Abstract
Background
Idiopathic intracranial hypertension (IIH) is a syndrome defined by elevated intracranial hypertension without radiographic evidence of a mass lesion in the brain. Dural venous sinus stenosis has been increasingly recognized as a treatable cause, and dural venous sinus stenting (DVSS) is increasingly performed.
Methods
A 5 year single-center retrospective analysis of consecutive patients undergoing DVSS for medically refractory IIH.
Results
There were 43 patients with a mean imaging follow-up of 6.5 months and a mean clinical follow-up period of 13.5 months. DVSS was performed as the first procedure for medically refractory IIH in 81.4% of patients, whereas 18.6% of patients included had previously had a surgical procedure (ventriculoperitoneal (VP) shunt or optic nerve sheath fenestration (ONSF)). Headache was present in all patients and after DVSS improved or remained stable in 69.2% and 30.8%, respectively. Visual acuity changes and visual field changes were present in 88.4% and 37.2% of patients, respectively. Visual field improved or remained unchanged in 92%, but worsened in 8% after stenting. There was a stent patency rate of 81.8%, with an 18.2% re-stenosis rate. Of the 43 procedures performed, there was a 100% technical success rate with zero major or minor complications.
Conclusion
Based on this single-center retrospective analysis, DVSS can be performed with high technical success and low complication rates. A majority of patients presented primarily with headache, and these patients had excellent symptom relief with DVSS alone. Patients presenting with visual symptoms had lower success rates, and this population, if stented, should be carefully followed for progression of symptoms.
Keywords: Idiopathic intracranial hypertension, pseudotumor cerebri, dural venous sinus stenting
Background
Idiopathic intracranial hypertension (IIH) is a syndrome defined by elevated intracranial hypertension without radiographic evidence of a mass lesion in the brain.1 The currently accepted standard of care for medically refractory IIH patients is a cerebrospinal flow diversion procedure (lumboperitoneal (LP) shunt, ventriculoperitoneal (VP) shunts or optic nerve sheath fenestration (ONSF)).2,3 ONSF is a procedure usually performed by an ophthalmologist, whereby a slit is made in the optic nerve sheath to reduce the local pressure around the optic nerve, usually a first-line treatment for patients with acute visual loss symptoms rather than headaches.3
Dural venous sinus stenosis has been increasingly recognized as a treatable cause of elevated intracranial pressure, and DVSS is increasingly performed.4,5 We present a single-center retrospective review of all patients who underwent DVSS for IIH.
Methods
Institutional review board (IRB) approval was obtained for a retrospective chart review. A single-center retrospective analysis of all consecutive patients undergoing DVSS for IIH at the Medical University of South Carolina (MUSC) from November 2009 to July 2014 was performed. Initially, only patients who were deemed medically refractory by a referring neurologist underwent stenting. Medically refractory patients all underwent lumbar puncture. Lumbar puncture confirmed elevated opening pressures and normal cerebrospinal fluid (CSF) analysis. Towards the mid-point of our experience at MUSC, any patients with a stenosis on direct venography with a significant gradient across the stenosis were treated with stenting prior to surgical CSF flow diversion (CSFFD). Data for presenting symptoms, baseline patient demographics, presence of headache (HA), visual changes, Diamox dose, location of stenosis and pressure gradient were obtained by manual chart review.
Procedure
All patients considered for DVSS had previously undergone non-invasive evaluation with either magnetic resonance venography (MRV) or computed tomographic (CT) venography. Patients with medically refractory IIH, absence of deep venous sinus thrombosis, and venous stenosis on non-invasive imaging were referred for diagnostic venography.
Diagnostic venography
If an appropriate stenosis was identified on non-invasive imaging, all patients underwent diagnostic angiography and venography under local anesthesia. Neither conscious sedation nor endotracheal anesthesia was used, as both of these could decrease the patient’s blood pressure and could potentially change the venous pressure gradient across the stenosis.
Arterial and venous femoral access was obtained using bilateral groins to minimize risk of arteriovenous fistula. Arterial angiography was performed using a 5 fr. Diagnostic catheter and was used to evaluate flow dynamics and to perform an indirect venogram, which was used for guide catheter and microcatheter navigation (Boston Scientific Renegade, Natick, MA, USA Hi-Flo 3.2 F microcatheter over a Boston Scientific Fathom 0.016 in microwire) into the superior sagittal sinus. Direct dural sinus pressure measurements were performed at the superior sagittal sinus, transverse sinus, sigmoid sinus and jugular bulb. Direct venography also excluded the presence of deep venous sinus thrombosis.
