Abstract
Stroke is associated with vulnerable carotid artery plaques showing specific histopathologic features, namely a lipid-rich necrotic core, intraplaque hemorrhage, ulceration, and thin fibrous cap. While ultrasound and computed tomography (CT) can identify carotid plaques and determine the extent of stenosis, magnetic resonance imaging (MRI) provides further information regarding plaque composition and morphology.
In this feasibility study, three patients with symptomatic, moderately stenosed plaques were imaged with CT angiography (CTA) and MRI (3T and 1.5T) without a dedicated receiver coil. The patients subsequently underwent carotid endarterectomy with en-bloc excision of the plaque. The CT and MR images were analyzed independently by three neuroradiologists to identify vulnerable plaque features. The images were correlated with the histopathology to confirm the findings.
All three patients had one or more vulnerable plaque features on histopathology. MRI allowed for better characterization of these features when compared to CTA. The pre- and post-contrast T1-weighted (T1W) images were most helpful for identifying the lipid-rich necrotic core and thin fibrous cap, while the time of flight-magnetic resonance angiography (TOF-MRA) and contrast-enhanced (CE)-MRA were excellent for detecting plaque hemorrhage and ulceration, respectively. The 3T images showed superior spatial and contrast resolution compared to the 1.5T images for all sequences.
By providing direct correlation between imaging and histopathology, this study demonstrates that 3T MRI without a dedicated surface coil is an excellent tool for assessing plaque vulnerability. In smaller hospitals or those with limited resources, it is reasonable to consider conventional MRI for patient risk stratification. Further studies are needed to determine how MRI and plaque vulnerability can be incorporated into routine clinical practice.
Keywords: Stroke, carotid stenosis, plaque, atherosclerotic, carotid artery disease, endarterectomy, carotid
Introduction
Stroke risk is determined by a number of biological factors, among them the presence of extracranial atherosclerotic disease. Carotid artery stenosis is a well-established risk factor for ischemic stroke, estimated to account for 10%–20% of strokes or transient ischemic attacks (TIAs).1 However, there is increasing evidence to suggest that the degree of stenosis measured on ultrasound is not sufficient to accurately predict stroke risk secondary to plaque burden.1 The landmark European Carotid Surgery Trial (ECST) demonstrated that of the 3019 participants with symptomatic carotid disease, nearly half (43.8%) had <30% stenosis.2 This suggests that other major factors, including plaque morphology and composition, play a key role in predicting future ischemic events.1
The current literature defines vulnerable carotid plaques as unstable lesions associated with an increased risk of rupture and thromboembolic events.3 The key characteristics associated with increased plaque vulnerability that have been identified on post-endarterectomy histopathologic specimens include intraplaque hemorrhage (IPH), ulceration, a thin fibrous cap, and the presence of a lipid-rich necrotic core (LRNC).1
While computed tomography (CT) and ultrasound can identify carotid plaques, magnetic resonance imaging (MRI) has the ability to further characterize plaques based on their composition and morphology.1 The aforementioned histopathologic features of vulnerable plaques can be appreciated on conventional MRI.4 In addition to direct visualization of the diseased vessel with excellent soft tissue contrast, MRI can be used to monitor the progression of disease and response to pharmacologic treatment.5
We present the CT and MR images (3T and 1.5T) and corresponding histopathology for three patients with symptomatic carotid atherosclerotic disease and moderate stenosis (50%–70%) on ultrasound. The goal of this feasibility study was to identify vulnerable carotid plaque features on MRI without a dedicated surface coil, providing direct correlation with histopathology to confirm the imaging findings and obtain consensus. MR images both from 1.5T and 3T scanners are also presented to determine whether magnet strength affects the reader’s ability to identify vulnerable plaque features.
Materials and methods
Study design
This study followed the policies of our local research ethics board. Three patients with symptomatic carotid atherosclerosis were selected for this study. All participants had moderate carotid artery stenosis (50%–70%) on ultrasound. Each patient had a CT angiogram and MRI study in addition to the ultrasound. Patients subsequently underwent revascularization with carotid endarterectomy and carotid plaques were excised en bloc.
