Abstract
Background
The primary neuroendocrine skin cancer, Merkel cell carcinoma (MCC), has a well-known predilection to metastasize systemically. However, the experience of systemic metastases in MCC is mainly disseminated through case reports due to the rarity of MCC.
Purpose
To elucidate the frequency and locations of systemic metastasis in MCC by reviewing the imaging of patients with metastatic MCC in a national cohort.
Material and Methods
Patients with diagnosed metastatic MCC by imaging studies in Finland during 1999–2012 were included in this study. We reviewed their imaging studies to evaluate the most frequent sites for systemic metastasis and determined the latency between the primary tumor diagnosis and systemic metastasis. The material includes 30 MCC patients with complete imaging series and 187 examinations, of which 102 (54%) were CT images.
Results
The mean latency from the primary tumor diagnosis to systemic metastasis was 2.1 years and the mean latency between the radiologic diagnosis of the metastases and death was 299 days. Metastases were recorded in several organ systems in most of the cases, and at least two separate metastatic sites in 63% of the cases. Metastatic spread was noted in 60% of the cases in distant lymph nodes. Liver and lungs were the most affected solid organs.
Conclusion
Systemic metastasis in MCC has no predilection site, basically every organ system can be involved. Most of the systemic metastases were recorded during the first two years after the MCC diagnosis.
Keywords: Neuroendocrine carcinoma, skin, systemic metastasis, latency
Introduction
Merkel cell carcinoma (MCC) is a rare neuroendocrine skin cancer that occurs mainly in fair-skinned, elderly individuals. Globally, 80% of the tumors are initiated by Merkel cell polyoma virus (MCV) DNA integration into the cancer cells early in MCC development (1). MCC has an inherent capacity for early and aggressive local and systemic dissemination (2). Approximately 65–70% of the patients present with clinically localized disease to the skin (American Joint Committee on Cancer [AJCC] stage I or II), 25–26% have palpable regional lymphadenopathy AJCC stage III, and 5–8% have distant metastasis, AJCC stage IV (3,4). The draining lymph node basin is most commonly the first site of metastasis, in 27–60% of the cases (5,6). Distant dissemination occurs in up to 40–50% of patients that develop visceral metastasis, particularly prevalent in the lungs, liver, and bone (7,8). Owing to the aggressive course of the disease, its mortality exceeds those of other forms of skin cancers (9). About one-third of the patients die of MCC including all stages and courses of disease (10).
Current treatment guidelines for MCC entail imaging studies during the course of the disease (11), from the preoperative stage to the postoperative follow-up. In addition to the clinical examination, ultrasound (US) of the loco regional nodes and total body positron emission tomography–computed tomography (PET-CT) will complete the staging in preoperative examinations (11) and direct the choice of the surgical treatment modality. In the follow-up, nodal US and CT or PET-CT are proposed (11). However, it is not clear whether imaging has any role in the follow-up of MCC patients.
The rarity of the prevalence of MCC limits the amount of information on the experiences on systemic metastases in MCC and the available information is mainly case reports. Reasons for this paucity of information might lie in the fact that when the disease has metastasized, it is considered incurable (11). This retrospective study was designed to assess the most frequent sites for systemic dissemination in MCC and to determine the latency between the primary tumor diagnosis and systemic metastasis by imaging.
Material and Methods
The study was approved by the Ethics Committee of the Helsinki University Hospital. The Ministry of Health and Social Affairs granted authors the permission to collect the patient data for study purposes. Permission to retrieve all images for study purpose was granted by the National Institute for Health and Welfare. Inclusion criteria for this study was that patient was diagnosed with systemic metastases MCC and images were available for review. No informed consent was required as all the patients had deceased prior to the study commencing.
Our group has gathered primary MCC tumor samples available in Finland since 1978. Immunohistochemistry served to validate all of the diagnoses. To accompany the tumor samples, comprehensive patient records have been gathered from hospital files and Finnish Cancer Registry records. The ongoing MCC projects of our research group continue to use this database.
A total of 57 MCC patients diagnosed between 1979 and 2013 with systemic metastases were identified. Imaging studies of these patients were retrieved for analysis. When autopsy was performed, the autopsy report was compared with the radiologic findings. All medical records and images were reviewed and detailed data on patient and tumor characteristics, including tumor size, location, stage of disease at the time of diagnosis, local recurrence, local and systemic metastasis, and survival, were obtained from the hospital and primary healthcare center files of the patients fitting the inclusion criteria. All included patients were staged according to the AJCC classification for this study (3). A total of 27 patients were excluded from this study because, due to archiving regulations, no imaging studies were available.
