.14 |
| • Osteocalcin | 19.6 ± 6.9 |
Vitamin D biomarkers available for n=149
Hip BMD available for n=140
Biomarkers available for n=144
Table 3.
Lipophilic statin frequency and duration of use
| Lipophilic statin type | Frequency (n=44) | Mean years of statin use* (range) |
|---|---|---|
| Atorvastatin (Lipitor) | 4 (9.1%) | 8.9 (4.0–16.5) |
| Lovastatin (Mevacor) | 3 (6.8%) | 8.7 (3.7–13.6) |
| Simvastatin (Zocor) | 37 (84.1%) | 7.1 (1.7–21.5) |
Based on length of use through follow-up date
Clinical Factors Associated with Change in Lower Extremity Bone Density
The mean time to follow-up testing was 21 months (range 16–44 months). In univariate mixed model analyses at the knee (Table 2), there was no association between longitudinal change in bone density at the knee and age, gender, injury duration, baseline BMI, total body weight, percent total body fat, vitamin D level or status (deficient versus normal), SCI motor completeness, or walking status (p=0.10–0.98). Statin use, baseline BMD, and change in total body mass were significantly associated with change in bone density at the knee (p=0.07, p=0.003 and p=0.001, respectively).
Table 2.
Univariate factors associated with mean change in bone density* (%/year)
| Variable | β ± SE | p |
|---|---|---|
|
| ||
| Age (years) | 0.011 ± 0.022 | 0.63 |
|
| ||
| Injury duration (years) | 0.011 ± 0.024 | 0.65 |
|
| ||
| BMI (kg/m2) | −0.009 ± 0.056 | 0.87 |
|
| ||
| Total body mass (kg) | 0.00037 ± 0.016 | 0.98 |
|
| ||
| Change in total body mass (kg) | 0.18 ± 0.055 | 0.0011 |
|
| ||
| Percent total body fat | −0.038 ± 0.038 | 0.31 |
|
| ||
| Vitamin D (ng/mL)1 | −0.065 ± 0.039 | 0.10 |
|
| ||
| Baseline BMD* (g/cm2) | −3.46 ± 1.16 | 0.0034 |
|
| ||
| Mean change in BMD (%/year) ± SE | p | |
|
| ||
| Gender | 0.64 | |
| • Female | −0.571 ± 0.898 | |
| • Male | −0.127 ± 0.339 | |
|
| ||
| Vitamin D status1 | 0.20 | |
| • Normal (≥20 ng/mL) | −0.379 ± 0.363 | |
| • Deficient (<20 ng/mL) | 0.510 ± 0.590 | |
|
| ||
| Current lipophilic statin use | 0.007 | |
| • Yes | 1.162 ± 0.576 | |
| • No | −0.722 ± 0.368 | |
|
| ||
| Motor completeness | 0.93 | |
| • Complete | −0.216 ± 0.455 | |
| • Incomplete | −0.156 ± 0.444 | |
|
| ||
| Walking status | 0.44 | |
| • Walk with or without aid | 0.110 ± 0.501 | |
| • Wheelchair user | −0.383 ± 0.410 | |
Mean bone density was obtained from a repeated measures regression model assessing the effects of each factor on distal femur and proximal tibia BMD within an individual
Biomarkers available for n=149
In a multivariable mixed model at the knee (Table 4), baseline knee BMD (p=0.0002), change in total body weight (p=0.005) and statin use (p=0.01) remained significantly associated with change in BMD. When removing baseline BMD from the analysis the statin effect remains significant (p=0.02).
Table 4.