Based on the available literature5 and our experience, patients may be considered for DVSS if there is a pressure gradient of ≥8 mm Hg. The three configurations of dural sinus stenosis amenable to intervention include hemodynamically significant:
Bilateral transverse/sigmoid sinus stenosis;
Unilateral transverse/sigmoid sinus stenosis with hypoplastic contralateral transverse/sigmoid sinus stenosis;
Uncommonly, isolated superior sagittal sinus stenosis.
Dural venous sinus stenting technique
Patients with medically refractory IIH with dural sinus stenosis and a pressure gradient of ≥8 mmHg were offered DVSS. All DVSS procedures were performed under general anesthesia. Via common femoral vein access, a guiding catheter, generally Neuron Max 088 (Penumbra Inc., Alameda, CA, USA), was navigated into the proximal internal jugular vein. In some cases, a Renegade HiFlo catheter was advanced into the superior sagittal sinus and direct sinus pressure measurements were obtained. Using a conduit technique,6 either the Neuron Max was advanced coaxially over a 6F diagnostic insert (Penumbra Inc.) or a tri-axial system composed of an 038 wire, 5F diagnostic catheter, 6F neuron 070 guiding catheter (or 071 Chaperone guide catheter (Microvention Inc., Tustin, CA, USA)) (Figure 1) and Neuron max catheter were used to cross the stenosis (Figure 2), generally at the transverse-sigmoid junction, and either the Neuron Max or the 070 guide catheter was left across the stenosis. With this large stable access platform, a self-expanding carotid stent (Cordis Precise ProRx (Codman Neurovascular, Raynham, MA, USA)) was advanced through the guide catheter across the stenosis. The Neuron Max was then gently withdrawn proximally (Figure 3) and the stent delivered using standard technique across the stenosis (Figure 4). Follow-up venography and pressure measurement were performed to confirm both morphological and hemodynamic resolution of the stenosis. If a there was a persistent stenosis, angioplasty was performed.
Figure 1.
Proximal to the venous stenosis in sigmoid sinus (long dashed arrow), 5F diagnostic catheter across stenosis in the distal transverse sinus (solid arrow), and tip of 035 guide wire in proximal superior sagittal sinus (small dashed arrow).
Figure 2.
Venogram showing tip of Neuron Max 088 guiding catheter distal to the venous stenosis in distal transverse sinus (long dashed arrow) after advancing over diagnostic catheter and 035 wire.
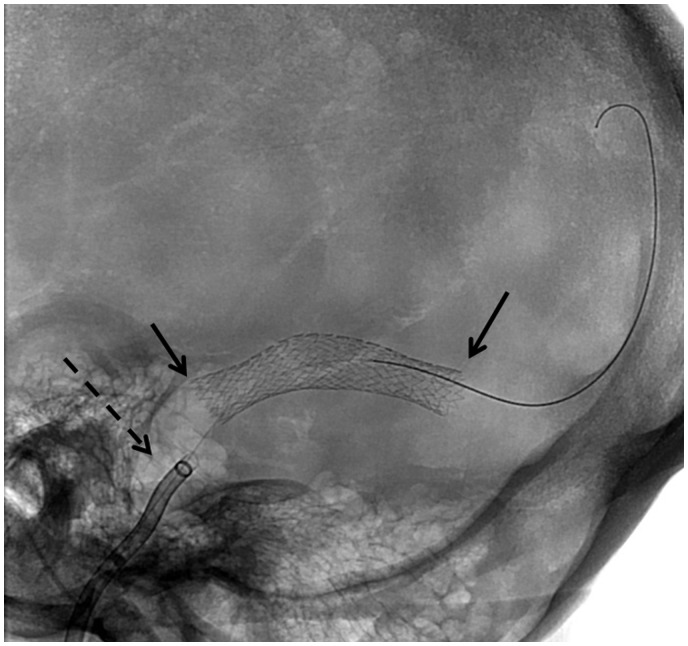
Figure 3.
Road map image demonstrating position of stent across target stenosis (short solid arrow at distal and proximal ends of stent delivery) over 014 microwire prior to stent delivery and after proximal withdrawal of Neuron Max 088 guiding catheter (long dashed arrow).
Figure 4.
Native unsubtracted image demonstrating position of self-expanding stent across target stenosis (short solid arrow at distal and proximal ends of stent delivery) with no residual stenosis and stable position of Neuron Max 088 guiding catheter (long dashed arrow).
Results
There were 43 patients included in this study, of whom 90.7% were female and 48.8% were African American (the remainder were Caucasian). The mean body mass index (BMI) was 34.8 kg/m2 (range 21–56 kg/m2) and age was 34.9 years (21–54 years). The follow-up period was 13.5 months (range 1–63 months) (Table 1).
