Abstract
There is considerable evidence from experimental studies in animals, as well as from clinical reports, that low-dose radiation hormesis is effective for the treatment of cancer and ulcerative colitis. In this study, we present 3 case reports that support the clinical efficacy of low-dose radiation hormesis in patients with these diseases. First, a patient with prostate cancer who had undergone surgical resection showed a subsequent increase in prostate-specific antigen (PSA). His PSA value started decreasing immediately after the start of repeated low-dose X-ray irradiation treatment and remained low thereafter. Second, a patient with prostate cancer with bone metastasis was treated with repeated low-dose X-ray irradiation. His PSA level decreased to nearly normal within 3 months after starting the treatment and remained at the low level after the end of hormesis treatment. His bone metastasis almost completely disappeared. Third, a patient with ulcerative colitis showed a slow initial response to repeated low-dose irradiation treatment using various modalities, including drinking radon-containing water, but within 8 months, his swelling and bleeding had completely disappeared. After 1 year, the number of bowel movements had become normal. Interest in the use of radiation hormesis in clinical practice is increasing, and we hope that these case reports will encourage further clinical investigations.
Keywords: low-dose radiation, hormesis, clinical trial, cancer, ulcerative colitis
Introduction
According to the “linear-no-threshold (LNT)” hypothesis, it has been thought that ionizing radiation is harmful to living organisms no matter how low the dose may be.1 However, recent studies have revealed that low-dose radiation can be beneficial to living organisms, since the mild oxidative stress induced by low-dose radiation induces adaptive responses, including enhanced cell damage repair/protection.
Various areas of the world have extraordinarily high radiation background levels, such as Guarapari (in Brazil), Kerala (in India), Ramsar (in Iran), Yangjiang (in China), and others.2-8 Many studies have been conducted to examine the health of residents in these areas, especially cancer incidence/death rates. Contrary to initial expectations, cancer incidence/death rates in these areas were lower than those of low background areas, and some were lower than those in average background areas. Reviews of epidemiological and other findings also indicate that cancer incidence after irradiation due to medical X-rays,9 contamination of houses with radioactive cobalt,10 radon exposure in houses,11,12 and nuclear shipyard workers,13-16 is much lower than would be expected based on LNT extrapolation. Indeed, Dobrzyński et al17 recently concluded that “claims that elevated natural background radiation levels lead to cancer or early childhood deaths are unjustified and misleading. The risk to the individual and society that is estimated by adhering to the LNT model is greater than the risk from doses and dose rates at which the LNT model cannot be validated!”17
There is extensive evidence that low-dose radiation is effective against cancer and inflammatory diseases in various animal models.18-31 Clinical trials also support efficacy in humans.32-38
In this article, we report 3 cases of radiation hormesis treatment in patients with prostate cancer, prostate cancer with bone metastasis, and ulcerative colitis.
Materials and Methods
X-Ray Irradiation
X-ray irradiation was applied at 20 to 50 mGy/min with a total dose of 150 mGy (125 kV, 3 mA, Winscope 2000; Toshiba Medical, Co, Japan). The distance from the body surface was 86 cm. The conditions were chosen in line with those used to treat patients with malignant lymphoma.36 In 1 case, irradiation was carried out 30 times in total at a frequency of once a week. In another case, the treatment was carried out 10 times in total at a frequency of 3 times a week.
Hormesis Room
The hormesis therapy room supplies both direct radiation from the wall surface and indirect radiation via inhalation of radon released into the air (Lead & Company Co, Yokohama, Japan). Released radon gas is retained by water sprinkled over the inside of the wall, which contains natural monazite excavated from a mountainous area in Japan. The average radiation dose rate is 11 μGy/h inside the room and the average concentration of radon is 9800 Bq/m3 (Figure 1). Temperature and humidity in the room are set to 39°C to 40°C and 70%, respectively. The patient usually remains in the room for 40 minutes.
Figure 1.

Hormesis room. The room is constructed using natural monazite excavated from a mountainous area in Japan. The average radiation level is 11 μGy/h at any point inside the room, even at a distance of 30 cm from the wall. The average concentration of radon is 9800 Bq/m3.