Multivariable mixed model of factors associated with percent change in bone density* (%/year)
| Crude model | Fully adjusted model* | Fully adjusted male only model** | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Mean Change in BMD (%/year) [95% CI] | p | Mean Change in BMD (%/year) [95% CI] | p | Mean Change in BMD (%/year) [95% CI] | p |
|
| ||||||
| Difference in Statin use | ||||||
| Statin user | 1.64 [0.30, 2.99] | 0.01 | 1.92 [0.43, 3.42] | 0.01 | 1.96 [0.36, 3.56] | 0.02 |
| Non user | reference | reference | reference | |||
|
| ||||||
| Difference in Walking Status | ||||||
| Wheelchair user | −1.45 [−2.86, −0.04] | 0.04 | −1.75 [−3.22, −0.28] | 0.02 | −1.72 [−3.36, −0.08] | 0.04 |
| Walker | reference | reference | reference | |||
|
| ||||||
| Baseline BMD (g/cm2) | −4.84 [−7.37, −2.30] | 0.0002 | −4.90 [−7.44, −2.35] | 0.0002 | −5.14 [−7.86, −2.44] | 0.0003 |
|
| ||||||
| Change in total body mass (kg) | 0.15 [0.05, 0.26] | 0.005 | 0.17 [0.06, 0.28] | 0.002 | 0.17 [0.05, 0.28] | 0.005 |
adjusted for age (p=0.21) and gender (p=0.28)
adjusted for age (p=0.31)
Given their clinical relevance to bone metabolism, age, injury completeness, walking status, and gender were individually assessed in multivariate mixed models with baseline knee BMD, change in total body weight, and statin use. In a multivariable mixed model assessing change in BMD at the knee (Table 4, crude model), a greater baseline knee BMD (p=0.0002) and wheelchair use (p=0.04) were associated with bone loss. Increases in total body weight (p=0.005) and statin use compared to no statin use (p=0.01) were significantly associated with an increase in BMD. Statin users gained BMD compared to non-users at a rate of 1.64% (95%CI 0.30% to 2.99%) per year. Wheelchair users lost BMD compared to walkers at a rate of 1.45% (95% CI −2.86% to −0.04%) per year. There was no association between age, gender, or injury completeness with bone loss. The results were similar when adjusting for age and gender or excluding women from the analysis (Table 4, fully adjusted and male only models).
DISCUSSION
We prospectively assessed factors associated with change in bone density at the knee over a period of 16 to 44 months in 152 individuals with SCI. There is no consensus-based protocol for DXA scanning at the knee. However, in this study we used a well-validated knee DXA method used in prior biomarker and bone strength studies in SCI [2, 8–12]. We found no association between traditional osteoporosis risk factors, including age, gender, body weight, %-total fat, vitamin D level, or vitamin D status (normal or deficient) and change in bone density at the knee. In univariate and multivariable analyses that included adjustment for baseline BMD at the knee, wheelchair use was associated with bone loss compared to walking while lipophilic statin use for one year or more was associated with a gain in BMD compared to no statin use. We found no association between change in BMD and SCI motor completeness, suggesting that ambulatory status contributes more to bone health than injury severity after SCI. An association between hydrophilic statin users and bone loss could not be assessed in the current study, as there were only 12 active hydrophilic statin users in this cohort.
The FRASCI study is the largest longitudinal study of disuse osteoporosis and the first to assess the impact of multiple risk factors for bone loss during the chronic phase of SCI. Although we studied participants for a relatively short time compared to their overall injury duration, we were still able to detect effects of lipophilic statin use and differences in ambulatory mode. In addition, as our cohort included participants whose follow-up began at different times after injury, adjustment for initial BMD allowed us to account for differences in bone loss trajectories, since our results indicate that persons with higher BMD values are at risk for greater degrees of bone loss. The differences we detected based on statin use (1.5–1.8%) are of the same magnitude reported in both cohort-based studies [13] and clinical trials [14, 15]. Moreover, our finding of a significant difference in bone loss between wheelchair users and walkers demonstrates the importance of mechanical loading or the lack thereof on bone metabolism following lower extremity paralysis. Contrary to the general population, age and gender were not risk factors for longitudinal bone loss in this study. We did not find associations between traditional risk factors and rates of bone loss in this study, consistent with prior reports that distinguish SCI-induced osteoporosis from postmenopausal or age-related bone loss. Both men and women of all ages develop severe osteoporosis following SCI with rapid bone loss ensuing immediately after cessation of walking due to paralysis. While 40% sublesional bone loss is common within the first 3 years after SCI, bone loss of this magnitude is rare in postmenopausal or age related osteoporosis. Our findings suggest that mechanical loading, and the loss thereof, is an important determinant of bone health following paralysis. It is possible that a weaker association exists between bone loss and traditional osteoporosis risk factors, such as age and gender, which might be detected by a larger study. Further work is needed to determine this. There were few women in the cohort, limiting our ability to study the effect of gender. It is also possible that a longer duration of follow-up was necessary to demonstrate an effect of aging in this population, as compared to stronger effects of statin use and differences in walking status. We also identified a potential protective bone effect of lipophilic statin use. There was a significant difference in longitudinal change in bone density based on statin use as non statin-users lost bone and lipophilic statin users did not. This perhaps suggests an anabolic effect on bone as has been suggested by prior studies. As questions have been raised about relationships between statin use and bone health being explained by differences in the health status of statin users and nonusers [16], our study design permitted us to adjust for factors that may represent these differences [17]. We were able to adjust for factors reflecting health status, such as age, SCI motor completeness, and weight.