Table 1.
Patient Summary .
|
Presenting symptoms
|
Post Stent Symptoms
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Age (Years) | Sex 1 = F, 2 = M | Race 1 = W, 2 = B, 3 = other | Body Weight (kg) | Body Mass Index (BMI) | CSF Opening Pressure (cm H2O) | HA Score 0 = Absent, 1 = present | PAP Grade 0 = Absent, 1-5 = Diff grades of PAP, 6 = optic atrophy | VC 0 = Absent, 1 = Present | VF Changes 0 = Absent, 1 = present | Previous medical treatment 0 = No 1 = Yes | Previous surgical treatment 0 = none, 1 = ONSF, 2 = CSF flow diversion, 3 = other | Mean Pre-stent Pressure gradient (mm Hg) | Location of Stenosis* | Location of stent placement 1 = right 2 = left 3 = both | Complications 0 = None | HA Score 1 = same, 2 = better, 3 = worse | PAP Grade 0 = Absent 1 = same, 2 = better, 6 = Optic atrophy after stent); Blank - NA | VC 1 = same, 2 = better, 3 = worse | VF Changes 1 = same, 2 = better, 3 = worse | Follow-up (months) | Time to Angiographic Follow-up from Treatment (Months) | Stent Patency (at the time of first angiographic F/U) 1 = yes 2 = stent stenosis |
| 1 | 45 | 1 | 1 | 108.5 | 39 | NA | 1 | 0 | 1 | 0 | 0 | 0 | NA | 2 | 1 | 0 | NA | 1 | 1 | NA | 63 | NA | NA |
| 2 | 35 | 1 | 1 | 82.3 | 34 | 35 | 1 | 1 | 1 | 1 | 0 | 1 | 26 | 1 | 1 | 0 | 1 | 2 | 3 | 2 | 24 | NA | NA |
| 3 | 28 | 1 | 2 | 99 | 37 | 31 | 1 | 6 | 1 | 1 | 1 | 2 | 11 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 29 | NA | NA |
| 4 | 30 | 1 | 2 | 99 | 35 | 53 | 1 | 1 | 1 | 0 | 0 | 0 | 10 | 2 | 1 | 0 | 2 | 1 | 1 | NA | 9 | 8 | 1 |
| 5 | 28 | 1 | 1 | 65.8 | 23 | NA | 1 | 0 | 1 | 0 | 0 | 0 | 20 | 2 | 1 | 0 | 2 | 0 | 2 | NA | 14 | NA | NA |
| 6 | 43 | 1 | 2 | 63.3 | 27 | 38 | 1 | 1 | 1 | 0 | 1 | 0 | NA | 2 | 1 | 0 | 2 | 2 | 2 | NA | 32 | 4 | 2 |
| 7 | 31 | 1 | 2 | 81.1 | 29 | 38 | 1 | 1 | 1 | 1 | 0 | 0 | 22 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 19 | NA | NA |
| 8 | 48 | 1 | 2 | 131.4 | 48 | 28 | 1 | 2 | 1 | 1 | 0 | 0 | 20 | 2 | 1 | 0 | 2 | 2 | 1 | NA | 45 | NA | NA |
| 9 | 50 | 1 | 2 | 113.4 | 45.7 | 33 | 1 | 0 | 1 | 0 | 0 | 0 | 14 | 1 | 1 | 0 | 2 | NA | 2 | NA | 25 | 22 | 1 |
| 10 | 48 | 1 | 2 | 98.1 | 34 | 33 | 1 | 0 | 1 | 0 | 1 | 0 | 17 | 1 | 1 | 0 | 1 | NA | 1 | NA | 24 | NA | NA |
| 11 | 48 | 1 | 1 | 90 | 33 | NA | 1 | 4 | 1 | 0 | 0 | 0 | 15 | 2 | 1 | 0 | 2 | 1 | 2 | NA | 3 | 15 | 1 |
| 12 | 33 | 1 | 2 | 132.2 | 49 | 14 | 1 | 2 | 1 | 1 | 0 | 0 | 13 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 37 | NA | NA |
| 13 | 26 | 1 | 1 | 83 | 33 | NA | 1 | 2 | 1 | 1 | 0 | 1 | 12 | 1 | 1 | 0 | 1 | NA | 1 | NA | 1 | NA | NA |
| 14 | 23 | 1 | 2 | 65 | 24 | 39 | 1 | 3 | 1 | 0 | 0 | 0 | 24 | 2 | 1 | 0 | 2 | 2 | 1 | NA | 14 | NA | NA |
| 15 | 31 | 1 | 1 | 99 | 37 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 11 | 1 | 1 | 0 | 2 | 1 | 2 | 1 | NA | 4 | 1 |