Imaging series were re-evaluated blindly by an experienced radiologist (EL), and lesions were categorized on the basis of the anatomical locations. Distant lymph node metastasis was classified as systemic metastasis to the lymph nodes beyond the nearest regional area of the primary tumor.
Results
The study cohort included 30 MCC patients with 187 accompanying imaging series (Table 1). The imaging studies were taken during 1999–2012. There were equal numbers of men and women. The mean age of the patients at the time of the MCC diagnosis was 75 years (age range, 50–89 years). The majority of the patients presented with cutaneous tumors (n = 12/40%) located in the head and neck region. Two patients in this series presented with unknown primary tumor. Cutaneous primary tumor sizes were in the range of 6–100 mm, with a mean of 25 mm. All patients died during the follow-up, with a mean follow-up time of 1088 days (range, 60 days–14.8 years). An autopsy report was available for four patients.
Table 1.
Demographic, treatment, tumor, and latency data for 30 patients with MCC.
| Patient no. | Age/ gender | Location of the primary tumor | Primary tumor size (mm) | AJCC stage at presentation | Treatments before metastasis | Imaging method | Distant metastasis | Latency from diagnosis to metastas(days) | Latency from metastasis to death (days) | Follow-up (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 82 F | Head and neck | 10 | IV | Palliative surgery | Body CT, head MRI | Liver, lung, DLN, ST, orbita | 104 | 101 | 205 |
| 2 | 72 M | Unknown primary | NA | IV | Palliative radiation therapy | US neck, abdomen | DLN | 101 | 21 | 122 |
| 3 | 60 F | Lower extremity | 17 | I | Surgery, radiation therapy | THX CT | Heart | 984 | 34 | 1018 |
| 4 | 84 M | Upper extremity | 30 | II | Surgery, radiation therapy | Body CT, MRI | Bone, DLN, ST | 400 | 57 | 457 |
| 5 | 86 F | Lower extremity | 11 | I | Surgery | Body CT, abdominal US | DLN | 544 | 242 | 786 |
| 6 | 56 F | Lower extremity | 15 | I | Surgery | Body CT | DLN | 5194 | 213 | 5407 |
| 7 | 81 F | Head and neck | 13 | I | Surgery | BSc | Bone | 84 | 2693 | 2777 |
| 8 | 87 F | Upper extremity | 26 | III | Palliative surgery, palliative radiation therapy | BSc | Kidney, bone | 4420 | 57 | 4477 |
| 9 | 77 M | Parotis/head and neck | 25 | IV | Surgery, chemo therapy | Neck, THX CT | Liver | 11 | 166 | 177 |
| 10 | 83 F | Head and neck | 20 | II | Surgery including SNB, radiation therapy | Body CT | DLN | 335 | 315 | 650 |
| 11 | 78 F | Head and neck | 20 | III | Surgery | Body CT | Adrenal gland, DLN | 1985 | 28 | 2013 |
| 12 | 72 F | Lower extremity | 13 | I | Surgery | Abdominal, Head CT | DLN, brain | 1196 | 578 | 1774 |
| 13 | 80 M | Head and neck | 14 | I | Surgery, radiation therapy | FDG-PET-CT | Liver, DLN | 221 | 95 | 316 |
| 14 | 73 M | Upper extremity | 26 | II | Surgery, radiation therapy | Body CT | Liver | 948 | 99 | 1047 |
| 15 | 61 M | Lower extremity | 35 | III | Surgery, radiation therapy | Body CT | Liver, pancreas, lung, adrenal | 1106 | 293 | 1399 |
| 16 | 86 F | Posterior torso | 40 | II | Surgery | THX CT | Lung | 361 | 18 | 379 |
| 17 | 78 M | Head and neck | 18 | I | Surgery, radiation therapy | Body CT | Pancreas, lung, anus, retroperitoneal and peritoneal cavity | 859 | 46 | 905 |
| 18 | 78 M | Lower extremity | 40 | II | Surgery | Body CT | DLN, ST | 616 | 191 | 807 |
| 19 | 76 M | Lower extremity | 100 | II | Surgery, radiation therapy | Body CT, MRI | Stomach, ST | NA | 143 | 376 |