A positive association between lipophilic statin use and bone density has been reported in cross-sectional studies of post-menopausal women [18, 19] and older men [13]. However, there is controversy in the literature regarding statin use and longitudinal change in bone density. A one-year randomized controlled trial of 40mg simvastatin failed to demonstrate significant increases in bone density in post-menopausal women with no known hypercholesterolemia at study entry [15]. Similarly, an 8-week course of 20mg daily atorvastatin did not cause significant changes in markers of bone turnover in post-menopausal women compared to placebo [20]. However, the remodeling index (ratio of C-telopeptide to osteocalcin) was reduced. This effect was seen only in participants age 62 or older in the atorvastatin group, suggesting an age-dependent effect of statins on bone turnover. An anabolic bone effect was reported in hypercholesterolemic post-menopausal women taking 40 mg of simvastatin for 1 year [14] or 2 years [21]. Similarly, statin use has been associated with decreased risk of hip fracture [22] and vertebral fracture [23] in a dose dependent fashion. Recent meta-analyses conclude that statins maintain and improve bone density in the general population and recommend prospective randomized clinical trials to confirm this in different populations [6, 7]. We assessed associations between statin use and longitudinal bone loss in a unique population predominantly of men predisposed to severe osteoporosis. These findings are in line with other observational findings and must be confirmed in large, well-controlled randomized clinical trials.
In the current study the majority of statin users were actively taking the lipophilic statin simvastatin. Statins have low bioavailability in bone, but lipophilic statins may have greater capacity to enter bone cells than hydrophilic statins [24]. Statins competitively inhibit HMG-CoA reductase to block mevalonic acid synthesis. Inhibition of downstream products of mevalonic acid, including farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP), indirectly blocks farnesylation and/or geranylgeranylation of small G proteins. Small G proteins regulate gene expression, cytokine production, and vesicular trafficking. The osteogenic activity of statins has been attributed to both activation and inhibition of Ras/Rho family small G proteins. Varying statin doses may preferentially affect specific small G proteins with different effects on bone cell activities. In vitro studies have demonstrated inhibition of both osteoclast differentiation and osteoblast apoptosis following statin treatment [24–27].
Cardiovascular disease is a leading cause of mortality following SCI [28–34]. While statins are routinely prescribed to optimize lipid profiles and reduce cardiovascular risk, they are known to have pleiotroipic effects in the nervous system, immune system, and the skeleton. Statins are also thought to have neuroprotective properties that are independent of their lipid-lowering activity with improved outcomes reported in neurological disorders including Parkinson’s disease [35], Alzheimer’s disease [36], hemorrhagic [37] and ischemic stroke [38], and in rodent models of spinal cord injury, multiple sclerosis, and traumatic brain injury [39–41]. The neuroprotective effect of statins may be due to prevention of secondary neurodegeneration thereby preserving function. There are no reports in the literature of randomized control trials to determine the effect of statins on any outcome (cardiovascular, neuroprotection, or osteogenic) following SCI.