Radon Sheet
Radon sheet is a flexible silicone sheet containing monazite, 44 cm × 93 cm in size. Its radiation dose is about 37 μGy/h at the surface. In the clinic, it can be attached to the front and back of the body or laid under the bed for home medical treatment.
Radon-Containing Water
Radon-containing water was prepared by saturating tap water overnight with 222Rn from a radon ball (Lead & Company Co). The radioactivity of α-rays, measured with an ALPHA-Scint, TRACERLAB GmbH (Koeln, Germany), was about 400 Bq/L.
Treatment details of each patient are specified in the case reports. It should be noted that the treatments had no apparent side effects.
Results
Before visiting Takara Clinic, all 3 patients were receiving chemotherapy at other hospitals, but they did not show any improvement in their condition. Then, they visited Takara Clinic. Therefore, Clinician Takara suggested them a hormesis therapy and accepted this proposal. Consequently, he attempted radiation hormesis as an experimental treatment to these patients based on clinical data of Sakamoto36 and our animal experiment data.19-29
Radiation Treatment for Prostate Cancer
It was reported that repeated low-dose (0.5 Gy every 4 days; total dose 2 Gy) γ-irradiation significantly delayed tumor growth in mice implanted with Ehrlich tumor cells.19,20 The average tumor size of the irradiated mice was significantly smaller than that of the nonirradiated control mice at each time point, and the tumor size was reduced by 46% on the 30th day in the irradiated mice.
First case report
The patient was a 60-year-old man who had been diagnosed with prostate cancer and underwent extirpation of the prostate at a major hospital. The level of the tumor marker PSA declined after surgery but subsequently increased to over 5. The patient was extremely uneasy and visited the Takara Clinic. Radiation hormesis treatment was started with low-dose X-ray irradiation at a dose of 150 mGy once a week, 30 times in total, basically according to the method of Sakamoto.36 The PSA value started decreasing immediately after the start of treatment and had declined to 0.085 by the sixth treatment. A low value has been maintained since then (Figure 2).
Figure 2.
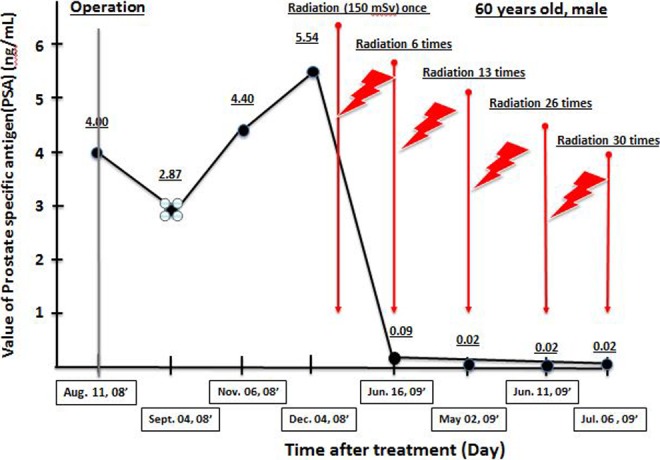
Changes in prostate-specific antigen (PSA) of a patient with prostate cancer after radiation hormesis treatment. The patient was irradiated with X-rays once a week at a dose of 150 mGy, 30 times in total. The distance from body surface was 86 cm. Serum PSA was assayed periodically. The X-ray irradiation treatment periods are shown.
Radiation Treatment for Inoperable End-Stage Prostate Cancer With Bone Metastasis
Ohshima et al29 have shown that repeated 0.5 Gy whole-body γ-irradiation (total dose of 2 Gy) significantly suppressed colony formation in the lungs in a B16 melanoma pulmonary metastasis model in mice.