The CT and MR images were analyzed independently by three fellowship-trained neuroradiologists, with an average of 10 years of experience, who were blinded to clinical data and other imaging tests. Vulnerable plaque features were identified using the data from Underhill et al.2 and Gupta et al.6 as a reference, in order to improve the accuracy of interpretation. The imaging findings were relayed to a research assistant who was not involved in interpretation. The histopathology was reviewed by a pathologist with expertise in cardiovascular disease. The research assistant generated a single file for each patient, allowing for side-by-side comparison of the imaging and histopathology. Each file was reviewed by all three neuroradiologists to resolve discrepancies and reach a consensus.
CT acquisition and processing
The scans were performed on a Toshiba Aquilion One CT scanner. The start of the acquisition was determined using a bolus tracking method following the administration of 60 ml of iodinated contrast medium (Isovue 370, Bracco Imaging). The source data were then reconstructed into 1 mm axial, 7 × 3 mm axial, sagittal and coronal maximum intensity projection (MIP) images and sent to picture archiving and communication system (PACS) for review.
MRI acquisition and processing
Imaging of the carotid arteries was performed on 1.5T and 3T scanners (Siemens), using a Siemens Standard Neck Matrix Coil for MAGNETOM Symphony and MAGNETOM Trio, Siemens Healthcare GmbH, Germany. A standardized carotid plaque protocol was used to produce the image of the carotid vessel that included the following sequences: three-dimensional (3D) time of flight (TOF) (repetition time (TR): 4.9, echo time (TE): 2.0), axial turbo spin echo (TSE) fast spin (FS) T1WI (2 mm thick), axial TSE FS T2WI, axial TSE FS proton density-weighted imaging (PDWI), long-axial TSE FS T1WI (2 mm thick), Dixon volumetric interpolated breath-hold examination (VIBE) (in phase, out of phase, fat image and water image; TR: 15.2, TE: 4.9, matrix: 320 × 320, slice thickness: 0.76, flip angle (FA): 9, NEx: 1, echo: 1), CE MRA (TR: 3, TE: 1.15, matrix: 512 × 80%, slice res: 60%, FA: 24, NEx: 1) and post-contrast axial TSE FS T1WI.
Results
Case 1
Clinical information: 60-year-old male presenting with transient left-sided visual loss with prior TIA. The CTA was performed first, followed by the MRI eight days later.
The large plaque in the left common carotid artery on CTA is noted to have a thin fibrous cap, LRNC, and focal area of ulceration on histopathology (Figure 1). The 3T pre- and post-contrast T1W images show a hypointense LRNC, best seen on the post-contrast image (Figure 2(b)). Plaque ulceration is seen on both the TOF and contrast-enhanced (CE)-MRA images (Figure 3), although TOF MRA underestimates the area of ulceration because of turbulent flow and subsequent signal dropout. The LRNC can also be appreciated on TOF-MRA as an isointense region (Figure 3).
Figure 1.
Case 1. Comparison between computed tomography angiography (CTA) and histopathology. The CTA ((a) enlarged on (c)) shows a large plaque in the left common carotid artery, with the asterisk indicating the vessel lumen. The lipid-rich necrotic core (green arrow) seen on histopathology (b) is also noted on CTA as a hypodense region surrounded by hyperdense plaque calcification (arrowheads). There is a focal area of ulceration (dashed arrow) that extends into the plaque wall (c). The thin fibrous cap (chevron) noted on histopathology (b) is not appreciated on CTA.
Figure 2.
Case 1. Comparison between 1.5T and 3T. (a) Sagittal 3.0T T1-weighted (T1W) image localizer on the left with corresponding 3T (top row) and 1.5T (bottom row) axial magnetic resonance (MR) images of a left internal carotid artery plaque in the same patient, just above the bifurcation. The vulnerable plaque is indicated by the dashed yellow line. (b) Enlarged pre- and post-contrast 3T T1W images demonstrate a hypointense lipid-rich necrotic core (green arrow), better appreciated on the post-contrast study. The lumen of the vessel is indicated by an asterisk.
