Abstract
Isolated aneurysms of spinal arteries are rare. Spinal artery aneurysms are commonly found in association with spinal cord arteriovenous malformation and coarctation of aorta and rarely with aortic arch interruption and Klippel-Trenaunay syndrome. Spinal angiograms are the gold standard for diagnosing these spinal artery aneurysms but with the advances in computed tomography technology these aneurysms can also be very well demonstrated in computed tomography angiograms. We describe three cases of anterior spinal artery aneurysm, those are flow related aneurysms, associated with coarctation of aorta and with Takayasu arteritis.
Keywords: Anterior spinal artery, aneurysm, computed tomography angiography, coarctation of aorta, Takayasu arteritis
Introduction
Isolated aneurysms of spinal arteries are rare, but can be found in association with spinal cord arteriovenous malformation (AVM) and coarctation of aorta (CoA) and rarely with aortic arch interruption and Klippel- Trenaunay syndrome.1 We describe three cases of anterior spinal artery (ASA) aneurysm associated with CoA and with Takayasu arteritis that are attributed to increased flow though collateral channels due to aortic obstruction at different levels.
Case details
Case 1
An 18-year-old male patient developed sudden onset severe neck pain which was followed by hyperextension of neck and bilateral upper limb weakness (power 2/5 medical research council (MRC)) that was managed conservatively. His symptoms were relieved after a few days and upper limb power regained after three weeks to MRC 4/5. His blood pressure was 160/90 mm Hg in both arms. Computed tomography (CT) scan of the neck was performed and revealed no evidence of bone injury. Magnetic resonance imaging (MRI) of the spine demonstrated prominent flow void in cervical and upper dorsal level with subarachnoid hemorrhage in lower cervical region causing cord edema. Subarachnoid hemorrhage (SAH) was also noted in the lumbar canal (Figure 1). A ruptured spinal vascular malformation was suspected, based on the classical clinical manifestation and imaging findings.
Figure 1.
Sagittal T2 weighted image (T2 WI) of the cervical spine shows acute subarachnoid hemorrhage (SAH) causing cord edema at lower cervical level. (a) Multiple flow voids are also seen in lower cervical and upper dorsal spinal canal. (b) SAH also noted in lumbar canal.
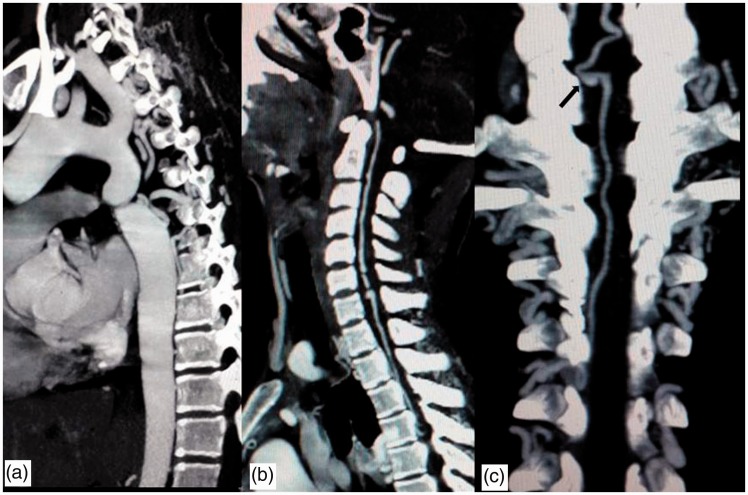
A CT angiogram was performed in a 64-slice scanner from base of skull up to aortic bifurcation. The CT angiogram revealed severe focal narrowing of the proximal descending thoracic aorta 3.8 cm after the origin of left subclavian artery (Figure 2(a)). Multiple collateral pathways were demonstrated including internal mammary, lateral thoracic, paravertebral and anterior spinal arterial axis. Dilated and tortuous ASA arising at vertebrobasilar axis is seen joining to radiculomedullary artery at D4 level and subsequently communicating with the descending thoracic aorta distal to the obstruction. The enlarged cervico-thoracic anterior spinal axis was acting as part of the collateral circulation supplying the aorta below the coarctation. A small wide-necked saccular aneurysm of size 5.6 × 3.3 mm was noted in the ASA at the level of upper border of the C7 vertebra (Figure 2(b), (c)) at the level of maximum bleed.
