Abstract
Background
Intracranial dural arteriovenous fistulae (DAVF) may present a treatment challenge. Endovascular embolization is in most cases the first line of treatment but does not always achieve cure. Gamma Knife (GK) radiosurgery represents an alternative treatment option, and the purpose of this study was to further evaluate its utility.
Methods
We reviewed all cases of DAVF treated between 2009 and 2016 at our institution with GK radiosurgery independently, or following failed/refused endovascular or surgical management. Patients’ clinical files, radiological images, catheter angiograms, and surgical DAVF disconnection reports were retrospectively reviewed.
Results
Sixteen DAVF (14 patients) treated by GK radiosurgery were identified. Eleven fistulae were aggressive and five were benign. Marginal doses ranged from 15 to 25 Gy. Target volumes ranged from 0.04 to 4.47 cm3. In all symptomatic patients, GK treatment resulted in symptom palliation. In 13/15 lesions, cure of symptoms (86.0%) was reported. One lesion was asymptomatic. Angiographic cure was achieved in eight cases (50%), small residual DAVF occurred in four, and four were unchanged. One patient developed headache that resolved at one year. No hemorrhage occurred during the follow-up period. There was no significant association between Borden type and cure rate. Prior failed endovascular treatment and small target volume were associated with lower rates of cure.
Conclusions
Stereotactic radiosurgery is viable treatment for DAVF. It is very effective in palliating symptoms as a de novo approach or adjunctive to endovascular therapy. In our experience it is only somewhat effective in achieving complete angiographic cure.
Keywords: Dural arteriovenous fistula, central nervous system vascular malformations, Gamma Knife radiosurgery, treatment outcome
Background
Dural arteriovenous fistulae (DAVF) are acquired shunts between dural arteries and intracranial veins. Intracranial DAVFs are rare and felt to be less common than brain arteriovenous malformations (BAVMs).1
Neurological symptoms of DAVFs are variable, and relate highly to lesion location and the presence or absence of cortical venous reflux (CVR).2 Accordingly, patients can present with complaints of pulsatile tinnitus or bruit, exophthalmos with or without chemosis, vision loss or symptoms relating to venous congestion and hemorrhage such as headaches, loss of consciousness, neurological deficits or decline and seizures. Lesions that result in hemorrhage are invariably aggressive, with CVR.
Treatment for DAVF is virtually always indicated for patients at risk of hemorrhage or vision loss,1,3,4 while treatment of “benign” dural AVF is in our practice indicated only if symptoms (such as tinnitus) are unbearable for the patient. To this end, embolization and microsurgery are the most common and effective treatments for DAVF.3,5 However, in some cases treatment risks via these approaches are prohibitive, thus fueling the interest in alternate treatment options. For brain (pial) arteriovenous malformations, Gamma Knife (GK) radiosurgery has been an established treatment modality for more than three decades. GK treatment for DAVF is less established, and we thus describe our experience in this report.
Materials and methods
We retrospectively evaluated the presentation, treatment and outcomes of patients presenting to our center with cranial DAVF from 2009 to 2016. These included Borden I–III lesions spanning any angioarchitecture. The evaluation included reviewing the patients’ clinical files and surgical reports as well as thorough evaluation of their radiological images including planar imaging and catheter angiograms. This study was approved by our institution’s research ethics board (REB) committee. A total of 197 DAVF presented to our center between 2009 and 2016. There were 170 (86%) patients treated. A total of 115 (68%) were treated by endovascular embolization, 23 (13.5%) by microsurgery, and six patients (3.5%) were treated with GK alone. In addition, 13 patients (7.6%) were treated with embolization and microsurgery, six patients (3.5%) with embolization followed by GK, and two patients (1.2%) with embolization, GK and microsurgery. Five (2.9%) patients were taught to perform manual compression of the ophthalmic vein to self-obliterate.6
The remaining 27 (14%) patients were managed conservatively. Twenty-six patients had benign DAVF that were asymptomatic or tolerating their symptoms (with or without medication). One patient with a malignant fistula was rendered palliative on presentation.