| 20 | 87 F | Upper extremity | 6 | I | Surgery | Abdominal CT | Liver | 502 | 21 | 523 |
| 21 | 89 F | Lower extremity | 30 | III | Surgery | Abdominal CT | Pancreas, DLN | 882 | 31 | 913 |
| 22 | 72 M | Head and neck | 10 | IV | Palliative radiation therapy, chemotherapy | Abdominal CT | Liver, stomach, lung right, ST, DLN retroperitoneal and peritoneal cavity | 527 | 81 | 608 |
| 23 | 81 M | Lower extremity | 50 | II | Surgery including SNB | Neck, body CT | DLN, pancreas | 230 | 242 | 472 |
| 24 | 66 F | Head and neck | NA | III | Surgery, radiation therapy | FDG-PET-CT | Bone, lung | 299 | 189 | 485 |
| 25 | 65 F | Upper extremity | 10 | I | Surgery | Head, body CT | Liver, lung, bone, brain, DLN | 119 | 471 | 590 |
| 26 | 50 M | Head and neck | 20 | II | Surgery | Body CT | DLN, ST | 366 | 482 | 848 |
| 27 | 76 M | Head and neck | 15 | I | Surgery including neck dissection radiation therapy | Neck, abdomen, THX CT | Spinal cord, bone, DLN, ST, retroperitoneal and peritoneal cavity | 303 | 83 | 386 |
| 28 | 86 M | Unknown primary | NA | IV | No treatment | Head, body CT | Lung, liver, spinal cord channel, bone, ST | 46 | 14 | 60 |
| 29 | 68 M | Upper extremity | 20 | II | Surgery | Body, THX CT, head MRI, neck US | Lungs, DLN, ST, pancreas, brain, pleura | 548 | 110 | 658 |
| 30 | 74 F | Head and neck | 40 | II | Surgery, radiation therapy | THX CT, neck MRI | DLN | 158 | 1857 | 2015 |
BSc, bone scintigraphy; CT, computed tomography; DLN, distant lymph nodes; FDG-PET-CT, fluoro deoxy glucose positron emission tomography–computed tomography; MRI, magnetic resonance imaging; NA, not available; ST, subcutaneous tissue; THX, thorax; US, ultrasound.
All patients received some type of treatment for their MCC before the detection of the metastases. In 28 patients (93%) the treatment was surgical intervention (Table 1). In 13 (43%) of the cases, surgery was the only treatment before the detection of metastatic spread. The most frequent adjuvant treatment was radiation therapy given to 14 (46%) cases followed by chemotherapy in two cases (7%).
Of the 187 imaging examinations, 102 (54%) were CT images, 62 (33%) were conventional chest X-ray images, 12 (6%) were magnetic resonance imaging (MRI), seven (3.7%) were ultrasound exams, two (1%) were PET-CT, and two (1%) were bone scintigraphy.
The mean latency from the primary tumor diagnosis to systemic metastasis by imaging was 2.1 years (range, 11 days–14.2 years). The mean latency between the radiologic diagnosis of the metastases and death was 299 days (range, 14 days–7.4 years) (Table 2).
Table 2.
Mean latencies between presentation and metastases diagnosis by imaging stratified by time and site of the metastases.
| Latency from the MCC diagnosis (years) | Location of tumor | Latency from the MCC diagnosis (months) |
|---|---|---|
| <2 | Subcutaneous tissue | 12 |
| Liver | 13 | |
| Lungs | 15 | |
| Distant lymph nodes | 16 | |
| Stomach | 17 | |
| Retroperitoneal and peritoneal cavity | 19 | |
| 2–3 | Pancreas | 24 |
| Brain and orbita | 25 | |
| Heart | 32 | |
| 3–4 | Kidneys and adrenal glands | 38 |
| Vertebral column and bones | 40 |
Table 3 presents the metastases stratified by their location and frequency. In most cases the patients had metastases in several organ systems, in 19/30 (63%) patients at least two separate metastatic sites were recognized. Metastasis affected the distant lymph nodes in the majority of the cases, 18/30 (60%); the liver and lungs were the most affected solid organs, with 9/30 (30%) cases each.
Table 3.