| 16 | 45 | 2 | 1 | 109 | 31 | 30 | 1 | 2 | 1 | 1 | 1 | 1 | NA | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 3 | 4 | 1 |
| 17 | 34 | 1 | 1 | 66.7 | 21.1 | 23 | 1 | 2 | 0 | 0 | 0 | 0 | 10 | 2 | 1 | 0 | 2 | 2 | NA | NA | 30 | NA | NA |
| 18 | 24 | 1 | 2 | 110.2 | 40.44 | NA | 1 | 5 | 1 | 1 | 1 | 0 | 12 | 1 | 1 | 0 | 2 | 1 | 3 | 3 | 14 | NA | NA |
| 19 | 22 | 1 | 2 | 96.4 | 37.6 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 20 | 1 | 1 | 0 | 1 | 2 | 2 | NA | 1 | NA | NA |
| 20 | 22 | 1 | 1 | 72.6 | 28.4 | NA | 1 | 1 | 0 | 1 | 1 | 0 | 16 | 1 | 1 | 0 | 1 | 1 | NA | 1 | 18 | NA | NA |
| 21 | 38 | 1 | 1 | 90.3 | 35.3 | NA | 1 | 0 | 0 | 0 | 1 | 0 | 20 | 1 | 1 | 0 | 2 | NA | 1 | NA | 4 | NA | NA |
| 22 | 41 | 1 | 2 | 154.2 | 53.2 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 12 | 1 | 1 | 0 | NA | NA | NA | NA | NA | NA | NA |
| 23 | 41 | 1 | 1 | 70.4 | 30.3 | NA | 1 | 0 | 1 | 0 | 0 | 0 | 21 | 1 | 1 | 0 | 1 | 0 | 1 | NA | 22 | 1 | 1 |
| 24 | 39 | 1 | 2 | 105.7 | 42.6 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 14 | 1 | 1 | 0 | 2 | 2 | 2 | NA | 16 | NA | NA |
| 25 | 54 | 1 | 1 | 87.6 | 28.12 | 31.5 | 1 | 1 | 1 | 0 | 1 | 0 | 25 | 1 | 1 | 0 | 2 | 1 | 1 | NA | 2 | 3 | 1 |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 26 | 29 | 1 | 1 | 72.6 | 27.45 | NA | 1 | 0 | 1 | 1 | 1 | 0 | 12 | 1 | 1 | 0 | 2 | NA | 2 | NA | 8 | 6 | 1 |
| 27 | 49 | 1 | 2 | 106.4 | 36 | NA | 1 | 3 | 1 | 0 | 1 | 0 | 21 | 2 | 1 | 0 | 2 | 2 | 2 | NA | 7 | NA | NA |
| 28 | 33 | 1 | 2 | 179.6 | 56 | NA | 1 | 6 | 1 | 1 | 1 | 2 | 46 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 4 | NA | NA |
| 29 | 33 | 1 | 2 | 114 | 41 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 16 | 1 | 1 | 0 | 2 | 2 | 1 | NA | 5 | NA | NA |
| 30 | 33 | 1 | 2 | 73 | 31.25 | NA | 1 | NA | 1 | 1 | 1 | 0 | 19 | 1 | 1 | 0 | 2 | NA | 1 | 1 | 3 | NA | NA |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 31 | 31 | 1 | 2 | 86 | 32.46 | NA | 1 | 1 | 1 | 0 | 1 | 0 | 22 | 1 | 1 | 0 | 2 | NA | 2 | 1 | 1 | NA | NA |
| 32 | 29 | 1 | 1 | 97 | 36.14 | 54 | 1 | 0 | 1 | 0 | 1 | 2 | 10 | 2 | 1 | 0 | 1 | NA | 1 | NA | 3 | 4 | 1 |
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 33 | 25 | 1 | 1 | 84.4 | 36.33 | NA | 1 | 0 | 1 | 0 | 1 | 0 | 13 | 3 | 1 | 0 | 2 | NA | 2 | NA | 3 | NA | NA |
| 34 | 37 | 2 | 2 | 67 | 23 | NA | 1 | 4 | 1 | 1 | 1 | 1 | 15 | 1 | 3 | 0 | 2 | 2 | 1 | 1 | 3 | NA | NA |
| 35 | 35 | 1 | 1 | 135 | 51 | 23 | 1 | 0 | 1 | 0 | 1 | 0 | 7 | 1 | 1 | 0 | NA | NA | NA | NA | NA | NA | NA |
| 36 | 38 | 1 | 1 | 116 | 41 | 30 | 1 | NA | 1 | 0 | 0 | 2 | 7 | 1 | 1 | 0 | 1 | NA | 2 | NA | 2 | NA | NA |
| 37 | 38 | 1 | 3 | 93 | 34 | NA | 1 | 0 | 1 | 1 | 0 | 0 | 20 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 5 | NA | NA |
| 38 | 28 | 1 | 1 | 102 | 33 | NA | 1 | NA | 1 | NA | 0 | 0 | 20 | 1 | 1 | 0 | 2 | NA | 1 | NA | 1 | NA | NA |
