Abstract
Background and aims
Arterial stiffness seems to be influenced by the dialysis method, but studies are sparse and the results discordant. High substitution volume online hemodiafiltration appears to have beneficial cardiovascular effects in dialysis patients, but its effects on arterial stiffness are not investigated. We aimed to analyze arterial stiffness parameters in high substitution volume post-dilution online hemodiafiltration and compare results to high-flux hemodialysis.
Methods
We studied arterial stiffness parameters using the oscillometric method (Arteriograph IrDA, TensioMed, Budapest, Hungary) in 23 non-diabetic patients on high substitution volume online postdilution hemodiafiltration and 23 non-diabetic patients on high-flux hemodialysis. Dialysis vintage was at least 6 months in all subjects.
Results
Hemodiafiltration-treated patients showed a more favorable arterial stiffness profile. Pulse wave velocity was significantly higher in hemodialysis compared to hemodiafiltration patients (10.39±2.29 m/s vs 9.0±1.7 m/s, p=0.026). Augmentation indexes and the diastolic reflection area were also significantly elevated hemodialysis patients compared to hemodiafiltration patients.
Conclusions
High substitution volume online postdilution hemodiafiltration could have a beneficial effect on arterial stiffness and should be assessed in properly sized controlled trials.
Keywords: arterial stiffness, hemodialysis, malnutrition, inflammation, diastolic dysfunction
Background and aims
Life expectancy of hemodialysis (HD) patients is greatly reduced even when compared to some forms of cancer. In this dire situation, cardiovascular disease (CVD) is ultimately the principal culprit. Arterial stiffness is an independent predictor of CVD morbidity and mortality in end-stage renal disease (ESRD) [1–5,18–19]. The various dialytic renal replacement therapies appear to have different effects on arterial stiffness parameters. Renal transplantation ameliorates arterial stiffness in adults and children [6–9]. Prolonged HD time and frequency seem to improve arterial stiffness parameters but have little influence on CVD progression [10,11]. High substitution volume on-line hemodiafiltration (HDF) proved effective in reducing CVD morbidity and mortality: DOPPS, CONTRAST, RISCAVID and the TURKISH HDF STUDY [12–17]. However, there is limited knowledge on the effects of HDF on arterial stiffness parameters. Therefore, we aimed to investigate whether HDF patients have better arterial stiffness parameters compared to HD patients. The objectives of the study were to compare pulse wave velocity and augmentation indexes in the two groups, in relation to demographic, biological and dialysis related factors.
Methods
Forty-six non-diabetic, stable, high-flux hemodialysis patients with dialysis vintage of more than twelve months were selected from a single dialysis center for a cross-sectional, case-control study. Twenty-three of these patients were randomly switched to high substitution volume (>20 L/dialysis session) online postdilution hemodiafiltration treatment and were included into the study population. Arterial stiffness parameters were assessed after a period of at least 6 months of stable treatment. High-flux Fresenius Helixone dialyzers (FX 60, 80 or 100) and Fresenius dialysis machines were used (4008S for HD and 5008 for HDF). Eligibility criteria were: age 18 to 80, stable, thrice weekly four hours’ bicarbonate dialysis sessions, blood flow rates over 250 mL/min, dialysis vintage over six months. Exclusion criteria were: diabetes mellitus, active malignancy, active infection, autoimmune disease, immunosuppressive treatment, chronic viral infections (hepatitis B, hepatitis C, HIV), auricular fibrillation, severe cardiac valve disease, aortic prosthesis. A single measurement of arterial stiffness was carried out before the midweek HD session using the oscillometric method (Arteriograph IrDA, TensioMed, Budapest, Hungary) [20]. Pulse wave velocity (PWV), central (AixAo) and brachial (AixBr) augmentation indexes, ejection duration (ED), pulse wave return time (RT), diastolic reflection area (DRA) and aortic systolic blood pressure (SBPao) were recorded. PWV values were interpreted as: optimal (<7 m/s), normal (7 to 9.7 m/s), elevated (9.7 to 12 m/s), and abnormally elevated (> 12 m/s), and the Aix were interpreted as: optimal (< −30%), normal (−30 to −10 %), elevated (−10 to +10%), and abnormally elevated (> 10%).