After GK, all patients received the same follow-up plan. Clinical assessment was performed at 3, 6, 12, 24 and 36 months. Imaging follow-up includes time-resolved magnetic resonance angiography (MRA) once every six months up to three years from treatment with a dedicated time-resolved imaging of kinetics (TRICKS, GE Healthcare) sequence at 3T. Patients with aggressive fistulae always received one digital subtraction angiography (DSA) follow-up at 36 months after radiosurgery; however, this was optional for benign lesions.
Each lesion received one session of GK radiosurgery. Marginal doses ranged from 15 to 25 Gy with an average dose of 20 Gy. Target volume ranged from 0.04 to 4.47 cm3. The target planning included fusion of contrast-enhanced magnetic resonance imaging (MRI), MRA, computed tomography angiography (CTA) and DSA. Table 1 summarizes the target volumes, marginal doses, and outcomes.
Table 1.
Gamma Knife target volume, marginal dose and outcome per lesion.
| Lesion | Target volume | Marginal dose | Complication | Clinical cure | Angiographic cure |
|---|---|---|---|---|---|
| 1 | 0.30 cm3 | 25 Gy | No | Yes, three months | No, unchanged |
| 2 | 1.33 cm3 | 25 Gy | No | Yes, six months | Yes, two years |
| 3 | 1.01 cm3 | 25 Gy | No | Yes, six months | Yes, two years |
| 4 | 1.17 cm3 | 25 Gy | Yes | No | Yes, three years |
| 5 | 0.12 cm3 | 15 Gy | No | Yes, six months | Yes, one year |
| 6 | 1.52 cm3 | 20 Gy | No | Yes, one year | No, regressed |
| 7 | 1.10 cm3 | 25 Gy | No | No | Yes, three years |
| 8 | 4.47 cm3 | 20 Gy | No | Yes, one year | Yes, three years |
| 9 | 1.24 cm3 | 25 Gy | No | Yes, one year | Yes, three years |
| 10 | 0.90 cm3 | 15 Gy | No | Yes, two years | No, unchanged |
| 11 | 0.72 cm3 | 25 Gy | No | Yes, two years | No, unchanged |
| 12 | 1.02 cm3 | 20 Gy | No | Yes, three months | No, regressed |
| 13 | 0.04 cm3 | 20 Gy | No | Yes, one year | No, unchanged |
| 14 | 3.55 cm3 | 25 Gy | No | Yes, six months | No, regressed |
| 15 | 3.43 cm3 | 15 Gy | No | Yes, six months | Yes, two years |
| 16 | 1.24 cm3 | 25 Gy | No | No | No, regressed |
 Clinical cure: five years
Clinical cure: five years
 Clinical cure: three years or less
Clinical cure: three years or less
All neurovascular cases are discussed at a multidisciplinary conference. DAVF are usually offered embolization or microsurgical treatments. GK is offered following failed attempts at conventional treatment, patient refusal, or in the setting of high procedural/surgical risk. A dose of 25 Gy is administered to volumes less than 4 cm3 and a dose of 20 Gy to volumes greater than 4 cm3. The radiation dose is scaled back, as required, to avoid a dose of greater than 15 Gy to eloquent brain (sensorimotor, language, visual, thalamus, hypothalamus, internal capsule, brain stem, cerebellar peduncles, and deep cerebellar nuclei as per modified Spetzler classification.7).
For planning of GK treatment a Leksell (Elekta, Sweden) stereotactic frame is placed on the patient’s head under local anesthesia. The patient then undergoes CTA, MRA and DSA. These three modalities are then digitally fused. The target is first defined on DSA and after fusion is defined on one of the consecutive planar axial images by a multidisciplinary team. For DAVF, the target typically consists of the junction between the dural arterial supply and the entry zone to the draining vein or dural sinus (i.e. the nidus or shunt).
Results
Lesion characteristics
The various lesion characteristics are summarized in Table 2.
Table 2.