Second case report
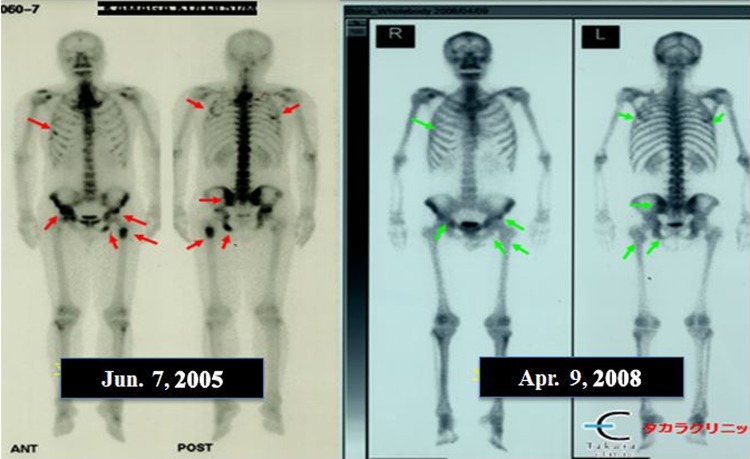
The patient was a 54-year-old man diagnosed with inoperable end-stage prostate cancer with bone metastasis. He reported hip joint pain. X-ray examination showed darkening of the bones, and his PSA was 860. He was diagnosed with metastatic prostate cancer. Treatment at several hospitals temporarily reduced his PSA value to 5.8, and then to 1.0, but it subsequently rose to 4.8, and he visited our clinic in July 2007. Electronic immunotherapy and radiation hormesis therapy were started in our clinic. He received X-ray irradiation 3 times a week at a dose of 150 mSv each (10 times in total) and used a radon sheet for 6 hours at bedtime from July 28, 2006, to May 29, 2007. His PSA value remained at about 1 during July/August 2006, then decreased to 0.5 in September, and to 0.1 in October. After the end of hormesis therapy on May 29, 2007, his PSA value was as low as 0.008 and remained at about that level (Figure 3). His doctor reported no apparent further accumulation of cancer cells in the whole body, suggesting that his bone metastasis had been successfully treated (Figure 4).
Figure 3.
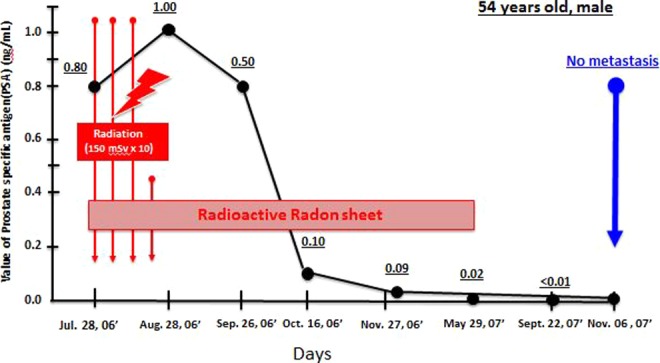
Changes in prostate-specific antigen (PSA) of a patient with prostate cancer with bone metastasis after radiation hormesis treatment. The patient received X-ray irradiation 3 times a week at a dose of 150 mGy each, 10 times in total, and used a radon sheet at bedtime. The treatment times are shown.
Figure 4.
Bone scintigraphy with 99mTc-hydroxymethylene diphosphonate (99mTc-HMDP) of the patient with prostate cancer with bone metastasis before and after radiation hormesis treatment.
Radiation Hormesis Treatment for Ulcerative Colitis
We have previously reported that low-dose radiation reduces inflammation in experimental models, including hepatitis.39,40 It has also been reported that irradiation attenuates some autoimmune diseases such as rheumatism, systemic lupus erythematosus, and collagen-induced arthritis in mouse models.23,24,26-28,41
Third case report
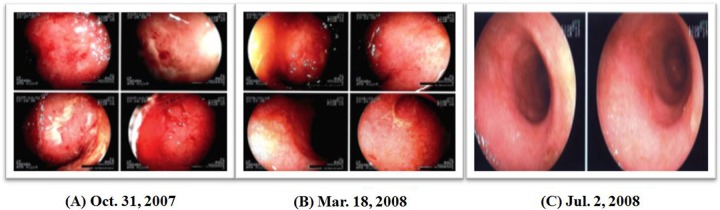
The patient was a 31-year-old man who had intractable ulcerative colitis since the age of 15. When he visited our clinic, he complained of frequent diarrhea. He had compressive fracture of the lumbar vertebrae and had severe depression. He had been taking prednisolone for 17 years. We started comprehensive immunotherapy, including the use of the hormesis room twice a week for 40 minutes, together with ingestion of about 200 mL of radon-containing water (330 Bq/L) with every meal and exposure to a radon sheet at bedtime. At 2 months after starting the treatment (October 2007), endoscopy showed slight bleeding of the colonic mucosal membrane, but bleeding and swelling had completely disappeared 8 months later (Figure 5A-C). In addition, his stools became regular, and the number of visits to the toilet gradually decreased. One year after starting the treatment, the number of bowel movements was 3 to 4 times a day (Figure 6). Further, his bone density increased from 50% before treatment to 60% as a result of stopping prednisolone. Since then he has remained healthy with no apparent inflammation until now.