Sites, numbers of metastases, and imaging modalities in 30 patients with MCC.
| Sites of metastasis | Number of patients (n (%)) | Multiple/ Solitary (n) | Imaging modality (n) | |
|---|---|---|---|---|
| Distant lymph nodes | 18 (60) | 15/3 | CT MRI US PET-CT | 22 3 2 1 |
| Liver | 9 (30) | 7/2 | CT PET-CT | 8 1 |
| Lungs | 9 (30) | 1/8 | CT PET-CT | 8 1 |
| Subcutaneous tissue | 8 (27) | 5/3 | CT MRI | 7 1 |
| Vertebral column and bones | 7 (23) | 4/3 | CT BSc MRI PET-CT | 3 2 1 1 |
| Pancreas | 6 (20) | 0/6 | CT | 6 |
| Brain or orbita | 4 (13) | 1/3 | CT MRI | 2 2 |
| Kidneys or adrenal glands | 3 (10) | 0/3 | CT BSc | 2 1 |
| Stomach | 2 (7) | 0/2 | CT | 2 |
| Heart | 1 (3) | 0/1 | CT | 1 |
| Retroperitoneal and peritoneal cavity | 3 (10) | 2/1 | CT | 3 |
Typically, metastases in the distant lymph nodes, retroperitoneal and peritoneal cavity, liver, subcutaneous tissue, and bones presented with multiple metastatic foci (Figs. 1 and 2). In the lungs, pancreas, stomach, and heart, the metastasis usually presented as a solitary focus.
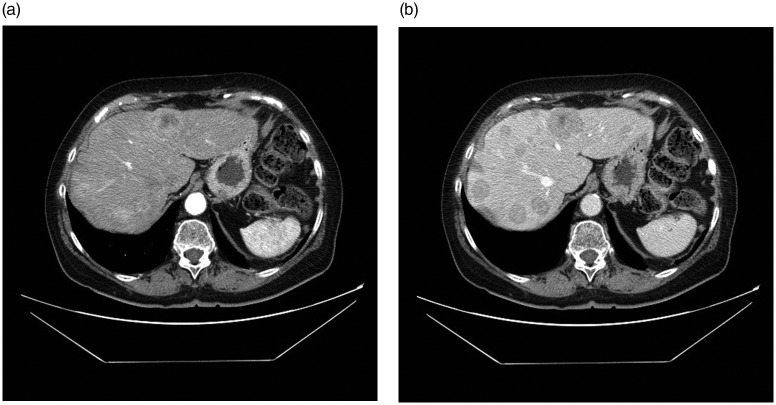
Fig. 1.
Multiple liver metastases in a patient with primary tumor in the neck (patient 1). Most of the metastases are enhanced by contrast medium in the arterial phase images (a) and show washout in venous phase images (b).
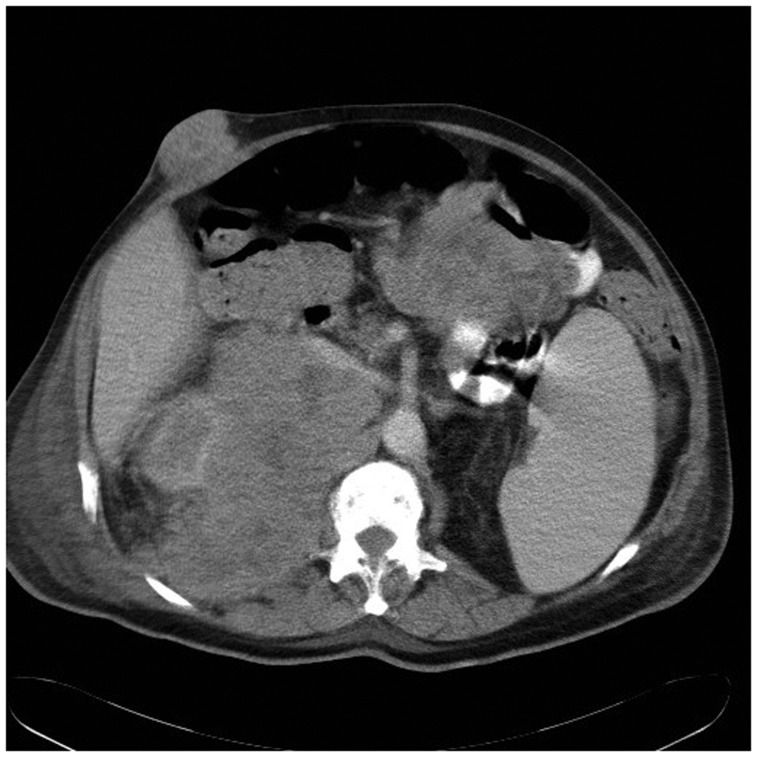
Fig. 2.