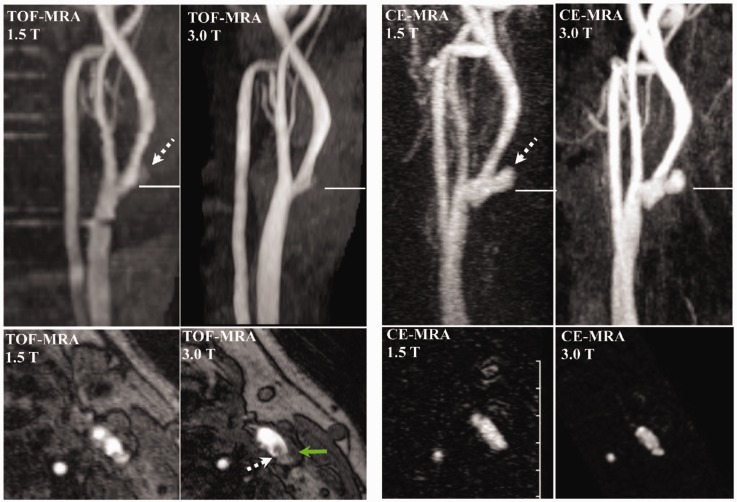
Figure 3.
Case 1. Comparison between time of flight (TOF) and contrast-enhanced magnetic resonance angiography (CE-MRA) in 1.5T and 3T. Coronal and axial T and 1.5 T TOF-MRA (left panel) and contrast-enhanced MRA maximum intensity projection (MIP) (right panel) images of the plaque in the left carotid artery bifurcation. The solid line in the coronal images denotes the level of the axial images below. Of note, the axial TOF-MRA images presented here are below the level of the MR images and histopathology presented in Figures 1 and 2. A plaque ulceration (dashed arrow) is noted both on the TOF and contrast-enhanced MRA images. The area of ulceration is underestimated on TOF-MRA because of turbulent flow causing signal dropout. The isointense region (green arrow) on the 3T axial TOF-MRA represents the lipid-rich necrotic core.
Case 2
Clinical information: 65-year-old male presenting with left arm and facial weakness that resolved after one hour. The CTA was acquired five days after the MRI.
There is a large plaque in the right common carotid artery with several vulnerable features identified on histopathology, including an LRNC, calcifications, a thin fibrous cap, and small area of IPH (Figure 4). The LRNC is once again demonstrated on the 3T T1W images as a hypointense area (Figure 5). The high-intensity band on the post-contrast TIW image represents the thin fibrous cap. The IPH appears iso- to hyperintense on the post-contrast T1W image. The intact, thin fibrous cap is also seen on TOF-MRA as a dark band (Figure 6). The hyperintense signal noted on TOF-MRA is consistent with IPH.
Figure 4.
Case 2. Comparison between histopathology and computed tomography angiography (CTA). Large plaque in the right common carotid artery, where the asterisk denotes the vessel lumen. Vulnerable plaque characteristics seen on histopathology (b) include a lipid-rich necrotic core (green arrows), calcifications (arrowhead), thin fibrous cap (chevron) and a small area of intraplaque hemorrhage (red arrow). The lipid-rich necrotic core (green arrow) is easily identifiable on the CTA ((a) enlarged on (c)) as a hypodense area surrounded by peripheral calcification (arrowhead). The area of hemorrhage (red arrow, (b)) cannot be reliably distinguished from the surrounding fat necrosis on CTA (green arrow, (c)).
Figure 5.
Case 2. Comparison between 1.5T and 3T. (a) 3T time of flight-magnetic resonance angiography (TOF-MRA) localizer with corresponding 3T (top row) and 1.5T (bottom row) axial MR images of the right carotid artery plaque (dashed yellow line), located at the carotid bifurcation. (b) Enlarged pre- and post-contrast 3T T1-weighted (T1W) images demonstrate a hypointense lipid-rich necrotic core (green arrow) and adjacent intraplaque hemorrhage (red arrow), which appears iso- to hyperintense on the post-contrast image. The fibrous cap (chevron) is noted as a high-intensity band on the post-contrast T1W image.
Figure 6.
Case 2. Comparison between 1.5T and 3T coronal contrast-enhanced (CE) magnetic resonance angiography (MRA) and axial time of flight (TOF)-MRA images of the plaque located at the carotid bifurcation. The solid line on the CE-MRA indicates the level of the axial TOF-MRA images below. The dark band on the axial TOF-MRA represents a thin fibrous cap (chevron), which appears to be intact. The wall of the plaque is bright on TOF-MRA, consistent with intraplaque hemorrhage (red arrow).
Case 3
Clinical information: 50-year-old male presented with headache, left arm weakness, and dysarthria. The majority of his symptoms resolved after 24 hours, with some residual left arm weakness. The patient had the MRI 19 days after the CTA.
