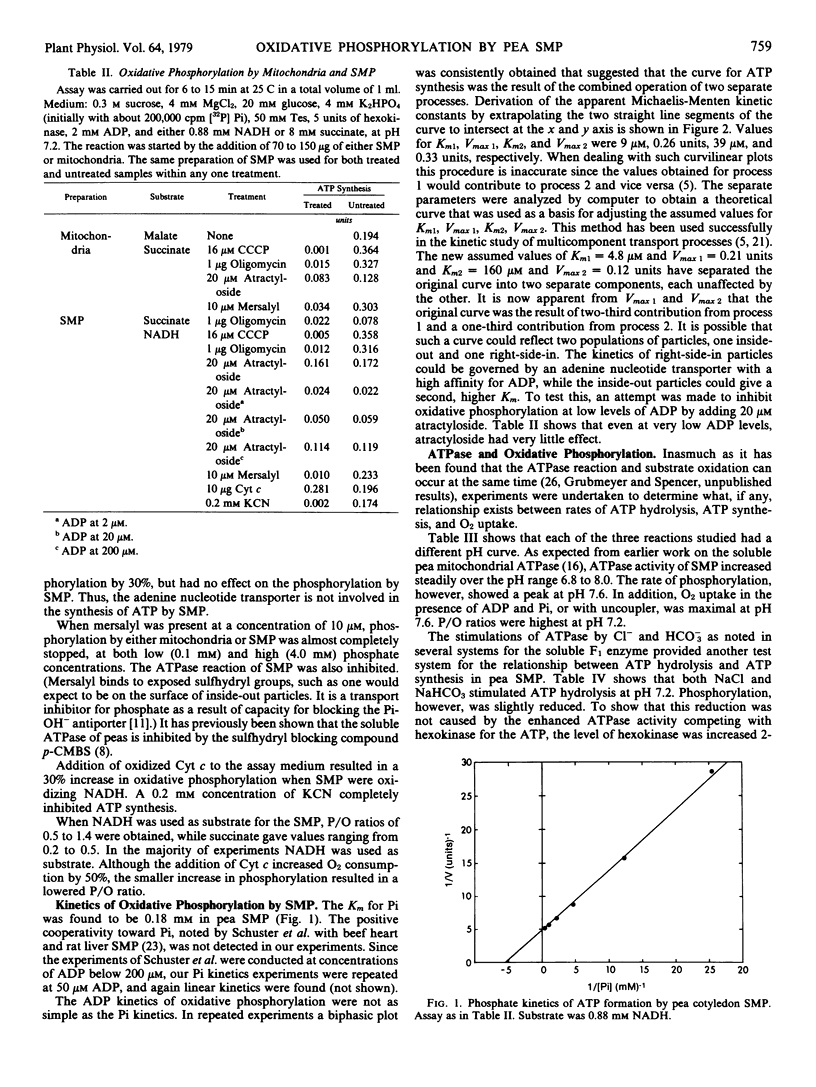
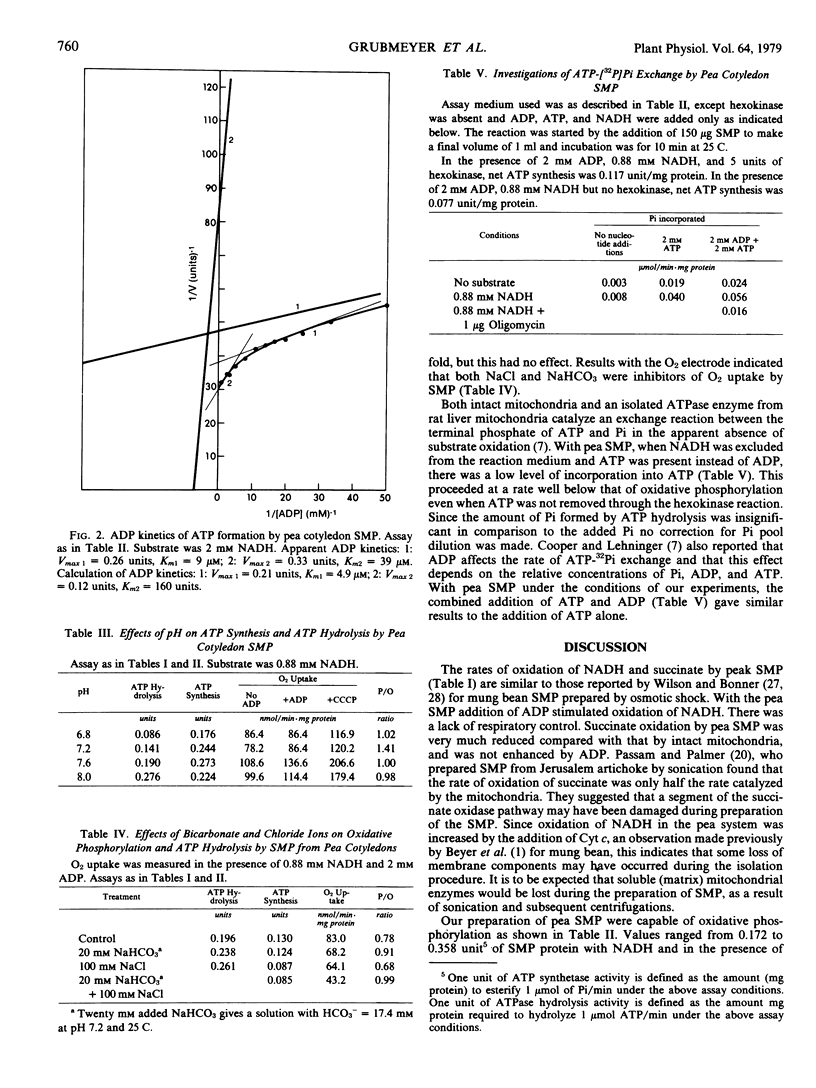
Abstract
Mitochondria and submitochondrial particles (SMP) from pea cotyledons were shown to catalyze oxidative phosphorylation as measured by 32Pi uptake into phosphate esters. ATP synthesis was sensitive to the electron transport inhibitor KCN, the uncoupler carbonyl cyanide m-chlorophenylhydrazone, and the coupling factor inhibitor oligomycin. Experiments with the adenine nucleotide translocator inhibitor atractyloside indicated the SMP were inside-out. Mersalyl completely inhibited ATP synthesis by SMP, and a separate experiment indicated that mersalyl has a direct effect on the ATPase complex. The kinetics of ATP synthesis indicated a high affinity for phosphate (Km = 0.18 millimolar). ADP kinetics gave a biphasic curve with Km values of about 4.8 and 160 micromolar. O2 uptake and ATP synthesis had a pH maximum of 7.6 while the ratio of micromoles phosphate esterified to microatoms O2 taken up was highest at pH 7.2. Sodium chloride inhibited both ATP synthesis and O2 uptake but stimulated the ATPase reaction. The SMP also catalyzed a slow ATP-phosphate exchange reaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G. H., Spencer M. Optimum pH and ionic strength for the assay of cytochrome c oxidase from pea cotyledon mitochondria. Can J Biochem. 1977 Oct;55(10):1114–1117. doi: 10.1139/o77-165. [DOI] [PubMed] [Google Scholar]
- Bygrave F. L., Lehninger A. L. The affinity of mitochondrial oxidative phosphorylation mechanisms for phosphate and adenosine diphosphate. Proc Natl Acad Sci U S A. 1967 May;57(5):1409–1415. doi: 10.1073/pnas.57.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER C., LEHNINGER A. L. Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. V. The adenosine triphosphate-phosphate exchange reaction. J Biol Chem. 1957 Jan;224(1):561–578. [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. II. Interaction with adenosine diphosphate. J Biol Chem. 1972 Dec 25;247(24):7969–7976. [PubMed] [Google Scholar]
- Christiansen R. O., Loyter A., Racker E. Effect of anions on oxidative phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1969 May;180(1):207–210. doi: 10.1016/0005-2728(69)90212-6. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C., Duncan I., Spencer M. Partial characterization of a soluble ATPase from pea cotyledon mitochondria. Can J Biochem. 1977 Aug;55(8):812–818. doi: 10.1139/o77-120. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C., Spencer M. Oligomycin-sensitive ATPase of Submitochondrial Particles from Corn. Plant Physiol. 1978 Apr;61(4):567–569. doi: 10.1104/pp.61.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Bertagnolli B. L., Shepherd W. D. Phosphate-induced Stimulation of Acceptorless Respiration in Corn Mitochondria. Plant Physiol. 1972 Sep;50(3):347–354. doi: 10.1104/pp.50.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Miller R. J., Dumford S. W. Uncoupling of respiration-linked contraction in corn mitochondria. Plant Physiol. 1968 May;43(5):811–814. doi: 10.1104/pp.43.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malhotra S. S., Spencer M. Effects of ethylene, carbon dioxide, and ethylene - carbon dioxide mixtures on the activities of "membrane-containing" and "highly purified" preparations of adenosine triphosphatase from pea-cotyledon mitochondria. Can J Biochem. 1974 Dec;52(12):1091–1096. doi: 10.1139/o74-153. [DOI] [PubMed] [Google Scholar]
- Malhotra S. S., Spencer M. Preparation and properties of purified (Na+ plus K+)-stimulated mitochondrial ATPase from germinating pea seeds. Can J Biochem. 1974 Jun;52(6):491–499. doi: 10.1139/o74-073. [DOI] [PubMed] [Google Scholar]
- Miller G. W., Hsu W. J. Effects of carbon dioxide-bicarbonate mixtures on oxidative phosphorylation by cauliflower mitochondria. Biochem J. 1965 Dec;97(3):615–619. doi: 10.1042/bj0970615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozersky S. M., Pettinati J. D., Kolman S. D. An improved method for the determination of orthophosphate suitable for assay of adenosine triphosphatase activity. Anal Chem. 1966 Aug;38(9):1182–1187. doi: 10.1021/ac60241a015. [DOI] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Schuster S. M., Reinhart G. D., Lardy H. A. Studies on the kinetic mechanism of oxidative phosphorylation. J Biol Chem. 1977 Jan 25;252(2):427–432. [PubMed] [Google Scholar]
- Solomos T., Malhotra S. S., Prasad S., Malhotra S. K., Spencer M. Biochemical and structural changes in mitochondria and other cellular components of pea cotyledons during germination. Can J Biochem. 1972 Jul;50(7):725–737. doi: 10.1139/o72-101. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y. Respiration-department uncoupler-stimulated ATPase activity in castor bean endosperm mitochondria and submitochondrial particles. Biochim Biophys Acta. 1975 Mar 20;376(3):505–518. doi: 10.1016/0005-2728(75)90171-1. [DOI] [PubMed] [Google Scholar]