Figure 5.
(A) Endoscopic image of October 31, 2007; (B) Endoscopic image of March 18, 2008; (C) Endoscopic image of July 2, 2008.
Figure 6.
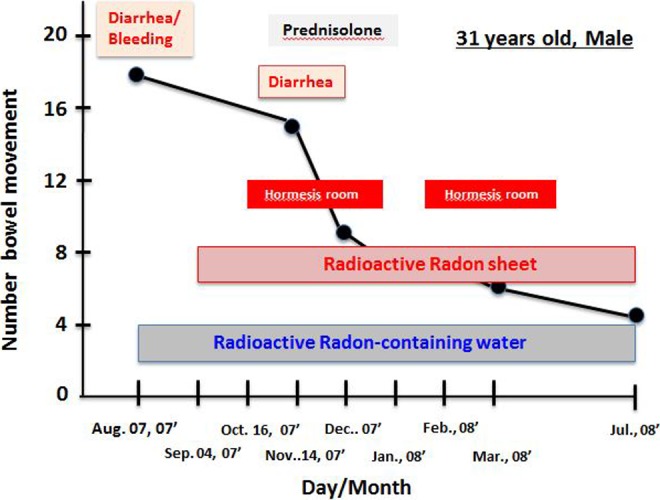
Changes in the number of bowel movements of the patient with ulcerative colitis after radiation hormesis treatment. The patient was treated in the hormesis room twice a week for 40 minutes, ingested about 200 mL of radon-containing water during every meal, and was exposed to a radon sheet at bedtime. The treatment times are shown.
Discussion
The use of natural radiation sources for the treatment of human diseases is well established in Austria’s Bad Gastein Clinic. Their medical facility uses an air at a temperature of 37.0°C to 41.5°C, relative humidity of 70% to almost 100%, and natural radon content of 44 kBq/m3. Our hormesis room was designed to provide similar conditions as far as possible. High-concentration radon inhalation therapy is recognized as a medical practice in Austria, and the inhalation amount and duration, and so on, are set out in a comprehensive treatment plan for each patient. The therapy is carried out in a gallery of a former gold mine, where the radon concentration is high (about 50 000 Bq/m3). Radon treatment at the facility has been reported to be effective in patients with ankylosing spondyloarthritis, rheumatic chronic polyarthritis, osteoarthritis, asthma, and atopic dermatitis.42,43
There are several explanations regarding the mechanism of action of this therapy.38,44,45 Radon gas enters the body from the respiratory or digestive system, reaches the blood, and then is carried to the whole body. Finally, the affected tissue/cell is stimulated by α-rays emitted from this nuclide. In addition, dilation of the peripheral blood vessels by hot water in the hot spring baths and galleries enhances blood circulation of the skin, joints, tendons, muscles, and so on.
In our previous animal experiments, we have confirmed that immunological activity of living body is markedly increased by whole-body irradiation of low doses of radiation. We conducted a clinical experimental trial in order to confirm these basic data, expecting a mechanism to kill cancer cells by raising cellular immunity rather than direct action of radiation itself.