| 39 | 21 | 1 | 2 | 81 | 35 | 55 | 1 | 2 | 1 | 1 | 0 | 0 | NA | 1 | 1 | 0 | 2 | 6 | 2 | 2 | 9 | NA | NA |
| 40 | 34 | 1 | 1 | 93 | 29 | NA | 1 | NA | 1 | 1 | 0 | 0 | 23 | 1 | 2 | 0 | 2 | NA | 2 | 2 | 12 | NA | NA |
| 41 | 28 | 2 | 1 | 70 | 21 | 56 | 1 | 1 | 1 | 0 | 0 | 0 | 8 | NA | 2 | 0 | NA | NA | NA | NA | NA | NA | NA |
| 42 | 29 | 1 | 2 | 101 | 35 | NA | 1 | 1 | 0 | 0 | 1 | 0 | 11 | 2 | 1 | 0 | 2 | 1 | 2 | NA | 8 | NA | NA |
| 43 | 44 | 2 | 1 | 68 | 24 | NA | 1 | 1 | 0 | 0 | 1 | 0 | 18 | 1 | 1 | 0 | 1 | 1 | NA | NA | 2 | 0 | 2 |
HA = Headache; PAP = Papilledema; VC = Visual changes; VF = Visual Field; ONSF-Optic nerve sheath fenestration; CSF cerebrospinal fluid; *Location of Stenosis: 1 = TSJ, 2 = Superior sagittal sinus 3 = Tandem superior sagittal sinus and R T/S Dural venous sinus, NA = Not reported.
DVSS was performed as the first procedure for medically refractory IIH in 81.4% (35/43 cases). DVSS was performed in 18.6%, or 8 of 43 patients, who had previously undergone a surgical procedure (Table 2). Half of these prior procedures were ONSF and half were CSFFD procedures.
Table 2.
Repeat procedure.
|
Presenting symptoms
|
Post Stent Symptoms
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # | Age (Years) | Sex (1 = F, 2 = M) | Race 1 = W, 2 = B, 3 = other) | Body Weight (kg) | Body Mass Index (BMI) | CSF Opening Pressure (cm H2O) | HA Score 0 = Absent, 1 = present | PAP Grade 0 = Absent, 1-5 = Diff grades of PAP, 6 = optic atrophy | VC 0 = Absent, 1 = Present | VF Changes 0 = Absent, 1 = present | Previous medical treatment 0 = No 1 = Yes | Previous surgical treatment 0 = none, 1 = onsf, 2 = csf flow diversion, 3 = other | Mean Pre-stent Pressure gradient (mm Hg) | Location of Stenosis | Location of stent placement 1 = right 2 = left 3 = both | Complic ations 0 = None | HA Score 1 = same, 2 = better, 3 = worse | PAP Grade 0 = Absent 1 = same, 2 = better, 6 = Optic atrophy after stent); Blank - NA | VC 1 = same, 2 = better, 3 = worse | VF Changes 1 = same, 2 = better, 3 = worse | Follow-up (months) | Time to Angio graphic Follow-up from Treatment (Months) | Stent Patency (at the time of first angiographic F/U) 1 = yes 2 = stent stenosis |
| 25 | 54 | 1 | 1 | 87.6 | 28.12 | NA | 0 | 1 | 1 | 0 | 1 | 0 | 21 | 2 | Right TS stenosis & Right sigmoid stenosis | 0 | 2 | 2 | 2 | NA | 4 | NA | NA |
| 30 | 33 | 1 | 2 | 73 | 31.25 | NA | 1 | 1 | 1 | 1 | 1 | 0 | 7 | 2 | NA | 0 | NA | NA | NA | ||||
| 32 | 29 | 1 | 1 | 97 | 36.14 | NA | 1 | 0 | 1 | 0 | 1 | 2 | NA | 1 | NA | 0 | 2 | NA | 2 | NA | 1 | NA | NA |
NA: not applicable.
Headache was a presenting symptom in 100% of patients. On ophthalmologic examination, papilledema was present in 65%, visual acuity changes present in 88.4%, and visual field changes present in 37.2% of patients.