Figure 2.
Sagittal and coronal reconstruction MIP images of computed tomography (CT) angiogram. (a) Significant focal narrowing of the proximal descending thoracic aorta is noted 3.8 cm after the origin of the left subclavian artery. (b) The anterior spinal artery (ASA) is dilated and a small aneurysm is seen at the upper border of C7. (c) The dilated ASA joins to the radiculomedullary artery at D4 level and the aneurysm is visualized more clearly (arrow in (c)). MIP: maximum intensity projection.
Case 2
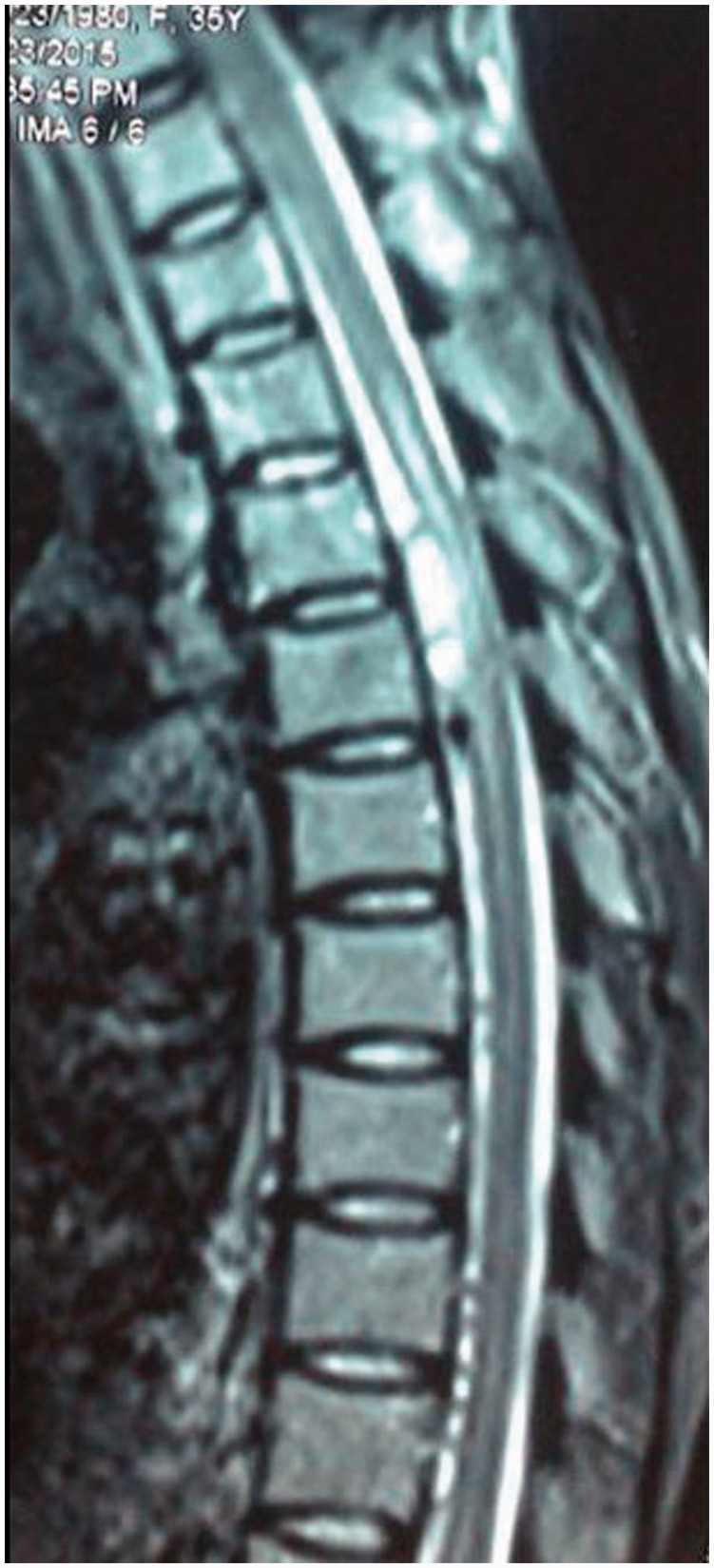
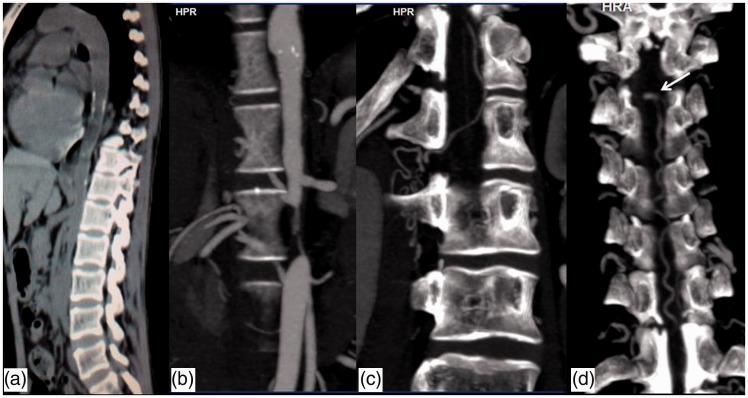
A 25-year-old lady presented with acute onset paraplegia and severe back pain. MRI of dorsal spine revealed subarachnoid hematoma at D8 and D9 level compressing the adjacent cord with focal cord edema (Figure 3). The patient underwent surgery and the hematoma was evacuated. Surgeons noticed some tortuous vessels around the cord surface at surgery and angiography was advised. Digital subtraction angiography (DSA) was carried out from the femoral route, but the guide wire could not be negotiated beyond the level of the lower abdominal aorta and the procedure was abandoned. CT angiography from the arch of the aorta to aortic bifurcation was carried out. CT angiogram revealed diffuse wall thickening of the aortic arch, descending thoracic and abdominal aorta as well as foci of aortic wall calcification (Figure 4(a)). Short segmental tight stenosis was noted in the infrarenal aorta for a length of 2.5 cm (Figure 4(b)). The right subclavian artery also showed wall thickening as well as luminal narrowing. A diagnosis of aorto-arteritis, type V Takayasu arteritis was made based on imaging findings. Multiple collateral channels (epigastric, mesenteric, paravertebral) were noted including enlarged anterior spinal arterial axis. The dilated ASA is communicating with the radicular arteries at L1 level and subsequently with the abdominal aorta caudal to the obstruction. An aneurysmal outpouching is seen from the ASA at upper border of D5 level (Figure 4(c), (d)).