Large retroperitoneal, peritoneal and subcutaneous metastases in a patient with primary tumor in the neck (patient 27).
Discussion
The imaging studies in patients with metastatic MCC were reviewed. No predilection site for distant metastases were found, as every visceral organ, skeletal system, subcutaneous tissue, and distant lymph nodes were involved. However, there is presently no clear agreement on the role of imaging in the management and follow-up of MCC (12). A recent European consensus advocates follow-up with nodal US together with once a year CT or PET-CT for up to five years (11). The NCCN Clinical Practice Guidelines in Oncology on MCC recommends imaging studies to be performed as clinically indicated during the follow-up (13).
The most frequent metastatic site found in this study was distant lymph nodes. This finding was in concordance with previous studies (12). The liver and lungs were the most frequently affected solid organs, which was in line with previous literature (7,8). Current treatment guidelines for MCC consider surgery the mainstay of treatment (11,13). Sentinel node biopsy is indicated for patients with clinically node negative disease, whatever the size of the tumor, in combination with wide excision of the primary tumor (11,13). Sentinel node biopsy may reveal thus patients with occult metastasis and predict unfavorable course of disease (14,15). Recent data point to the direction that primary tumor size does not predict nodal involvement, which is contrary to an earlier paradigm (16,17). However, when the disease has metastasized, there is currently no established curative treatment (11).
The median time to recurrence in MCC patients was approximately eight months, with 90% of the recurrences occurring within 24 months (5,18,19). Subcutaneous metastases in this series had the shortest mean latency from the MCC diagnosis with a time span of only 12 months, a further 66% of the patients were diagnosed with metastases within 24 months. All patients in this study died a mean of just ten months (range, 14 days–7.4 years) after distant metastases were confirmed. This falls well within the range reported in previous literature, where survivals were just nine to 12 months after metastatic disease was recognized, depending on the study (5,20–22).
MCC was once regarded as an indolent skin tumor (23–25), but it has since proven to be one of the deadliest of skin cancers. Although rare in incidence, in Europe with an annual incidence rate of 1.3/1,000,000 (26), MCC is the second most common cause of skin cancer deaths after melanoma, with an estimated cause-specific death rate of 0.43 per 100,000 persons (27). Most of the MCC patients die with non-localized, i.e. metastatic disease (28), which accords with the findings in other cancers (29). Most of the patients present with localized disease (4). Nevertheless, MCC grows rapidly within just few months (2) and tumor doubling times are five to 12 days, or even as rapid as one to five days in the most aggressive tumor subtypes (30).
This study has several limitations that should be acknowledged. One inherent limitation lies in the retrospective design and relatively small number of patients. Further, most of our imaging studies were performed as clinically indicated. The archiving of images is only 20 years in Finland; therefore, we were not able to get access to all the images of MCC patients with metastatic disease. Although MCC has been recognized and characterized since 1972 (31), it was not until the discovery of the Merkel cell polyoma virus in 2008 (1) that an enormous interest in MCC arose, both in research and reporting clinical experience. The rapidly expanding body of knowledge regarding MCC has just recently generated treatment recommendations (11,13). Apart from studies in the 1980s and 1990s, there has been little interest in reporting the metastatic disease due to the fact that there is no curative treatment for metastatic MCC.
In conclusion, this current study showed that systemic metastasis in MCC has no predilection site or organ, as basically every organ system was involved in our study. Most of the systemic metastases were recognized during the first two years after the MCC diagnosis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding from Cancer Foundation grant.