Post procedure, improvement was defined by the improvement in the presenting symptoms, including headache, papilledema, visual acuity and visual field. After intervention, headache improved in 69.2% and remained stable in 30.8%. Although no patient had documented worsening headaches, data was unavailable for four patients. Papilledema improved in 59% of patients (13/22 cases). Visual acuity changes improved in 43% (15/35 cases) and remained the same in 51% (18/35 cases) after treatment. Acuity worsened in only 6% (2/35 cases). Visual field improved in 46% (6/13 cases), stayed the same in another 46% (6/13 cases), and worsened in 8% (1/13 cases) of the patients after treatment.
Opening pressure was only documented in 18 patients, but the mean was 35.8 mmHg (range 14–56 mmHg). The mean pre-stent pressure gradient was 16.74 mmHg (range 7–46 mmHg), but data was not reported for four patients. Patient 37 was reported as >20 mmHg, and for calculation consistency we assumed this to be 20 mmHg. Sixty-nine percent (29/42) of patients had right transverse sinus stenosis, 28.6% (12/42) had left transverse sinus stenosis, and 2.4% had bilateral stenosis. Ninety-three percent (40/43) of the stents were placed in the right transverse sinus junction, 4.7% (two) stents were placed in the superior sagittal sinus alone, and one patient had two stents placed (superior sagittal sinus and right transverse dural venous sinus).
Mean time to angiographic follow-up was 6.5 months, with a range of 0–22 months. A second angiographic follow-up was done for selected patients (patient 15 (8 months) and patient 23 (6 months)). There was a stent patency rate of 81.8% for 9/11 patients and an 18.2% re-stenosis rate. Data was not available for 32 patients because routine follow-up venography was not performed. Repeat stenting was performed in two patients (patients 25 and 30) for in-stent stenosis. Patient 32 had recurrent stenosis but a pressure gradient of only 7 mmHg, and elected not to have additional stent placement.
There was a 100% technical success rate with zero major and minor complications.
Discussion
IIH is a syndrome of exclusion and is currently defined by the Dandy criteria, which include an abnormal elevation of intracranial pressure with a normal composition of CSF and no intracranial mass or venous sinus thrombosis.1 Pseudotumor cerebri was first described by Quinke in 1893.7,8 The classic clinical triad was headache, blurred vision and vomiting. Papilledema was present in approximately 95% of patients, and if left untreated could progress to optic atrophy and irreversible blindness in a subset of patients.9,10
The pathophysiology of IIH has been attributed to either overproduction of CSF or decreased absorption of CSF. Some early hypotheses for IIH included serous meningitis (Quinke); increased CSF in the subarachnoid space (Passot); hydrocephalus due to remote effects of bacterial toxins or otitis media (Warrington); and altered vasomotor control of the intracranial bed (Dandy).11 In a subset of patients, increased intracranial pressure may be due to a focal stenosis in a dural venous sinus. Since CSF is normally absorbed by the pressure-sensitive arachnoid granulations, a stenosis of any sinus may lead to impaired venous drainage, resulting in decreased cerebral venous drainage, which ultimately results in cerebral venous hypertension and impaired CSF reabsorption.3,4,12,13 However, the causal relationship between dural venous sinus stenosis and IIH is not well understood and may be more complex than simple mechanical flow impairment. Dural venous sinus stenosis in the setting of IIH may represent a common presentation of a heterogeneous group of underlying pathologies.
Several series document resolution of transverse sinus stenosis (in the setting of IIH) after CSFFD with VP shunt placement.14–16 In 2006, Scoffings et al.17 described a patient with IIH on diuretics and acetazolamide who underwent catheter-based venography and direct pressure measurements across a transverse sinus stenosis with a gradient of 19 mmHg (29 to 10 mmHg). After a high volume tap, 45 ml CSF, the pressure decreased from 29 mmHg to 2 mmHg.17 Immediately following LP shunt , repeat catheter-based venography and direct pressure measurements demonstrated resolution of stenosis and the pressure gradient.17 The transverse sinus pressures were 7 mmHg.17 Although the patient had symptomatic improvement for 2 weeks, she ultimately required CSFFD with VP shunt, with symptomatic relief at 1 year follow-up.17
Indications for surgical intervention included progressive visual symptoms or intractable symptoms, usually headaches.3,18,19 ONSF is considered the first-line treatment if patients have visual symptoms with minimal headaches.2,3,20–24 ONSF makes logical sense in this subset, because the procedure rapidly reduces pressure on the optic nerve. Limitations of the procedure include patients presenting with severe headaches as primary symptom, availability of ophthalmologists who are comfortable with performing this technically challenging procedure, and recovery, which is not trivial.