Gamma Knife DAVF demographic information, lesion location, symptoms and characteristics.
| Lesion | Age | Sex | Location | Symptoms/ Rationale | Borden type | Cognard type | Geibprasert type | Dural/ Osteodural | Pial-induced angiogenesis Yes/No | Venous pouch | Venous stenosis | Hemorrhage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | Vein of Galen, origin of straight sinus | Headache, reflux | Type III | IV | Lat. epidural | Dural | No | No | Yes | No |
| 2 | 63 | F | Distal sigmoid sinus-right jugular fossa | Tinnitus, dizziness | Type I | IIa | Vent. epidural | Dural | No | No | Yes | No |
| 3 | 44 | M | Vein of Galen | Headache, ptosis | Type III | IV | Lat. epidural | Dural | No | Yes | Yes | No |
| 4 | 45 | F | Petrous ridge | Malignant, tinnitus | Type III | IV | Lat. epidural | Dural | No | Yes | Yes | No |
| 5 | 71 | F | Left cavernous sinus | Malignant reflux, eye swelling | Type II | IIa + b | Vent. epidural | Dural | No | No | No | No |
| 6 | 66 | F | Torcular, right transverse sinus | Bruit | Type I | I | Dors. epidural | Osteodural | Yes | No | No | No |
| 7 | 54 | M | Torcular | Cerebellar hemorrhage, bruit | Type III | III | Dors. epidural | Dural | No | No | No | Yes |
| 8 | 37 | F | Torcular, right transverse sinus | Tinnitus, headache, eye congestion | Type III | IV | Dors. epidural | Dural | No | No | Yes | No |
| 9 | 64 | F | Torcular, left transverse sinus | Bruit, ocular pain | Type II | IIb | Dors. epidural | Dural | No | No | No | Yes |
| 10 | 67 | F | Transverse sigmoid junction | Bruit, nausea/ vomiting | Type I | I | Dors. epidural | Dural | No | No | No | No |
| 11 | 67 | F | Torcula and left transverse sinus | Bruit, nausea/ vomiting | Type II | IIa + b | Dors. epidural | Osteodural | Yes | No | No | No |
| 12 | 64 | M | Left falx and straight sinus | Ocular pain, dizziness | Type II | IIb | Lat. epidural | Dural | No | No | Yes | No |
| 13 | 44 | M | Right parietal, anterior tentorial margin | No symptoms | Type III | III | Lat. epidural | Dural | No | No | No | No |
| 14 | 64 | M | Vermian cerebellar vein | Cerebellar hemorrhage | Type III | III | Lat. epidural | Osteodural | No | Yes | Yes | Yes |
| 15 | 51 | M | Left sigmoid region | Tinnitus | Type I | IIa | Lat. epidural | Osteodural | No | No | No | No |
| 16 | 71 | F | Left sigmoid region | Bruit | Type I | IIa | Lat. epidural | Osteodural | No | No | No | No |
 Clinical cure: five years
Clinical cure: five years
 Clinical cure: three years or less
Clinical cure: three years or less
M: male; F: female; DAVF: dural arteriovenous fistulae; Lat.: lateral; Dors.: dorsal; Vent.: ventral.
The 14 DAVF patients we treated with GK radiosurgery included eight females and six males between the ages of 44 and 71 (mean 57.2). Two patients had more than one DAVF. The 16 lesions treated spanned various angioarchitectures, including five Borden I, four Borden II and seven Borden III. The distribution in terms of Cognard type were two Cognard I, three Cognard IIa, two Cognard IIb, two Cognard Ia + b, three Cognard III and four Cognard IV. There were eight lateral epidural, two ventral epidural and six dorsal epidural fistulae.8 Five out of 16 DAVF were osteodural type, rather than purely dural. Of the 16 lesions, three presented with intracranial hemorrhage, two with headache, two with ocular congestion, and eight with irritating bruit or pulsatile tinnitus. One lesion was incidentally discovered. Of the non-hemorrhagic lesions, seven were aggressive with CVR. In total, 11 of the DAVF that we treated with GK were “aggressive” and five were “benign” according to the van Dijk classification.9
Venous pouches were present in three lesions, all of which were Borden III lesions of the lateral epidural type. All of these lesions had venous stenosis and outflow restrictions. A stenosis was defined as a narrow segment in the vein compared to the diameter of this draining vein proximally, distally or in both locations. One patient had a flow-related aneurysm associated with a Borden type II lesion. Overall, seven lesions possessed outflow restrictions and their presence was not reliably associated with Borden type.