As described in our case reports, we obtained good responses to low-dose radiation in a patient with prostate cancer and also in a patient with prostate cancer and bone metastasis. Their PSA values were decreased and remained low even after the end of hormesis therapy. It is especially noteworthy that the bone metastasis almost completely disappeared. The irradiation dose used was set to 150 mGy based on the report of Sakamoto.36 This seemed reasonable because adaptive responses to ionizing radiation were observed in the dose range of 100 to 500 mGy in our animal experiments using mouse and animal cells.46-48 Since human sensitivity to radiation is twice as high as that of mice,49 the dose of 150 mGy used here should be effective in inducing an adaptive response. However, the response is transient and usually disappears within 48 hours. Consequently, it is necessary to repeat the irradiation, and it seems possible that the 6-hour exposure to the radon sheet in the patient with cancer with bone metastasis contributed to his improvement. In our animal studies on the tumor-suppressive efficacy of low-dose radiation, we suggested involvement of a shift of the immunity balance, since the cytotoxic activities of natural killer (NK) and cytotoxic T lymphocytes (CTLs) were enhanced after repeated irradiation. That is, irradiation at 0.5 Gy significantly increased interferon (IFN) γ production by splenocytes of tumor-bearing mice, while interleukin-4 (IL-4) was unchanged, resulting in an increased IFN-γ/IL-4 (type 1 helper T cell to type 2 helper T cell, Th1/Th2) ratio, a hallmark of Th1 shift.22 Irradiation also increased the IL-12 production and glutathione (a cellular antioxidant) level of macrophages.29 It is well established that elevation of intracellular antioxidants activates cell-mediated immunity, including NK cells and CTLs.46,50-52 Therefore, a shift in the immunity balance toward Th1 and consequently enhancement of antitumor activity might be one of the mechanisms of the efficacy of low-dose irradiation in the present cases. Furthermore, we found that the levels of matrix metalloproteinase (MMP) 2 protein and activity were significantly decreased in lung tissues of a B16-melanoma metastasis mice model exposed to low-dose γ-irradiation.53 Thus, a decrease in MMP could also be involved in the suppression of tumor metastasis in humans.
As for the effect of low-dose radiation on ulcerative colitis, our patient took radon-containing water besides using the hormesis room and radon sheet. However, it is not clear how much radon contributed to the improvement of the pathology, although mouse models of atopy, hyperuricemia, and so on, were improved by ingestion of radon-containing water amounting to around 200 to 300 Bq/L.21,54 However, it remains to be established what the optimum radiation dose and schedule might be.
A series of experiments on suppression of autoimmune rheumatism by low-dose radiation revealed induction of regulatory T cell (Treg), which normalizes biased helper T cell (Th1/Th2) activity. Moreover, it is well established that inflammation involves the mitogen-activated protein kinase (MAPK) signaling pathway and that MAPK phosphatase-1 (MKP-1) inhibits activation of the MAPK pathway. Although the activities of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 MAPK were decreased within a short period of time in RAW26.7 cells exposed to 0.5 Gy γ-rays, an increase in MKP-1 activity was found. Therefore, the effects of low-dose γ-rays on p38 MAPK activation and on lipopolysaccharide-induced production of inflammatory cytokine tumor necrosis factor α (TNF-α) were examined. Activation of p38 MAPK and production of TNF-α were suppressed by γ-irradiation, indicating that MKP-1 is involved in the signal transduction pathway of anti-inflammatory action of low-dose γ-rays.28 These mechanisms might also operate in humans.
Interest in the use of radiation hormesis in clinical practice is increasing, and basic data are being accumulated.9,43,54-58 We hope that our case reports will encourage further clinical studies.