CSF diversion techniques (VP or LP shunts) are usually the first surgical intervention for medically refractory patients with IIH.3,21,25,26 The most common indication is severe headache, but it may be more urgent if the patient presents with progressive visual changes.2,19,25,27,28 There are no prospective randomized studies for CSFFD, although previously there was no need for one, given no other readily available option. Until recently, CSFFD was the only option available for this patient population. CSF diversion is an invasive procedure with high rates of revisions.21,26–28 Given the fact that most patients with IIH are young, obese women, the long-term cost of repeat procedures and complications should not be taken lightly. Serious complications described in the literature include shunt infections,21,25–27,29–32 tonsillar herniation,30 subdural hematoma,31 and CSF fistula.21 We were uncertain about the severity of “operative complications,” as they were not clearly described by Rosenberg et al.,26 so we included them in the minor complications category.
The authors would argue that CSFFD as the universal first-line intervention should be re-evaluated given the high technical and clinical success rates with low complication rate associated with DVSS in appropriately selected patients. We would suggest that all patients being considered for either CSFFD or ONSF for medically refractory IIH should undergo a contrast-enhanced MR venogram to exclude the presence of high-grade dural venous sinus stenosis. If present, diagnostic venography with pressure measurements should be performed to verify that the lesion is amenable to DVSS. Then, after review by a multidisciplinary team comprised of neurologists and specialists in ONSF, CSFFD and DVSS, an individualized treatment plan should be established for each patient. Given the fact that none of the procedures has a permanent 100% clinical and technical success rate, treatment failures for one procedure may be amenable to a different procedure. Currently, DVSS is not widely recognized as a potential treatment option for either first- or second-line treatment in this patient population.
Given the relatively small number of patients with medically refractory IIH requiring DVSS, a large randomized study may never be successfully performed. The strongest level of evidence for DVSS may be limited to Class III evidence; therefore, we would urge anyone performing these procedures to carefully collect pre- and post-procedural data (especially ophthalmological data) with long-term follow up and to share that data with the interventional community. This collective data may be pooled in future meta-analysis studies.
Limitations
This was a retrospective review; therefore, major limitations were related to inconsistent data collection and long-term clinical and imaging follow-up. There were inconsistent pre-procedural ophthalmological exams documenting baseline visual acuity and visual field changes. There was also a variable duration of follow-up, generally limited to patients re-presenting with new clinical symptoms. Follow-up imaging was not available in 32/45 patients (71.1%). Future prospective IRB-approved studies may benefit from routine clinical follow-up documenting symptoms and ophthalmologic exams with imaging follow-up (CT venography at 1, 3 and 5 years). CT venography would further clarify rates of in-stent stenosis, stenosis at the proximal or distal margins of the stent, and patency.
Conclusion
Based on our single-center retrospective analysis, DVSS can be performed with high technical success and low complication rates. A majority of patients presented primarily with headache, and these patients had excellent symptom relief with DVSS alone as the first-line treatment for medically refractory IIH. Patients presenting with visual symptoms had lower success rates, and this population should be carefully followed to ensure that alternative treatment with ONSF or VP shunting is pursued when appropriate.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SS reports non-financial support from Penumbra and grants/non-financial support from Stryker neurovascular, AS and IC report grants and non-financial support from Penumbra, outside the submitted work.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002; 59: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 2.Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2012; 83: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clin Neurol Neurosurg 2013; 115: 252–259. [DOI] [PubMed] [Google Scholar]
- 4.Higgins JN, Cousins C, Owler BK, et al. Idiopathic intracranial hypertension: 12 cases treated by venous sinus stenting. J Neurol Neurosurg Psychiatry 2003; 74: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: A review of 52 patients and of model predictions. AJNR Am J Neuroradiol 2011; 32: 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turkoglu E, Kazanci B, Karavelioglu E, et al. Intracerebral hematoma following lumboperitoneal shunt insertion: A rare case report. Turk Neurosurg 2011; 21: 94–96. [PubMed] [Google Scholar]