There are limitations of the current study that should be considered. We only studied bone loss at the knee and therefore these findings may not apply to other skeletal sites. As this was designed as an observational study, statin use is by self-report and lipid profiles were not assessed. Also, our findings are likely reflective of simvastatin use and therefore should be confirmed in other lipophilic statins. Statin-induced myositis is a concern, especially for individuals with reduced strength following SCI. Based on our study design, we have no information on side effects associated with statin use. However, there was no difference in change in either total body or lower extremity lean mass based on statin use. As has been suggested in other observational studies of statin use and bone health, it is possible that our findings reflect selection bias and residual confounding. However, we did assess many traditional predictors of bone loss including age, gender, vitamin D status, and body composition, suggesting this is not the case. Additionally, in this study the post-SCI osteoporotic fracture incidence was 1.3% while 20% of all participants and 77% of those with motor complete SCI reported prevalent post-SCI osteoporotic fractures. The majority of these fractures were at the knee (distal femoral metaphysis or proximal tibial metaphysis) and the location and fracture rates are similar to prior reports [2, 42]. However, there were too few fractures to assess the association between statin use and incident fracture, likely because of the relatively short observation period. Although a prospective study, these findings are hypothesis generating and must be confirmed in a well-designed randomized clinical trial.
Despite these study limitations, when considered in conjunction with recent meta-analyses, our findings support clinical trials to prospectively confirm the osteogenic benefits of statins and to identify optimal dosing in men and women with SCI. Our findings also suggest that therapies aimed at preserving the ability to ambulate, that introduce technology-assisted ambulation, or a combination of both may be osteoprotective after SCI.
Acknowledgments
We thank Rachael Burns and Kara Loo, research assistants, Boston VA Healthcare System, for collection of anthropometric data, and Merilee Teylan, Boston VA Healthcare System, analyst, for assistance with data cleaning and database management.
Footnotes
DISCLOSURES
Leslie Morse, Nguyen Nguyen, Ricardo Battaglino, A J Guarino, David Gagnon, Ross Zafonte, and Eric Garshick declare that they have no conflict of interest. This study received support from: The National Institute of Arthritis and Musculoskeletal and Skin Diseases [1R01AR059270-01], National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR 90SI5007-01-02), The Office of Research and Development, Rehabilitation Research and Development [Merit Review Grant B6618R and RX-000792-01A2], and The Massachusetts Veterans Epidemiology Research and Information Center, Cooperative Studies Program, Department of Veterans Affairs
References
- 1.National Spinal Cord InjuryStatistical C. Spinal Cord Injury Facts and Figures at a Glance. J Spinal Cord Med. 2014;37:355–356. doi: 10.1179/1079026814Z.000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone. 2012;51:600–605. doi: 10.1016/j.bone.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carbone LD, Chin AS, Burns SP, Svircev JN, Hoenig H, Heggeness M, Weaver F. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos Int. 2013;24:2261–2267. doi: 10.1007/s00198-013-2295-8. [DOI] [PubMed] [Google Scholar]