There is a large plaque in the right internal carotid artery that has an LRNC, central area of IPH, calcifications, thin fibrous cap and significant area of ulceration on histopathology (Figure 7). The hypodense area on CTA represents the LRNC and the hyperdense area is consistent with calcification. The IPH cannot be reliably identified on CTA. The hypointense region on the 3T pre- and post-contrast T1W images is consistent with the focal area of calcification and fat necrosis (Figure 8). An area of ulceration is noted both on TOF-MRA and CE-MRA, but the size of the ulceration is overestimated on TOF because of turbulent flow (Figure 9). The hyperintense region on the axial TOF-MRA represents an area of ulceration adjacent to the thin fibrous cap.
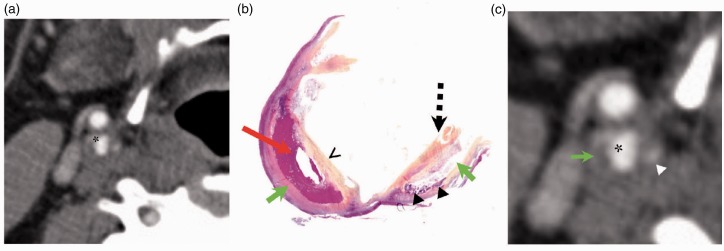
Figure 7.
Case 3. Comparison between computed tomography angiography (CTA) and histopathology. Large plaque in the right internal carotid artery, where the asterisk denotes the lumen of the artery ((a), (c)). The vulnerable plaque features identified on pathology (b) include a lipid-rich necrotic core (LRNC) (green arrows) with a central region of intraplaque hemorrhage (IPH) (red arrow), calcifications (arrowheads) and a very thin fibrous cap (chevron) with a notable area of ulceration (dashed arrow). The LRNC (green arrow) is seen as a hypodense area on CTA (c). The hyperdense area on CTA (arrowhead) corresponds with the calcifications seen on histopathology (c). The intraplaque hemorrhage (b) cannot be reliably identified on CTA.
Figure 8.
Case 3. Comparison between 1.5T and 3T. (a) 3T Contrast-enhanced magnetic resonance angiography (MRA) localizer with corresponding 3T (top row) and 1.5T (bottom row) axial MR images of the right internal carotid artery plaque outlined by the dashed yellow line. The vulnerable area has a variable appearance on MR while the calcifications are iso- to hypointense on all sequences. (b) Enlarged axial 3T pre- and post-contrast T1-weighted (T1W) images show a hypointense area with minimal enhancement, consistent with the focal area of lipid necrosis and calcification seen on histopathology.
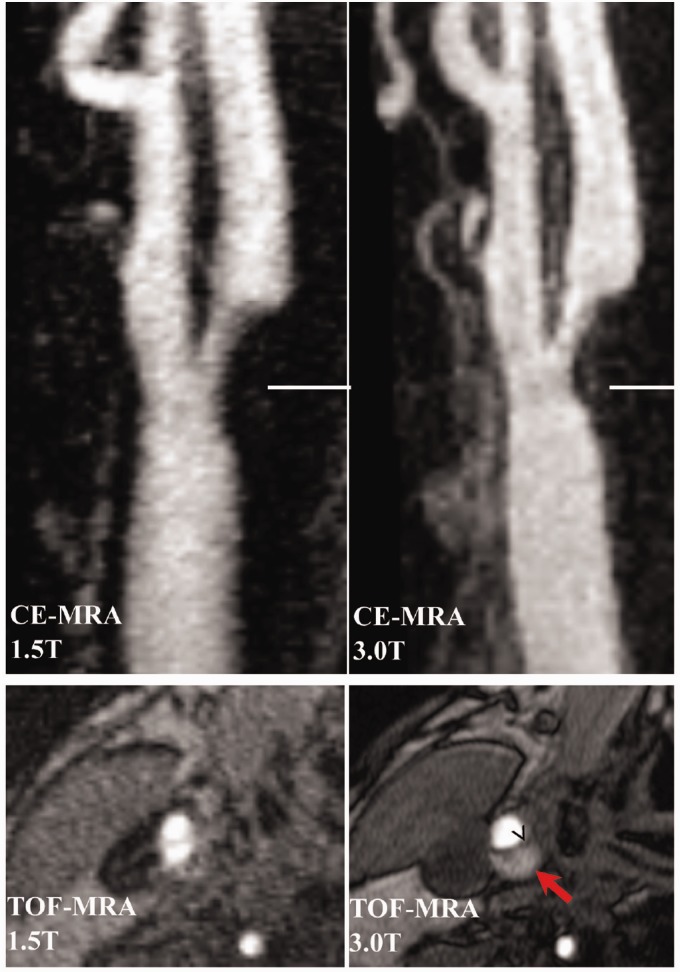
Figure 9.