Blood chemistries were obtained under fasting conditions before the midweek HD. All blood tests were performed by Lotus-Med Laboratories, Bucharest. Dialysis parameters were averaged over six months: urea, urea reduction ratio (URR), normalized protein catabolic rate (nPCR), serum potassium, sodium, bicarbonate, calcium, phosphorus levels and calcium-phosphorus product (CaxP), complete blood count, alanine transaminase (ALT), alkaline phosphatase (ALP), serum glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), pulse pressure (PP), heart rate (HR), processed blood volume, blood flow (Qs), effective treatment time (min), single-pool Kt/V (spKt/V), equilibrated Kt/V (eKt/V). Ferritin and high sensitivity C reactive protein (hsCRP) were measured every 3 months (3 measurements), lipid parameters, serum albumin and serum intact parathyroid hormone (iPTH) levels were measured every 6 months (2 measurements). Variables were expressed as mean ± standard deviation. We used the The Shapiro-Wilk test in SPSS Statistics for analysing the normality of data. Groups were compared using the unpaired Student t-test, Mann–Whitney U-test or Chi-square test, as appropriate. Statistical software: Microsoft Excel 2007, MedCalc v.12 and SPSS v20.
The study was approved by the University ethics committee, in accordance with the 2000 Declaration of Helsinki and the Declaration of Istanbul 2008. All subjects gave their informed consent prior to their inclusion in the study.
Results
Quality measurements of arterial stiffness were successfully conducted in 23 HD patients, 23 HDF patients. The demographics and clinical characteristics of the patients studied are presented in Table I. The two groups did not differ significantly in age, sex and dialysis vintage. Similar dialysis dose, dialysis adequacy and BP control were obtained in the seven months prior to arterial stiffness measurement in both study groups. All patients showed elevated CRP and ferritin levels. Serum creatinine, triglycerides and transferrin saturation were higher in HDF patients (Table I).
Table I.
Demographic, dialysis and laboratory parameters of the study groups.
| Parameters | HD | HDF | p | |
|---|---|---|---|---|
| Age (years) | 55.0±10.6 | 47.8±14.0 | 0.06 | |
| Gender (female, %) | 43.5 | 47.8 | 0.55* | |
| Dialysis vintage (months) | 57.0±36.0 | 69.3±56.7 | 0.43 | |
| BMI (kg/m2) | 27.2±5.2 | 27.6±4.2 | 0.73 | |
| Predialysis | SBP (mmHg) | 140.9±14.0 | 133.3±21.0 | 0.16 |
| DBP (mmHg) | 77.8±6.3 | 73.4±9.7 | 0.07 | |
| HR (/min) | 73.4±1.4 | 73.7±3.8 | 0.67 | |