Figure 3.
Sagittal T2 weighted image (T2 WI) of the dorsal spine shows hyperintense subacute subarachnoid hemorrhage (SAH) causing adjacent cord edema. Some flow voids are also noted in the lower dorsal canal.
Figure 4.
(a) NCCT thorax and abdomen, sagittal reformatted image demonstrates wall thickening and calcification involving thoracic and abdominal aorta. (b) Computed tomography (CT) angiography reveals focal tight stenosis involving infrarenal aorta. Collateral channels seen communicating the anterior spinal artery with aorta caudal to the obstruction through the radicular arteries ((b), (c) and (d)). A small aneurysmal outpouching from anterior spinal artery is seen at the level of upper border of D5 (arrow in (d)).
NCCT: Non contrast CT.
Case 3
A 46-year-old male patient presented with sudden onset of severe back pain. He also had a history of back pain and weakness of the left lower limb for one year. He also had tingling parasthesia of the left half of the body below the nipple for six months. On examination, he was hypertensive. Power in both upper limbs was 5/5 MRC, 3/5 in left hip and knee, 2/5 in left ankle and toes. Plantar was extensor bilaterally. Sensory system was normal.
MRI of the dorsal spine revealed T2 hypointense extramedullary lesion of size 18 × 15 mm anterior to the spinal cord at D1 level causing spinal cord compression and increased T2 signal in the adjacent cord. The lesion shows intense enhancement in post-contrast T1WI. Prominent flow voids were seen anterior to the cord above and the below the level of lesion in the intradural space. Prominent flow voids were also seen in the paraspinal muscles. The findings were suggestive of type IV dural arteriovenous fistula with a suspicion of ASA aneurysm (Figure 5(a), (b)). DSA was carried out that showed ASA aneurysm at D1 level, supplied by the right thyrocervical trunk. There was evidence of CoA with multiple collateral channels (Figure 5(c)). The patient was operated on and clipping of the ASA aneurysm done.