Case 3. 1.5T and 3T time of flight-magnetic resonance angiography (TOF-MRA) (left panel) and contrast-enhanced (CE)-MRA maximum intensity projection (MIP) (right panel) showing a right internal carotid artery plaque. The horizontal lines in the coronal images indicate the level of the axial images below. Of note, the CE-MRA images were acquired coronally so the reconstructed axial images could not be saved. An area of ulceration (dashed arrows) is noted both on TOF-MRA and CE-MRA, but the size of the ulceration is overestimated on TOF because of turbulent flow. The thin fibrous cap (chevron) is seen as a hypoattentuating signal on the axial TOF-MRA with an adjacent area of ulceration that appears hyperintense.
Discussion
Vulnerable carotid artery plaques are a major risk factor for TIAs and ischemic stroke. Imaging can play a critical role in predicting and preventing future ischemic events when the appropriate modalities are used. While ultrasound and CT are useful for determining the degree of stenosis, MRI provides additional information about plaque composition and morphology. To our knowledge, this is the first study providing direct comparison between the conventional MR images and histopathology of vulnerable carotid artery plaques.
All three patients in this study were found to have vulnerable carotid artery plaques on histopathology, including an LRNC, IPH, thin fibrous cap, and ulceration. This is consistent with the literature suggesting that symptomatic plaques have higher rates of IPH, LRNC, and a thin fibrous capsule compared to stable plaques, which generally have a thick capsule and no LRNC.1
Several vulnerable plaque features were identifiable on CTA including an LRNC, which appeared hypodense, and focal areas of calcification (Figures 1, 4, and 7). CTA was also useful for detecting plaque ulceration (Figures 1 and 7). One challenge with CTA is the overlapping Hounsfield units (HU) of the LRNC and IPH, where the median density ranges from 25 to 32.6 HU and −17 to 31 HU, respectively.4 The inability to distinguish between LRNC and IPH and the presence of calcification artifact limit the use of CTA for vulnerable plaque assessment.
The vulnerable carotid plaques had a variable appearance on MRI depending on the sequence. For this reason, a multi-sequence protocol was used in order to provide an accurate assessment of plaque composition. The T1W and T2W sequences were most useful for delineating the overall anatomy. As predicted, plaque calcification was hypointense on all sequences. The post-contrast T1W images provided improved differentiation between the thin fibrous cap and the LRNC, which was hypointense relative to the enhancing fibrous cap. At times, image interpretation was challenging because of motion and pulsation artifact; however, most of these issues were resolved by referencing the histopathology.
Hemorrhage can have a variable appearance on MRI, where recent hemorrhage (<6 weeks) is hyperintense on T1W imaging and organized hemorrhage (>6 weeks) is hypointense. Therefore, it was difficult to delineate intraplaque hemorrhage from an LRNC on T1W imaging as both can have a similar signal intensity on T1W imaging. This challenge can be overcome by acquiring the pre-gadolinium T1WI with and without fat-suppression and by TOF-MRA, which is useful for detecting oxyhemoglobin and methehemoglobin.1 Hyperacute and subacute IPH is hyperintense on TOF-MRA while LRNC is isointense, as demonstrated in Case 2 (Figures 3–6). The TOF-MRA axial images were also helpful for identifying the thin fibrous cap, which appeared as a dark band.
CE-MRA was superior to TOF-MRA for visualizing plaque ulceration, regardless of magnet strength. TOF-MRA both underestimated and overestimated the size of the ulceration when compared to CE-MRA in Case 1 (Figure 3) and Case 3 (Figure 6), respectively. This is likely due to turbulent flow and subsequent change of signal in the vessel because of the spin-dephasing phenomenon in TOF-MRA.