| Postdialysis | SBP (mmHg) | 134.9±15.5 | 125.4±21.6 | 0.09 |
| DBP (mmHg) | 75.2±6.1 | 71.0±9.8 | 0.09 | |
| HR (/min) | 74.0±1.2 | 74.1±2.3 | 0.82 | |
| Interdialytic weight gain (%) | 2.8±1.3 | 3.4±1.3 | 0.11 | |
| Processed blood volume (L) | 75.0±4.5 | 78.6±7.0 | 0.07 | |
| Blood flow (ml/min) | 312.2±18.8 | 324.5±25.8 | 0.07 | |
| Serum albumin (g/dL) | 3.9 ± 0.2 | 3.8±0.3 | 0.28 | |
| C-reactive protein (mg/L) | 17.6* ± 17.3 | 18.6*±35.7 | 0.91* | |
| nPCR (g/kg/day) | 1.0±0.1 | 1.0±0.1 | 0.08 | |
| ALT (UI/L) | 29.9*±14.3 | 29.1±7.1 | 0.82* | |
| ALP (UI/L) | 92.5±42.0 | 79.9±37.1 | 0.29 | |
| Glucose (mg/dL) | 86.4±18.0 | 91.4±12.9 | 0.29 | |
| Potassium (mEq/L) | 4.9±0.5 | 4.7±0.5 | 0.16 | |
| Sodium (mEq/L) | 137.0±1.8 | 136.5±1.8 | 0.38 | |
| Bicarbonate (mEq/L) | 21.0±1.9 | 20.7±1.6 | 0.56 | |
| Calcium (mg/dL) | 8.8±0.5 | 8.8±0.8 | 0.94 | |
| Phosphate (mg/dL) | 5.5±1.4 | 5.1±0.9 | 0.24 | |
| Calcium × Phosphate (mg2/dL2) | 48.4±12.5 | 44.8±9.2 | 0.25 | |
| iPTH (ng/L) | 454.8*±487.3 | 420.5*±423.1 | 0.80* | |
| Predialysis creatinine (mg/dL) | 8.6±2.3 | 10.1±1.9 | 0.02 | |
| Predialysis urea (mg/dL) | 126.8±23.3 | 139.8±26,5 | 0.08 | |
| Postdialysis urea (mg/dL) | 36.7±9.8 | 37.2±10.4 | 0.88 | |
| Urea reduction ratio (%) | 71.1±5.3 | 73.5±4.6 | 0.11 | |
| Cholesterol (mg/dL) | 178.3±37.9 | 184.2±48.6 | 0.94 | |
| LDL cholesterol (mg/dL) | 113.5*±31.6 | 114.0±42.4 | 0.80* | |
| HDL cholesterol (mg/dL) | 38.5±10.8 | 35.3±9.5 | 0.29 | |
| Triglycerides (mg/dl) | 131.6*±90.0 | 171.6*±122.4 | 0.22* | |
| Hemoglobin (g/dL) | 11.5±0.8 | 11.55±1.4 | 0.83 | |
| Red blood cells (no/mm3) | 3.7±0.3 | 3.7±0.5 | 0.90 | |
| Hematocrit (%) | 35.3± .6 | 35.2±4.3 | 0.92 | |
| Ferritin (μg/L) | 1283.4±508.4 | 1174.3*±695.7 | 0.55 | |
| Transferrin saturation (%) | 37.2±14.1 | 48.2±19.2 | 0.03 | |
| White blood cells (no/mm3) | 6766.6±1343.4 | 6079.0±1423.5 | 0.10 | |
| Platelet count (no/mm3) | 213905.0±68625.5 | 200617.9±49439.7 | 0.46 | |
| Equilibrated KT/V (ml/min) | 1.3±0.2 | 1.4±0.2 | 0.07 | |
| Single pool KT/V (ml/min) | 1.5±0.2 | 1.6±0.2 | 0.07 | |
non normal distribution (Mann–Whitney U-test);
BMI: body mass index; nPCR: normalized protein catabolic rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; iPTH: intact parathormone
Arterial stiffness and blood pressure parameters were normally distributed. We used the Student t-test to compare the means between the study groups.
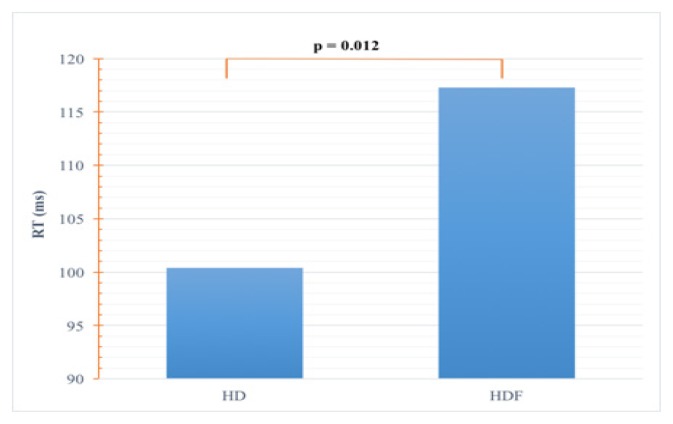
The RT was significantly shorter in HD compared to HDF patients (100.4±23 ms versus 117.3±20.5 ms, p=0.012) (Figure 1).