References
- 1.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008; 319: 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol 2008; 58: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, et al. Merkel cell carcinoma, New York, NY: Springer, 2010. [Google Scholar]
- 4.Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC Staging System. Ann Surg Oncol 2016; 23: 3564–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol 2005; 23: 2300–2309. [DOI] [PubMed] [Google Scholar]
- 6.Anderson SE, Beer KT, Banic A, et al. MRI of merkel cell carcinoma: histologic correlation and review of the literature. Am J Roentgenol 2005; 185: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 7.Wynne CJ, Kearsley JH. Merkel cell tumor. A chemosensitive skin cancer. Cancer 1988; 62: 28–31. [DOI] [PubMed] [Google Scholar]
- 8.Shack RB, Barton RM, DeLozier J, et al. Is aggressive surgical management justified in the treatment of Merkel cell carcinoma? Plast Reconstr Surg 1994; 94: 970–975. [DOI] [PubMed] [Google Scholar]
- 9.Becker JC. Merkel cell carcinoma. Ann Oncol 2010; 21(Suppl. 7): vii81–vii5. [DOI] [PubMed] [Google Scholar]
- 10.Rockville Merkel Cell Carcinoma Group. Merkel cell carcinoma: recent progress and current priorities on etiology, pathogenesis, and clinical management. J Clin Oncol 2009; 27: 4021–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebbe C, Becker JC, Grob JJ, et al. Diagnosis and treatment of Merkel cell carcinoma. European consensus-based interdisciplinary guideline. Eur J Cancer 2015; 51: 2396–2403. [DOI] [PubMed] [Google Scholar]
- 12.Hawryluk EB, O’Regan KN, Sheehy N, et al. Positron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer Center. J Am Acad Dermatol 2013; 68: 592–599. [DOI] [PubMed] [Google Scholar]
- 13.Bichakjian CK, Olencki T, Alam M, et al. Merkel cell carcinoma, version 1.2014. Journal of the National Comprehensive Cancer Network: JNCCN 2014; 12: 410–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgari MM, Sokil MM, Warton EM, et al. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol 2014; 150: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howle JR, Veness MJ. Outcome of patients with microscopic and macroscopic metastatic nodal Merkel cell carcinoma: an Australian experience. Dermatol Surg 2014; 40: 46–51. [DOI] [PubMed] [Google Scholar]
- 16.Tarantola TI, Vallow LA, Halyard MY, et al. Prognostic factors in Merkel cell carcinoma: analysis of 240 cases. J Am Acad Dermatol 2013; 68: 425–432. [DOI] [PubMed] [Google Scholar]
- 17.Iyer JG, Storer BE, Paulson KG, et al. Relationships among primary tumor size, number of involved nodes, and survival for 8044 cases of Merkel cell carcinoma. J Am Acad Dermatol 2014; 70: 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bichakjian CK, Lowe L, Lao CD, et al. Merkel cell carcinoma: Critical review with guidelines for multidisciplinary management. Cancer 2007; 110: 1–12. [DOI] [PubMed] [Google Scholar]
- 19.Ott MJ, Tanabe KK, Gadd MA, et al. Multimodality management of Merkel cell carcinoma. Arch Surg 1999; 134: 388–392. discussion 92–93. [DOI] [PubMed] [Google Scholar]
- 20.Voog E, Biron P, Martin JP, et al. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer 1999; 85: 2589–2595. [DOI] [PubMed] [Google Scholar]
- 21.Santamaria-Barria JA, Boland GM, Yeap BY, et al. Merkel cell carcinoma: 30-year experience from a single institution. Ann Surg Oncol 2013; 20: 1365–1373. [DOI] [PubMed] [Google Scholar]
- 22.Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst 2010; 102: 793–801. [DOI] [PubMed] [Google Scholar]
- 23.De Wolff-Peeters C, Marien K, Mebis J, et al. A cutaneous APUDoma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer 1980; 46: 1810–1816. [DOI] [PubMed] [Google Scholar]
- 24.Pilotti S, Rilke F, Lombardi L. Neuroendocrine (Merkel cell) carcinoma of the skin. Am J Surg Pathol 1982; 6: 243–254. [DOI] [PubMed] [Google Scholar]
- 25.Kroll MH, Toker C. Trabecular carcinoma of the skin: further clinicopathologic and morphologic study. Arch Pathol Lab Med 1982; 106: 404–408. [PubMed] [Google Scholar]
- 26.van der Zwan JM, Trama A, Otter R, et al. Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer 2013; 49: 2565–2578. [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald TL, Dennis S, Kachare SD, et al. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am Surg 2015; 81: 802–806. [DOI] [PubMed] [Google Scholar]
- 28.Ascoli V, Minelli G, Kanieff M, et al. Merkel cell carcinoma: a population-based study on mortality and the association with other cancers. Cancer Causes Control 2011; 22: 1521–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 30.Van Gele M, Boyle GM, Cook AL, et al. Gene-expression profiling reveals distinct expression patterns for Classic versus Variant Merkel cell phenotypes and new classifier genes to distinguish Merkel cell from small-cell lung carcinoma. Oncogene 2004; 23: 2732–2742. [DOI] [PubMed] [Google Scholar]
- 31.Toker C. Trabecular carcinoma of the skin. Arch Dermatol 1972; 105: 107–110. [PubMed] [Google Scholar]