Acknowledgments
The authors would like to thank our patients for granting permission to report their cases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Valentin J. Low-dose extrapolation of radiation-related cancer risk. Ann ICRP. 2005;35(4):1–140. [DOI] [PubMed] [Google Scholar]
- 2. Aliyu AS, Ramli AT. The world’s high background natural radiation areas (HBNRAs) revisited: a broad overview of the dosimetric, epidemiological and radiological issues. Radiat Measurements. 2015;73(3):51–59. [Google Scholar]
- 3. Attar M, Kondolousy YM, Khansari N. Effect of high dose natural ionizing radiation on the immune system of the exposed residents of Ramsar town, Iran. Iran J Allergy Asthma Imumnol. 2007;6(2):73–78. [PubMed] [Google Scholar]
- 4. Fornalski KW, Dobrzyński L. The cancer mortality in high natural radiation areas in Poland. Dose Response. 2012;10(4):541–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghiassi-Nejad M, Zakeri F, Gh.Assaei M, Kariminia A. Long-term immune cytogenetic effects of high level natural radiation on Ramsar inhabitants in Iran. J Environ Radioact. 2014;74(1-3):107–116. [DOI] [PubMed] [Google Scholar]
- 6. Hendry JH, Simon SL, Wojcik A, et al. Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Prot. 2009;29(2A):A29–A42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koya PK, Chougaonkar MP, Predeep P, et al. Effect of low and chronic radiation exposure: a case-control study of mental retardation and cleft lip/palate in monazite-bearing coastal areas of Southern Kerala. Radiat Res. 2012;177(1):109–116. [DOI] [PubMed] [Google Scholar]
- 8. Vaiserman AM. Radiation hormesis: historical perspective and implications for low-dose cancer risk assessment. Dose Response. 2010;8(2):172–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Treatment of Alzheimer disease with CT scans: a case report. Dose Response. 2016;14(2):1–7. doi:10.1177/1559325816640073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen WL, Luan YC, Shieh MC, et al. Effects of cobalt-60 exposure on health of Taiwan residents suggest new approach needed in radiation protection. Dose Response. 2007;5(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scott BR. Residential radon appears to prevent lung cancer. Dose Response. 2011;9(4):444–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thompson RE. Epidemiological evidence for possible radiation hormesis from radon exposure: a case-control study conducted in Worcester, MA. Dose Response. 2011;9(4):59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sponsler R, Cameron JR. Nuclear shipyard worker study (1980-1988): a large cohort exposed to low-dose-rate gamma radiation. Int Low Radiat. 2005;1(4):463–478. [Google Scholar]
- 14. Luckey TD. The health effects of low-dose ionizing radiation. J Am Phys Surg. 2008;13(2):39–42. [Google Scholar]
- 15. Cuttler JM, Pollycove M. Nuclear energy and health. Dose Response. 2009;7(1):52–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galván I, Bonisoli-Alquati A, Jenkinson S, et al. Chronic exposure to low-dose radiation at Chernobyl favors adaptation to oxidative stress in birds. Func Ecol. 2014;28(6):1311–1555. [Google Scholar]
- 17. Dobrzyński L, Fornalski KW, Feinendegen LE. Cancer mortality among people living in areas with various levels of natural background radiation. Dose Response. 2015;13(3):1559325815592391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosoi Y, Sakamoto K. Suppressive effect of low dose total body irradiation on lung metastasis: dose dependency and effective period. Radiother Oncol. 1993;26(2):177–179. [DOI] [PubMed] [Google Scholar]
- 19. Kojima S, Nakayama K, Ishida H. Low dose gamma-rays activate immune functions via induction of glutathione and delay tumor growth. J Radiat Res. 2004;45(1):33–39. [DOI] [PubMed] [Google Scholar]
- 20. Kojima S. Induction of glutathione and activation of immune functions by low-dose, whole body irradiation with γ-rays [in Japanese]. Yakugaku Zasshi. 2006;126(10):849–857. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi M, Kojima S. Suppression of atopic dermatitis and tumor metastasis in mice by small amounts of radon. Radiat Res. 2006;165(3):337–342. [DOI] [PubMed] [Google Scholar]