In all three cases, the 3T magnet was superior to the 1.5T for visualizing the vulnerable plaque features. For example, it was difficult to accurately identify the LRNC on the pre- and post-contrast T1W sequence on the 1.5T images, while the LRNC was readily seen on the 3T images in all three cases. This is in agreement with the existing literature, which suggests that 3T plaque imaging has several advantages over 1.5T, including higher signal-to-noise and contrast-to-noise ratio and overall improved image quality, scan time, and spatial resolution.7
Several carotid plaque imaging studies have used a four-channel phased-array carotid surface coil for plaque analysis.5 While the purpose of this study was not to compare MRI with and without a dedicated surface coil, it is interesting to note the findings of these studies. DeMarco et al.8 imaged both symptomatic and asymptomatic patients with 50%–99% stenosis with MRA at 3T using a research surface coil. The plaque characteristics identified in symptomatic patients with mild/moderate stenosis included a thin/ruptured fibrous cap, LRNC, IPH, and ulceration, which is comparable to the results of the current study. Of note, the surface coils used for 3T imaging of carotid plaques that are compatible with our MR scanner can be used only under a Health Canada investigational test authorization. In addition, there is no Health Canada-approved receiver coil dedicated for 1.5T imaging of carotid plaques9 so it is not current practice to routinely image patients with carotid surface coils.
There are several limitations of this study that must be addressed. The primary goal of this feasibility study was to identify vulnerable plaque features on conventional MRI without a surface coil by direct comparison with histopathology to confirm these findings. While it would be interesting to compare results with and without a dedicated surface coil, such a case-control study design would be difficult to implement because of the risks, costs, and inconvenience of imaging a patient multiple times with contrast administration. Another limitation of this study was the lack of quantitative, volumetric data. While the purpose of this study was to provide a qualitative analysis of vulnerable carotid artery plaques, in future investigations it would be interesting to determine the relationship between the volume of LRNC, IPH and calcification and the relative risk of ischemic events. Future studies with larger cohorts including both symptomatic and asymptomatic patients with variable degrees of stenosis indicated for surgical resection, should be considered.
Conclusions
Through direct histopathologic correlation, this study suggests that conventional MRI without a dedicated receiver coil is a useful modality to assess plaque vulnerability. The key vulnerable plaque features identified were an LRNC, IPH, ulceration, and a thin fibrous cap. The most useful MRI sequences were the pre- and post-contrast T1W, TOF, and CE-MRA. Three-T images were superior to 1.5T for delineating vulnerable features as a result of the improved signal-to-noise ratio and spatial resolution. This study suggests that MRI without a dedicated receiver coil is a reliable tool for identifying vulnerable plaques, with the potential to be used for risk stratification in smaller hospitals or in resource-limited areas that do not have access to dedicated surface coils. Future work is needed to determine how carotid MRI can be incorporated into the routine clinical practice for carotid atherosclerosis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brinjikji W, Huston J, 3rd, Rabinstein AA, et al. Contemporary carotid imaging: From degree of stenosis to plaque vulnerability. J Neurosurg 2016; 124: 27–42. [DOI] [PubMed] [Google Scholar]
- 2.Underhill HR, Hatsukami TS, Fayad ZA, et al. MRI of carotid atherosclerosis: Clinical implications and future directions. Nat Rev Cardiol 2010; 7: 165–173. [DOI] [PubMed] [Google Scholar]
- 3.Esposito-Bauer L, Saam T, Ghodrati I, et al. MRI plaque imaging detects carotid plaques with a high risk for future cerebrovascular events in asymptomatic patients. PLoS One 2013; 8: e67927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huibers A, de Borst GJ, Wan S, et al. Non-invasive carotid artery imaging to identify the vulnerable plaque: Current status and future goals. Eur J Vasc Endovasc Surg 2015; 50: 563–572. [DOI] [PubMed] [Google Scholar]
- 5.Balu N, Yarnykh VL, Scholnick J, et al. Improvements in carotid plaque imaging using a new eight-element phased array coil at 3T. J Magn Reson Imaging 2009; 30: 1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: A systematic review and meta-analysis. Stroke 2013; 44: 3071–3077. [DOI] [PubMed] [Google Scholar]
- 7.Yuan C, Oikawa M, Miller Z, et al. MRI of carotid atherosclerosis. J Nucl Cardiol 2008; 15: 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMarco JK, Ota H, Underhill HR, et al. MR carotid plaque imaging and contrast-enhanced mr angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: An in vivo study at 3T. AJNR Am J Neuroradiol 2010; 31: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health Canada. Medical Devices Active Licence Listing (MDALL)—Your reference tool for licensed medical devices in Canada, http://webprod5.hc-sc.gc.ca/mdll-limh/start-debuter.do?lang=eng (accessed March 2016).