- 4.Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20:385–392. doi: 10.1007/s00198-008-0671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morse LR, Giangregorio L, Battaglino RA, Holland R, Craven BC, Stolzmann KL, Lazzari AA, Sabharwal S, Garshick E. VA-based survey of osteoporosis management in spinal cord injury. PM R. 2009;1:240–244. doi: 10.1016/j.pmrj.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Zhu LP, Yang XL, Huang HL, Ye DQ. HMG-CoA reductase inhibitors (statins) and bone mineral density: a meta-analysis. Bone. 2013;54:151–156. doi: 10.1016/j.bone.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone. 2007;40:1581–1587. doi: 10.1016/j.bone.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Doherty AL, Battaglino RA, Donovan J, Gagnon D, Lazzari AA, Garshick E, Zafonte R, Morse LR. Adiponectin is a candidate biomarker of lower extremity bone density in men with chronic spinal cord injury. J Bone Miner Res. 2014;29:251–259. doi: 10.1002/jbmr.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morse LR, Sudhakar S, Lazzari AA, Tun C, Garshick E, Zafonte R, Battaglino RA. Sclerostin: a candidate biomarker of SCI-induced osteoporosis. Osteoporos Int. 2013;24:961–968. doi: 10.1007/s00198-012-2072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse LR, Lazzari AA, Battaglino R, Stolzmann KL, Matthess KR, Gagnon DR, Davis SA, Garshick E. Dual energy x-ray absorptiometry of the distal femur may be more reliable than the proximal tibia in spinal cord injury. Arch Phys Med Rehabil. 2009;90:827–831. doi: 10.1016/j.apmr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan CO, Battaglino RA, Doherty AL, Gupta R, Lazzari AA, Garshick E, Zafonte R, Morse LR. Adiponectin is associated with bone strength and fracture history in paralyzed men with spinal cord injury. Osteoporos Int. 2014;25:2599–2607. doi: 10.1007/s00198-014-2786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA. Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res. 2012;27:352–359. doi: 10.1002/jbmr.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleicher K, Cumming RG, Naganathan V, Seibel MJ, Sambrook PN, Blyth FM, Le Couteur DG, Handelsman DJ, Creasey HM, Waite LM. Lifestyle factors, medications, and disease influence bone mineral density in older men: findings from the CHAMP study. Osteoporos Int. 2011;22:2421–2437. doi: 10.1007/s00198-010-1478-9. [DOI] [PubMed] [Google Scholar]
- 14.Montagnani A, Gonnelli S, Cepollaro C, Pacini S, Campagna MS, Franci MB, Lucani B, Gennari C. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32:427–433. doi: 10.1016/s8756-3282(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 15.Rejnmark L, Buus NH, Vestergaard P, Heickendorff L, Andreasen F, Larsen ML, Mosekilde L. Effects of simvastatin on bone turnover and BMD: a 1-year randomized controlled trial in postmenopausal osteopenic women. J Bone Miner Res. 2004;19:737–744. doi: 10.1359/JBMR.040209. [DOI] [PubMed] [Google Scholar]
- 16.Ray WA, Daugherty JR, Griffin MR. Lipid-lowering agents and the risk of hip fracture in a Medicaid population. Inj Prev. 2002;8:276–279. doi: 10.1136/ip.8.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCandless LC. Statin use and fracture risk: can we quantify the healthy-user effect? Epidemiology. 2013;24:743–752. doi: 10.1097/EDE.0b013e31829eef0a. [DOI] [PubMed] [Google Scholar]
- 18.Edwards CJ, Hart DJ, Spector TD. Oral statins and increased bone-mineral density in postmenopausal women. Lancet. 2000;355:2218–2219. doi: 10.1016/s0140-6736(00)02408-9. [DOI] [PubMed] [Google Scholar]
- 19.Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC Geelong Osteoporosis S. Statin use, bone mineral density, and fracture risk: Geelong Osteoporosis Study. Arch Intern Med. 2002;162:537–540. doi: 10.1001/archinte.162.5.537. [DOI] [PubMed] [Google Scholar]
- 20.Berthold HK, Unverdorben S, Zittermann A, Degenhardt R, Baumeister B, Unverdorben M, Krone W, Vetter H, Gouni-Berthold I. Age-dependent effects of atorvastatin on biochemical bone turnover markers: a randomized controlled trial in postmenopausal women. Osteoporos Int. 2004;15:459–467. doi: 10.1007/s00198-004-1598-1. [DOI] [PubMed] [Google Scholar]
- 21.Lupattelli G, Scarponi AM, Vaudo G, et al. Simvastatin increases bone mineral density in hypercholesterolemic postmenopausal women. Metabolism. 2004;53:744–748. doi: 10.1016/j.metabol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Rejnmark L, Vestergaard P, Mosekilde L. Statin but not non-statin lipid-lowering drugs decrease fracture risk: a nation-wide case-control study. Calcif Tissue Int. 2006;79:27–36. doi: 10.1007/s00223-006-0024-4. [DOI] [PubMed] [Google Scholar]