- 22. Hayase H, Ohshima Y, Takahashi M, et al. The enhancement of Th1 immunity and the suppression of tumour growth by low-dose γ-radiation. Int J Low Radiat. 2008;5(4):275–289. [Google Scholar]
- 23. Nakatsukasa H, Tsukimoto M, Ohshima Y, et al. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 2008;49(4):381–389. [DOI] [PubMed] [Google Scholar]
- 24. Nakatsukasa H, Tsukimoto M, Tokunaga A, et al. Repeated gamma-ray irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells, but not by damaging lymphocytes directly. Radiat Res. 2010;174(3):313–324. [DOI] [PubMed] [Google Scholar]
- 25. Nakatsuaksa H, Tsukimoto M, Harada H, Kojima S. Adenosine A2B receptor antagonist suppresses differentiation to regulatory T cells without suppressing activation of T cells. Biochem Biophys Res Commun. 2011;409(1):114–119. [DOI] [PubMed] [Google Scholar]
- 26. Tago F, Tsukimoto M, Nakatsukasa H, Kojima S. Repeated 0.5-Gy gamma irradiation attenuates autoimmune disease in MRL-lpr/lpr mice with suppression of CD3+CD4-CD8-B220+ T-Cell proliferation and with up-regulation of CD4+CD25+Foxp3+ regulatory T cells. Radiat Res. 2008;169(1):59–66. [DOI] [PubMed] [Google Scholar]
- 27. Tsukimoto M, Nakatsukasa K, Sugawara K, Yamashita K, Kojima S. Repeated 0.5-Gy γ-irradiation attenuates experimental autoimmune encephalomyelitis with up-regulation of regulatory T cells and suppression of IL-17 production by Th17 cells. Radiat Res. 2008;170(4):429–436. [DOI] [PubMed] [Google Scholar]
- 28. Tsukimoto M, Homma T, Mutou Y, Kojima S. 0.5-Gy γ-ray irradiation suppresses production of TNF-α through up-regulation of MKP-1 in mouse macrophage RAW264.7 cells. Radiat Res. 2009;171(2):219–224. [DOI] [PubMed] [Google Scholar]
- 29. Ohshima Y, Tsukimoto M, Kojima S. The novel mechanism of metastasis inhibition by low-dose whole-body irradiation with gamma-rays. Int J Low Radiat. 2008;5(2):156–167. [Google Scholar]
- 30. Nowosielska EM, Cheda A, Wrembel-Wargocka J, Janiak MK. Immunological mechanism of the low-dose radiation-induced suppression of cancer metastases in a mouse model. Dose Response. 2010;8(2):209–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu HS, Liu ZM, Yu XY, Song AQ, Liu N, Wang H. Low-dose radiation induces antitumor effects and erythrocyte system hormesis. Asian Pac J Cancer Prev. 2013;14(7):4121–4126. [DOI] [PubMed] [Google Scholar]
- 32. Yamaoka K, Mifune T, Mitsunobu F, et al. Basic study on radon effects and thermal effects on humans in radon therapy. Physiol Chem Phys Med NMR. 2001;33(2):133–138. [PubMed] [Google Scholar]
- 33. Yamaoka K, Mitsunobu F, Kojima S, et al. The elevation of p53 protein level and SOD activity in the resident blood of the Misasa radon hot spring district. J Radiat Res. 2005;46(1):21–24. [DOI] [PubMed] [Google Scholar]
- 34. Falkenbach A, Kovacs J, Franke A, Jörgens K, Ammer K. Radon therapy for the treatment of rheumatic diseases—review and meta-analysis of controlled clinical trials. Rheumatol Int Clin Exp Invest. 2005;25(3):205–210. [DOI] [PubMed] [Google Scholar]
- 35. Mitsunobu F, Yamaoka K, Hanamoto K, et al. Elevation of antioxidant enzymes in the clinical effects of radon therapy for bronchial asthma. J Radiat Res. 2003;44(2):95–99. [DOI] [PubMed] [Google Scholar]
- 36. Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2(4):293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arens M, Sabater S, Hernández V, et al. Anti-inflammatory effects of low-dose radiotherapy: indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 2012;188(11):975–981. [DOI] [PubMed] [Google Scholar]
- 38. Rödel F, Frey B, Gaip U, et al. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 2012;19(12):1741–1750. [DOI] [PubMed] [Google Scholar]
- 39. Kojima S, Matsuki O, Nomura TA, Kubodera A, Yamaoka K. Elevation of mouse liver glutathione level by low-dose of γ-ray irradiation and its effect on CCl4-induced liver damage. Anticancer Res. 1998;18(4A):2471–2476. [PubMed] [Google Scholar]