Figure 1.
Comparison of RT.
RT: return time of the pulse wave
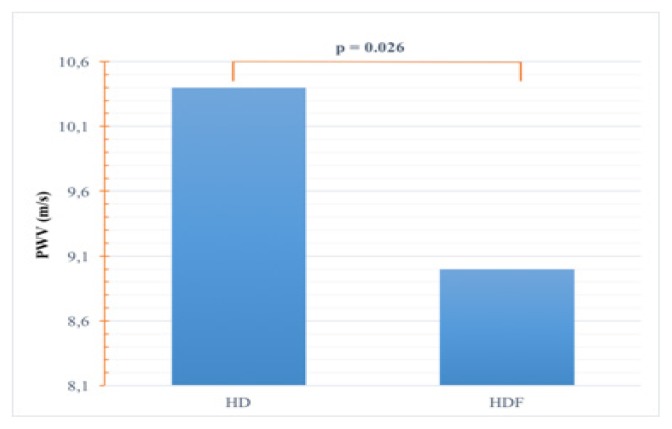
PWV was significantly higher in HD compared to HDF patients (10.4±2.3 m/s versus 9.0±1.7 m/s, p=0.026) (Figure 2).
Figure 2.
Comparison of PWV.
PWV: pulse wave velocity
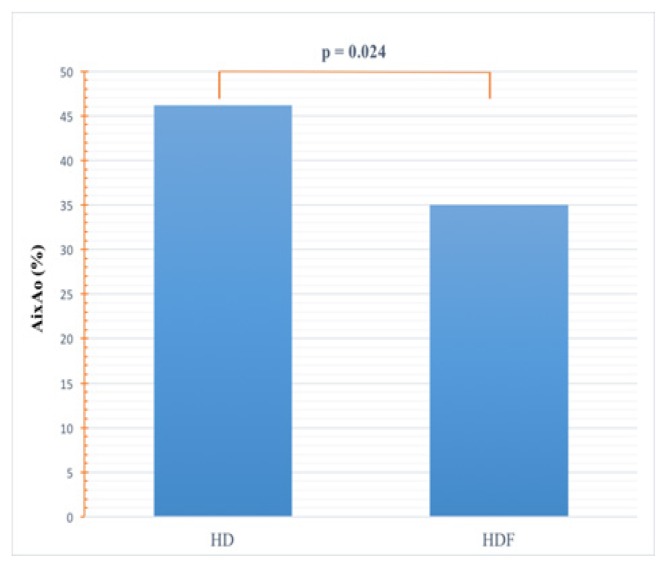
AixAo was abnormally elevated (>10%) in both dialysis patient groups but still significantly higher in HD compared to HDF patients (46.2±14.2% versus 35±18%, p=0.0243) (Figure 3).
Figure 3.
Comparison of AixAo.
AixAo: aortic augmentation index
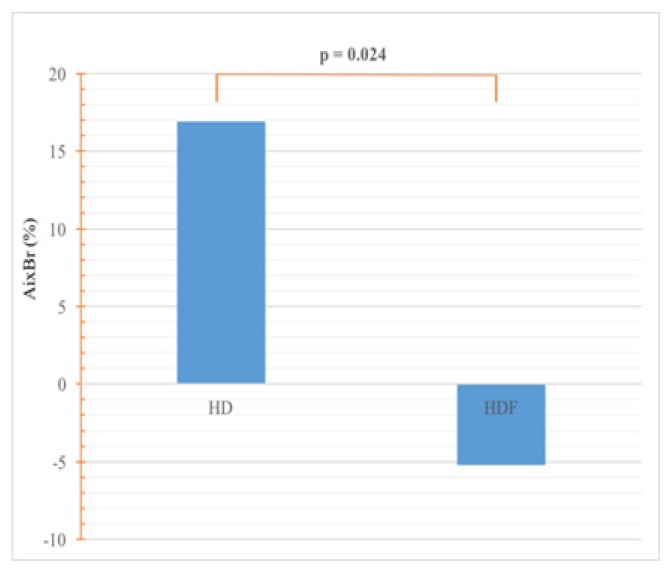
AixBr was elevated in HDF patients and abnormally elevated in HD patients, which represents a significant difference between groups (16.9±28% versus −5.19±35.58, p=0.0244) (Figure 4).