Figure 5.
Magnetic resonance imaging (MRI) and DSA in Case 3. (a) Saggital and (b) axial T2 weighted MRI shows a well-defined rounded hypointense lesion anterior to the spinal cord at D1 level compressing the spinal cord posteriorly. Increased signal intensity noted in the spinal cord at D1 and D2 level. Dilated flow voids are also noted cranial to the lesion as well as in the pre and paravertebral region. (c) DSA shows the spinal aneurysm and multiple dilated collateral channels. DSA: digital subtraction angiography.
Discussion
Although isolated ASA aneurysms are rare, they can be found in association with arteriovenous malformations of the spine, aortic coarctation, Moyamoya disease, vasculitides, or infections.1–3 These aneurysms are usually wide-necked saccular or fusiform and smaller in size. They have a tendency to bleed and commonly present with spinal, or rarely intracranial, subarachnoid hemorrhage.1,4,5 Spinal hemorrhage located in upper cervical region usually presents with the classic signs of intracerebral SAH, whereas hemorrhage at the lower cervical and thoracic or lumbar levels presents with sudden excruciating pain in neck or back respectively and spinal neurological symptoms.1 Our Case 1 and Case 2 presented with sudden onset neck and back pain, respectively, that corresponded to the site of the aneurysm.
When there is any chronic vascular obstruction, collateral channels develop and they potentially offer an important alternative source of blood supply when the original vessel fails to provide sufficient blood. The main collaterals in coarctation are through the internal mammary and the intercostal arteries, particularly from the intercostals and subclavian arterial branches, but also include other vessels such as the vertebral, spinal, suprascapular, long thoracic, and epigastric arteries.2,6 Common collateral channels in abdominal aortic stenosis include collaterals between superior and inferior epigastric arteries, and lower intercostals and lumber with circumflex iliac arteries.7 In addition to epigastric vessels, we found mesenteric as well as the ASA acting as collateral channel in our Case 2.
The ASA acting as a collateral channel is not common, but in our cases it acted as an important collateral channel that was supplying blood to the aorta distal to the obstruction and therefore led to the development of aneurysm as a result of hemodynamic stress from increased flow. Pathogenesis of spinal artery aneurysms associated with narrowing of descending thoracic or abdominal aorta appears to be related to hemodynamic factors and therefore classified as flow-related aneurysms.
The aorta distal to the obstruction was stealing blood from the ASA through the radicular artery, a phenomenon known as “aortic steal” has been previously described in CoA.8,9 The ASA acting as a collateral channel with subsequent development of aneurysm and rupture has been described in CoA probably because of the involvement in proximal thoracic aorta, but has not been reported in association with type V Takayasu arteritis. To the best of our knowledge, this is the first case of such an association.
Rarely, myelopathy can be the presenting feature of CoA.1,2,4 It could be attributed to compression due to dilated intraspinal collaterals, spinal or radicular artery aneurysm, or subarachnoid hemorrhage. The proposed mechanism of myelopathy includes compressive etiology in the relatively tight spinal canal of the lower cervical and upper thoracic regions, aortic steal or ischemic events that can occur due to microemboli from dilated collateral channels that may act as a source of a clot. Regression of symptoms have been noted after decompressive laminectomy10 as seen in our Case 2.
Most cases of ASA aneurysm associated with CoA were diagnosed by spinal angiogram, intraoperatively or at autopsy. But now with the improvement in CT technology, CT angiography can be used to define these aneurysms and other related vascular collateral channels. One such case has been described by Aoun et al., where the diagnosis was confirmed by CT angiography and subsequently the patient underwent therapeutic embolization.11 In two of our cases, the diagnosis of the ASA aneurysm was made on CT angiography and in the third case, there was suspicion of ASA aneurysm on MRI that was subsequently confirmed on DSA. Although DSA is the gold standard for diagnosis and treatment planning, with the advancement of CT angiographic technique, wider availability and greater area of coverage, it has now become a useful noninvasive modality for initial diagnosis of these aneurysms. In addition, CT angiography can provide complete information on the entire aorta as well as other collateral pathways. With multislice CT, thin sections can be acquired which are reconstructed and provide a very good depiction of the vascular tree along with collaterals where demonstration of ASA and other pathologies is made with perfection.