- 40. Kojima S, Matsumori S, Ishida H, Yamamoto A. Possible role of elevation of glutathione in the acquisition of enhanced proliferation of mouse splenocytes exposed to small-does γ-rays. Int J Radiat Biol. 2000;76(12):1641–1647. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka T, Tago F, Fang S, Shimura N, Kojima S. Repeated 0.5-Gy γ-ray irradiation attenuates autoimmune manifestation in MRL-lpr/lpr mice. Int J Radiat Biol. 2005;80(10):731–740. [DOI] [PubMed] [Google Scholar]
- 42. Erickson BE. The therapeutic use of radiation treatment in Europe: an “alternative” remedy in the United States. Dose Response. 2007;5(1):48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scott BR. Radiation-hormesis phenotypes, the related mechanisms and implications for disease prevention and therapy. J Cell Commun Signal. 2014;8(4): 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu SZ. Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: mechanisms and implication. Nonlinearity in Biol Toxicol Med. 2003;1(1):71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin SZ, Pan XN, Wu N, Jin GH, Liu SZ. Whole-body low-dose irradiation promotes the efficacy of conventional radiotherapy for cancer and possible mechanisms. Dose Response. 2007;5(4):349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kojima S, Matsuki O, Kinoshita I, Gonzalez TV, Shimura N, Kubodera A. Dose small-dose γ-ray induce endogenous antioxidant potential in vivo? Biol Pharm Bull. 1997;20(6):601–604. [DOI] [PubMed] [Google Scholar]
- 47. Tsukimoto M, Tamaishi N, Homma T, Kojima S. Low-dose gamma-ray irradiation induces translocation of Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res. 2010;51(3):349–353. [DOI] [PubMed] [Google Scholar]
- 48. Tamaishi N, Tsukimoto M, Kitami A, et al. P2Y6 receptors and ADAM17 mediate low-dose gamma-ray irradiation-induced focus formation (activation) of EGF receptor. Radiat Res. 2011;174(2):193–200. [DOI] [PubMed] [Google Scholar]
- 49. UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources, Effects and Risks of Ionizing Radiation, Annex G. 1988. Early effects in man of high doses of radiation; II Dose-Response Relationship in Man. Paragraph 111&112 pp. 570. [Google Scholar]
- 50. Kojima S, Matsuki O, Kubodera A, et al. Induction of mRNAs for glutathione synthesis-related proteins in mouse liver by low doses of γ-rays. Biochim Biophys Acta. 1998;1381(3):312–318. [DOI] [PubMed] [Google Scholar]
- 51. Kojima S, Shimomura H, Matsumori S. Effect of pre-irradiation with low-dose γ-rays on chemically induced hepatotoxicity and glutathione depletion. Anticancer Res. 2000;20(3A):1583–1588. [PubMed] [Google Scholar]
- 52. Kojima S, Ishida H, Takahashi M, Yamaoka K. Elevation of glutathione induced by low-dose γ rays and its involvement in increased natural killer activity. Radiat Res. 2002;157(3):275–280. [DOI] [PubMed] [Google Scholar]
- 53. Ohshima Y, Kitami A, Kawano M, Tsukimoto M, Kojima S. Induction of extracellular ATP mediates increase of intracellular thioredoxin in RWA264.7 cells exposed to low-dose γ-rays. Free Radic Biol Med. 2011;51(6):1240–1248. [DOI] [PubMed] [Google Scholar]
- 54. Etani R, Kataoka T, Kanzaki N, et al. Difference in the action mechanism of radon inhalation and radon hot spring water drinking in suppression of hyperuricemia in mice. J Radiat Res. 2016;57(3):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen W, Xu X, Bai L, et al. Low-dose gamma-irradiation inhibits IL-6 secretion from human lung fibroblasts that promotes bronchial epithelial cell transformation by cigarette-smoke carcinogen. Carcinogenesis. 2012;33(7):1368–74. [DOI] [PubMed] [Google Scholar]
- 56. Doss M. Low dose radiation adaptive protection to control neurodegenerative diseases. Dose Response. 2014;12(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oakley PA. Is use of radiation hormesis the missing link to a better cancer treatment? J Cancer Ther. 2015;6(7):601–605. [Google Scholar]
- 58. Yang G, Li W, Jiang H, et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics. Int J Cancer. 2016;139(10):2157–2168. [DOI] [PubMed] [Google Scholar]