Figure 4.
Comparison of AixBr.
AixBr: brachial augmentation index
Aortic and brachial blood pressure, and derivate parameters (MAP: mean arterial pressure; PP: pulse pressure) were elevated from normal values but did not differ significantly among the dialysis patient groups.
Discussion
Arterial stiffness is a comprehensive term encompassing the characteristics of the entire arterial system, including biochemical, structural, and mechanical changes in the wall of small and large arteries, as well as the comparative pressures. The direct wave traveling from the heart, the reflective wave and the systolic and diastolic periods can be determined from the pulse wave contour [20].
The pulse wave is affected by the elasticity of the aortic wall and the actual resistance of all peripheral vasculature. PWV is a marker of arterial stiffness and it reflects arterial wall structure and function [21]. The augmentation index is the mirror and the measure of the actual dilatation capacity of arterioles (peripheral arterial resistance) [22].
Most arterial stiffness parameters were pathologic in dialysis patients compared to controls. The increased arterial stiffness of dialysis patients translates into decreased aortic wall elasticity and peripheral artery (arteriolar) distensibility.
In our HD patients, taking into account the normal range, PWV was characterized as elevated. HD patients’ high arterial stiffness reflects aortic rigidity (high PWV) and severely increased peripheral arterial resistance as reflected by the increased augmentation indexes. By contrast, HDF patients had normal PWV, hence better aortic distensibility and a milder increase in peripheral arterial resistance (Table II).
Table II.
Summary of arterial stiffness status in dialysis patients.
| PWV | AixAo | AixBr | DRA | |
|---|---|---|---|---|
| HD | ↑ | ↑↑ | ↑↑ | ↓↓ |
| HDF | Normal | ↑ | ↑ | Normal* |
DRA normal range not defined by TensioMed
We found higher augmentation indexes in HD patients who had high blood pressure. As the mean blood pressure drop over the circulation occurs mostly along the small arteries and arterioles [20], the increased peripheral vascular resistance and endothelial dysfunction could account for the excess Aix in HD patients. Increasing the HD dose was shown to promote better BP control but had mixed effects on arterial stiffness and augmentation indexes [23,24]. The cross-sectional study design and relative small number of subjects could be the main limitations of the current study. Further prospective randomized studies with extended follow-up are needed to address the issue of arterial stiffness in HD versus HDF.
Conclusions
Arterial stiffness (PWV and Aix) was higher in HD patients compared to HDF treated patients, independent of age, dialysis vintage and the dialysis dose. These findings would suggest that postdilution online HDF could have an important favorable effect on arterial stiffness in dialysis patients.