The treatment strategy of ASA aneurysm remains controversial. In cases of ruptured ASA aneurysm associated with CoA, a surgical or endovascular treatment of aneurysm with immediate anatomical correction of the disease best prevents subsequent SAH. Since many of the reported spinal aneurysms have a fusiform rather than saccular shape, standard surgical (clip placement) or endovascular treatment (coiling) of the aneurysm becomes difficult, leaving the option for trapping of the aneurysm with occlusion of the parent vessel. Our first two cases have been kept on close follow-up and in Case 3 clipping of aneurysm was done. Spontaneous regression of ASA aneurysms has been described in dissecting type of aneurysm and those associated with vasculitis, however none of them were flow-related aneurysms.4,12
Conclusion
ASA aneurysms are very rare, however they can be seen in situations where the ASA acts as a collateral pathway as in CoA and Takayasu arteritis. Although spinal DSA is the gold standard, CT angiography is the best initial non-invasive imaging modality for diagnosis of such aneurysms and identification of various collateral pathways. Early diagnosis and management is required to prevent complications and morbidity associated with these cases.
Acknowledgement
The authors wish to thank the Department of Neurosurgery for patient management.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rengachary SS, Duke DA, Tsai FY, et al. Spinal arterial aneurysm: Case report. Neurosurgery 1993; 33: 125–129. [DOI] [PubMed] [Google Scholar]
- 2.Smith BS, Penka CF, Erickson LS, et al. Subarachnoid hemorrhage due to anterior spinal artery aneurysm. Neurosurgery 1986; 18: 217–219. [DOI] [PubMed] [Google Scholar]
- 3.Singh V, Naik S, Shetty GS, et al. Anterior spinal artery aneurysm in a case of Moyamoya disease. Acta Neurol Belg 2016; 116: 663–665. [DOI] [PubMed] [Google Scholar]
- 4.Berlis A, Scheufler KM, Schmahl C, et al. Solitary spinal artery aneurysms as a rare source of spinal subarachnoid hemorrhage: Potential etiology and treatment strategy. AJNR Am J Neuroradiol 2005; 26: 405–410. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CC, Bellon RJ, Ogilvy CS, et al. Aneurysms of the lateral spinal artery: Report of two cases. Neurosurgery 2001; 48: 949–953. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Kumar S. Hematomyelia due to anterior spinal artery aneurysm in a patient with coarctation of aorta. Neurol India 2010; 58: 675–676. [DOI] [PubMed] [Google Scholar]
- 7.Kendall BE, Andrew J. Neurogenic intermittent claudication associated with aortic steal from the anterior spinal artery complicating coarctation of the aorta. J Neurosurg 1972; 37: 89–94. [DOI] [PubMed] [Google Scholar]
- 8.Hardman RL, Lopera JE, Cardan RA, et al. Common and rare collateral pathways in aortoiliac occlusive disease: A pictorial essay. AJR Am J Roentgenol 2011; 197: W519–W524. [DOI] [PubMed] [Google Scholar]
- 9.Gill M, Pathak HC, Singh P, et al. A case of aortic coarctation presenting with quadriparesis due to dilated tortuous anterior spinal artery. Neurol India 2011; 59: 317–318. [DOI] [PubMed] [Google Scholar]
- 10.Tan KP, Ng FC, Ong PL. Paraparesis due to dilated spinal collaterals. Singapore Med J 1979; 20: 454–456. [PubMed] [Google Scholar]
- 11.Aoun SG, El Ahmadieh TY, Soltanolkotabi M, et al. Ruptured spinal artery aneurysm associated with coarctation of the aorta. World Neurosurg 2014; 81: 441.e17–e22. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez Romero D, Batista AL, Gentric JC, et al. Ruptured isolated spinal artery aneurysms. Report of two cases and review of the literature. Interv Neuroradiol 2014; 20: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]