Abstract
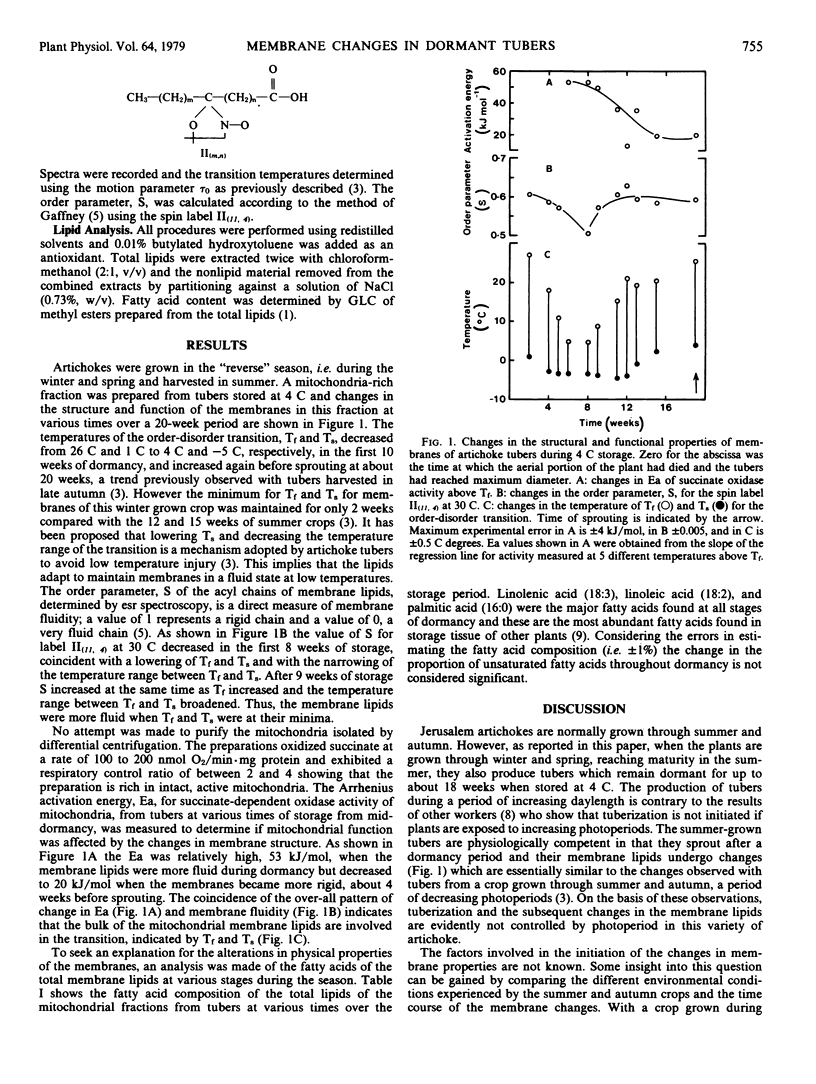
Changes in the temperature response, fluidity, function and the acyl fatty acid composition, were determined for a mitochondria-rich membrane fraction from Jerusalem artichoke (Helianthus tuberosus L.) tubers during dormancy for a crop which matured in midsummer. The temperature of both the upper and lower limits of the membrane lipid transition decreased during dormancy from 26 C and 1 C to 4 C and −5 C, respectively. This was similar to the changes observed with crops maturing in late autumn. The order parameter of a spin label intercalated into the membrane lipids decreased from about 0.6 to 0.5 during dormancy and returned to the original value before sprouting, showing that membrane fluidity increased during dormancy. The activation energy of succinate oxidase of tuber mitochondria was generally high at middormancy when membrane lipids were more fluid and decreased as the membranes became more rigid at the end of dormancy. The fatty acid composition of the membrane lipids did not alter significantly during dormancy. The results indicate that neither decreasing day length nor low soil temperature during tuber maturation is essential for the initiation of the membrane changes necessary for tubers to avoid low temperature injury during dormancy. The increase in membrane fluidity during dormancy could not be accounted for by an increase in the proportion of unsaturated fatty acids in the membrane lipids.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Smillie R. M. The effect of chloramphenicol and cycloheximide on lipid synthesis during chloroplast development in Euglena gracilis. Arch Biochem Biophys. 1970 Jul;139(1):179–189. doi: 10.1016/0003-9861(70)90059-7. [DOI] [PubMed] [Google Scholar]
- Chapman E., Wright L. C., Raison J. K. Seasonal Changes in the Structure and Function of Mitochondrial Membranes of Artichoke Tubers: A Requisite for Surviving Low Temperatures during Dormancy. Plant Physiol. 1979 Feb;63(2):363–366. doi: 10.1104/pp.63.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier G., Willemot C. Lipid changes in roots of frost hardy and less hardy alfalfa varieties under hardening conditions. Cryobiology. 1974 Aug;11(4):324–331. doi: 10.1016/0011-2240(74)90009-1. [DOI] [PubMed] [Google Scholar]
- Laggner P., Barratt M. D. The interaction of a proteolipid from sarcoplasmic reticulum membranes with phospholipids. A spin label study. Arch Biochem Biophys. 1975 Sep;170(1):92–101. doi: 10.1016/0003-9861(75)90100-9. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Functional properties of biological membranes: a physical-chemical approach. Prog Biophys Mol Biol. 1975;29(1):3–56. doi: 10.1016/0079-6107(76)90019-5. [DOI] [PubMed] [Google Scholar]
- Lyons J. M., Wheaton T. A., Pratt H. K. Relationship between the Physical Nature of Mitochondrial Membranes and Chilling Sensitivity in Plants. Plant Physiol. 1964 Mar;39(2):262–268. doi: 10.1104/pp.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchie E. J., Raison J. K. Membrane lipid fluidity and its effect on the activation energy of membrane-associated enzymes. Biochim Biophys Acta. 1979 Jul 5;554(2):364–374. doi: 10.1016/0005-2736(79)90377-8. [DOI] [PubMed] [Google Scholar]
- de la Roche I. A., Pomeroy M. K., Andrews C. J. Changes in fatty acid composition in wheat cultivars of contrasting hardiness. Cryobiology. 1975 Oct;12(5):506–512. doi: 10.1016/0011-2240(75)90032-2. [DOI] [PubMed] [Google Scholar]