References
- 1.Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int. 2003;63(5):1852–1860. doi: 10.1046/j.1523-1755.2003.00932.x. [DOI] [PubMed] [Google Scholar]
- 2.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 3.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM, et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39(3):735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 4.Munakata M, Sakuraba J, Tayama J, Furuta T, Yusa A, Nunokawa T, et al. Higher brachial-ankle pulse wave velocity is associated with more advanced carotid atherosclerosis in end-stage renal disease. Hypertens Res. 2005;28(1):9–14. doi: 10.1291/hypres.28.9. [DOI] [PubMed] [Google Scholar]
- 5.Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol. 2001;12(10):2117–2124. doi: 10.1681/ASN.V12102117. [DOI] [PubMed] [Google Scholar]
- 6.Kaur M, Lal C, Bhowmik D, Jaryal AK, Deepak KK, Agarwal SK. Reduction in augmentation index after successful renal transplantation. Clin Exp Nephrol. 2013;17(1):134–139. doi: 10.1007/s10157-012-0653-z. [DOI] [PubMed] [Google Scholar]
- 7.Tawadrous H, Kamran H, Salciccioli L, Schoeneman MJ, Lazar J. Evaluation of arterial structure and function in pediatric patients with end-stage renal disease on dialysis and after renal transplantation. Pediatr Transplant. 2012;16(5):480–485. doi: 10.1111/j.1399-3046.2012.01721.x. [DOI] [PubMed] [Google Scholar]
- 8.Hotta K, Harada H, Sasaki H, Iwami D, Fukuzawa N, Morita K, et al. Successful kidney transplantation ameliorates arterial stiffness in end-stage renal disease patients. Transplant Proc. 2012;44(3):684–686. doi: 10.1016/j.transproceed.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Buhaescu I, Covic M. Successful renal transplantation decreases aortic stiffness and increases vascular reactivity in dialysis patients. Transplantation. 2003;76:1573–1577. doi: 10.1097/01.TP.0000086343.32903.A8. [DOI] [PubMed] [Google Scholar]
- 10.Demirci MS, Celik G, Ozkahya M, Tumuklu M, Toz H, Asci G, et al. Effects of thrice weekly nocturnal hemodialysis on arterial stiffness. Atherosclerosis. 2012;220(2):477–485. doi: 10.1016/j.atherosclerosis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Chan CT, Jain V, Picton P, Pierratos A, Floras JS. Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int. 2005;68(1):338–344. doi: 10.1111/j.1523-1755.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 12.Canaud B, Bragg-Gresham JL, Marshall MR, Desmeules S, Gillespie BW, Depner T, et al. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006;69:2087–2093. doi: 10.1038/sj.ki.5000447. [DOI] [PubMed] [Google Scholar]
- 13.Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–1096. doi: 10.1681/ASN.2011121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28(1):192–202. doi: 10.1093/ndt/gfs407. [DOI] [PubMed] [Google Scholar]
- 15.Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008;23(7):2337–2343. doi: 10.1093/ndt/gfm951. [DOI] [PubMed] [Google Scholar]
- 16.Penne EL, van der Weerd NC, Bots ML, van den Dorpel MA, Grooteman MP, Lévesque R, et al. Patient- and treatment-related determinants of convective volume in post-dilution haemodiafiltration in clinical practice. Nephrol Dial Transplant. 2009;24:3493–3499. doi: 10.1093/ndt/gfp265. [DOI] [PubMed] [Google Scholar]
- 17.Penne EL, van Berkel T, van der Weerd NC, Grooteman MP, Blankestijn PJ. Optimizing haemodiafiltration: tools, strategy and remaining questions. Nephrol Dial Transplant. 2009;24:3579–3581. doi: 10.1093/ndt/gfp333. [DOI] [PubMed] [Google Scholar]
- 18.Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 19.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde Richard. Cardiovascular Physiology Concepts. Lippincott Williams & Wilkins; 2005. pp. 93–94. [Google Scholar]
- 21.Nichols WW, O‘Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 4th ed. London: Edward Arnold; 1998. Vascular impedance; pp. 54–97.pp. 243–283.pp. 347–395. [Google Scholar]
- 22.Baulmann J, Schillings U, Rickert S, Uen S, Dusing R, Illyes M, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26:523–528. doi: 10.1097/HJH.0b013e3282f314f7. [DOI] [PubMed] [Google Scholar]
- 23.van Eps CL, Jeffriess L, Haluska B, Hawley CM, Coombes J, Matsumoto A, et al. Cardiac and vascular structure and function parameters do not improve with alternate nightly home hemodialysis: an interventional cohort study. BMC Nephrol. 2011 Oct 3;12:51. doi: 10.1186/1471-2369-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber T, O’Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21(11):1194–1202. doi: 10.1038/ajh.2008.277. [DOI] [PubMed] [Google Scholar]