- 23.Schoofs MW, Sturkenboom MC, van der Klift M, Hofman A, Pols HA, Stricker BH. HMG-CoA reductase inhibitors and the risk of vertebral fracture. J Bone Miner Res. 2004;19:1525–1530. doi: 10.1359/JBMR.040607. [DOI] [PubMed] [Google Scholar]
- 24.Ruan F, Zheng Q, Wang J. Mechanisms of bone anabolism regulated by statins. Biosci Rep. 2012;32:511–519. doi: 10.1042/BSR20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282:4983–4993. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 26.Ohnaka K, Shimoda S, Nawata H, Shimokawa H, Kaibuchi K, Iwamoto Y, Takayanagi R. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287:337–342. doi: 10.1006/bbrc.2001.5597. [DOI] [PubMed] [Google Scholar]
- 27.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 28.Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81:723–728. doi: 10.1212/WNL.0b013e3182a1aa68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flank P, Wahman K, Levi R, Fahlstrom M. Prevalence of risk factors for cardiovascular disease stratified by body mass index categories in patients with wheelchair-dependent paraplegia after spinal cord injury. J Rehabil Med. 2012;44:440–443. doi: 10.2340/16501977-0964. [DOI] [PubMed] [Google Scholar]
- 30.Krum H, Howes LG, Brown DJ, Ungar G, Moore P, McNeil JJ, Louis WJ. Risk factors for cardiovascular disease in chronic spinal cord injury patients. Paraplegia. 1992;30:381–388. doi: 10.1038/sc.1992.87. [DOI] [PubMed] [Google Scholar]
- 31.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–152. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- 32.Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk factors in persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med. 2010;42:272–278. doi: 10.2340/16501977-0510. [DOI] [PubMed] [Google Scholar]
- 33.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Increased cardiovascular disease risk in Swedish persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42:489–492. doi: 10.2340/16501977-0541. [DOI] [PubMed] [Google Scholar]
- 34.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med. 2011;43:237–242. doi: 10.2340/16501977-0658. [DOI] [PubMed] [Google Scholar]
- 35.Sheng Z, Jia X, Kang M. Statin use and risk of Parkinson’s disease: A meta-analysis. Behav Brain Res. 2016;309:29–34. doi: 10.1016/j.bbr.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 36.Silva T, Teixeira J, Remiao F, Borges F. Alzheimer’s disease, cholesterol, and statins: the junctions of important metabolic pathways. Angew Chem Int Ed Engl. 2013;52:1110–1121. doi: 10.1002/anie.201204964. [DOI] [PubMed] [Google Scholar]
- 37.Wong GK, Liang M, Tan H, Lee MW, Po YC, Chan KY, Poon WS. High-dose simvastatin for aneurysmal subarachnoid hemorrhage: a multicenter, randomized, controlled, double-blind clinical trial protocol. Neurosurgery. 2013;72:840–844. doi: 10.1227/NEU.0b013e31828ab413. [DOI] [PubMed] [Google Scholar]
- 38.Miedema I, Uyttenboogaart M, Luijckx GJ. Statin use and lipid profile in relation to safety and functional outcome after thrombolysis in ischemic stroke. Expert Rev Neurother. 2012;12:907–910. doi: 10.1586/ern.12.88. [DOI] [PubMed] [Google Scholar]
- 39.Esposito E, Rinaldi B, Mazzon E, Donniacuo M, Impellizzeri D, Paterniti I, Capuano A, Bramanti P, Cuzzocrea S. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-alpha. J Neuroinflammation. 2012;9:81. doi: 10.1186/1742-2094-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han X, Yang N, Cui Y, Xu Y, Dang G, Song C. Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat. Neurosci Lett. 2012;521:136–141. doi: 10.1016/j.neulet.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 41.Shunmugavel A, Martin MM, Khan M, Copay AG, Subach BR, Schuler TC, Singh I. Simvastatin ameliorates cauda equina compression injury in a rat model of lumbar spinal stenosis. J Neuroimmune Pharmacol. 2013;8:274–286. doi: 10.1007/s11481-012-9419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]