- 7.Pearce JM. From pseudotumour cerebri to idiopathic intracranial hypertension. Pract Neurol 2009; 9: 353–356. [DOI] [PubMed] [Google Scholar]
- 8.Degnan AJ, Levy LM. Pseudotumor cerebri: Brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol 2011; 32: 1986–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol 2006; 5: 433–442. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuroophthalmol 2004; 24: 138–145. [DOI] [PubMed] [Google Scholar]
- 11.Bandyopadhyay S. Pseudotumor cerebri. Arch Neurol 2001; 58: 1699–1701. [DOI] [PubMed] [Google Scholar]
- 12.Puffer RC, Mustafa W, Lanzino G. Venous sinus stenting for idiopathic intracranial hypertension: A review of the literature. J Neurointerv Surg 2013; 5: 483–486. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque FC, Dashti SR, Hu YC, et al. Intracranial venous sinus stenting for benign intracranial hypertension: Clinical indications, technique, and preliminary results. World Neurosurg 2011; 75: 648–652; discussion 592–595. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JNP, Pickard JD. Lateral sinus stenosis in idiopathic intracranial hypertension resolving after CSF diversion. Neurology 2004; 62: 1907–1908. [DOI] [PubMed] [Google Scholar]
- 15.Baryshnik D, Farb R. Changes in the appearance of venous sinuses after treatment of disordered intracranial pressure. Neurology 2004; 62: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 16.Rohr A, Dorner L, Stingele R, et al. Reversibility of venous sinus obstruction in idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2007; 28: 656–659. [PMC free article] [PubMed] [Google Scholar]
- 17.Scoffings DJ, Pickard JD, Nicholas P, et al. Resolution of transverse sinus stenoses immediately after CSF withdrawal in idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry 2007; 78: 911–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corbett JJ, Thompson HS. The rational management of idiopathic intracranial hypertension. Arch Neurol 1989; 46: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 19.Thambisetty M, Lavin PJ, Newman NJ, et al. Fulminant idiopathic intracranial hypertension. Neurology 2007; 68: 229–232. [DOI] [PubMed] [Google Scholar]
- 20.Feldon SE. Visual outcomes comparing surgical techniques for management of severe idiopathic intracranial hypertension. Neurosurg Focus 2007; 23: E6. [DOI] [PubMed] [Google Scholar]
- 21.Burgett RA, Purvin VA, Kawasaki A. Lumboperitoneal shunting for pseudotumor cerebri. Neurology 1997; 49: 734–739. [DOI] [PubMed] [Google Scholar]
- 22.Kelman SE, Sergott RC, Cioffi GA, et al. Modified optic nerve decompression in patients with functioning lumboperitoneal shunts and progressive visual loss. Ophthalmology 1991; 98: 1449–1453. [DOI] [PubMed] [Google Scholar]
- 23.Sergott RC, Savino PJ, Bosley TM. Modified optic nerve sheath decompression provides long-term visual improvement for pseudotumor cerebri. Arch Ophthalmol 1988; 106: 1384–1390. [DOI] [PubMed] [Google Scholar]
- 24.Brourman ND, Spoor TC, Ramocki JM. Optic nerve sheath decompression for pseudotumor cerebri. Arch Ophthalmol 1988; 106: 1378–1383. [DOI] [PubMed] [Google Scholar]
- 25.El-Saadany WF, Farhoud A, Zidan I. Lumboperitoneal shunt for idiopathic intracranial hypertension: Patients’ selection and outcome. Neurosurg Rev 2012; 35: 239–243. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg ML, Corbett JJ, Smith C, et al. Cerebrospinal fluid diversion procedures in pseudotumor cerebri. Neurology 1993; 43: 1071–1072. [DOI] [PubMed] [Google Scholar]
- 27.Eggenberger ER, Miller NR, Vitale S. Lumboperitoneal shunt for the treatment of pseudotumor cerebri. Neurology 1996; 46: 1524–1530. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair AJ, Kuruvath S, Sen D, et al. Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia 2011; 31: 1627–1633. [DOI] [PubMed] [Google Scholar]
- 29.Johnston I, Besser M, Morgan MK. Cerebrospinal fluid diversion in the treatment of benign intracranial hypertension. J Neurosurg 1988; 69: 195–202. [DOI] [PubMed] [Google Scholar]
- 30.McGirt MJ, Woodworth G, Thomas G, et al. Cerebrospinal fluid shunt placement for pseudotumor cerebri-associated intractable headache: Predictors of treatment response and an analysis of long-term outcomes. J Neurosurg 2004; 101: 627–632. [DOI] [PubMed] [Google Scholar]
- 31.Abubaker K, Ali Z, Raza K, et al. Idiopathic intracranial hypertension: Lumboperitoneal shunts versus ventriculoperitoneal shunts – case series and literature review. Br J Neurosurg 2011; 25: 94–99. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Serieh B, Ghassempour K, Duprez T, et al. Stereotactic ventriculoperitoneal shunting for refractory idiopathic intracranial hypertension. Neurosurgery 2007; 60: 1039–1043. discussion 1043–1044. [DOI] [PubMed] [Google Scholar]