All fistulae were supplied by regional dural arterial branches of the external carotid arteries and vertebral arteries. Two lesions received additional arterial blood supply from pial arteries. Both of these were osteodural rather than dural fistulae, and corresponded to the dorsal epidural type, with a large number of arterial feeders.
Ten patients had undergone unsuccessful endovascular attempts at cure (three benign, seven aggressive), and four were abandoned without embolization for technical reasons. Three lesions had undergone an unsuccessful attempt at surgical cure (one benign, two aggressive). None had previously undergone radiosurgery. One patient was deemed a poor surgical candidate with a deep lesion (aggressive fistula), and two patients (one benign, one aggressive) refused all invasive treatments (one of which was the incidentally discovered fistula).
Eleven patients in our study were Borden II/III lesions treated with GK. Five of these underwent prior technically unsuccessful endovascular procedures that were aborted prior to cure because of technical difficulty with either access or penetration of embolic material. As mentioned, a further two patients were deemed not ideal for embolization because of angioarchitecture. One patient was considered a poor candidate for microsurgery and one for embolization because of the depth of the lesion and poor overall health. Finally, two patients refused all invasive treatment options.
Symptomatic cure
Symptomatic cure was achieved in 13 lesions accounting for 86.0% (13/15) of the total symptomatic lesions as one lesion was incidental. At six-month and one-year MRI, lesions that responded symptomatically were all seen to possess shrinking draining veins with later filling, thickened wall, and thrombosis within the venous pouch when applicable.
Among the 13 symptomatically cured lesions, five were Borden I, three were Borden II and five were Borden III. Failure to cure occurred in two Borden I, 0 Borden II and one Borden III lesion. Two dural and one osteodural lesion were among the failures. Osteodural fistulae would symptomatically improve at a minimum of one year, whereas many dural lesions clinically improved at three to six months.
Three lesions were treated with a marginal dose of 15 Gy with three successes and no failures. At 20 Gy, there were four successes and no failures. At 25 Gy, there were six successes and three failures. All three failures were targets of 1.17 cm3 or less. There were no similarities of the anatomical target characteristics for these failures (i.e. pial vein, sinus wall, arterial network, Borden or Cognard type).
Angiographic cure
Eight of 16 lesions achieved angiographic cure (50%), and a further four of 16 lesions significantly regressed. We reached the time further bracket of five years in four of our patients, three of whom were angiographically cured (75%), six cures occurred in malignant fistulae, and two in benign. Additionally, one fistula was reduced from malignant to benign by GK. Two osteodural lesions possessed a secondary pial blood supply, and neither of these lesions was cured angiographically, but both were cured symptomatically. Of the eight angiographic failures, one possessed one arterial feeder, three possessed three, one possessed five and three possessed nine or more.
Complications
In one patient, following GK treatment, new headache occurred. This resolved at one-year follow-up. No other complications were noted. No hemorrhage occurred during the follow-up period of 139 patient-years. There was no significant association between Borden or Cognard type and cure rate. Five out of eight angiographic failures received prior embolization.
Discussion
GK radiosurgery is employed for much the same purpose as microsurgery or transcatheter embolization, which is either to reduce the likelihood of hemorrhage in aggressive DAVF or to improve quality of life that is impaired by shunt-associated symptoms such as irritating bruit, headache, or ocular symptoms (Figure 1). While angiographic resolution of the shunt represents the ultimate goal, this is not always necessary in benign shunts in order to achieve clinical success.10 In our practice, the dose is the prescription dose, which is almost always the intended least dose that the target volume receives. If only 98% of the target is covered by the prescription dose, then 2% of the target volume receives less radiation. Ninety-eight percent is the minimum coverage that we allow, unless there is some sort of problem. With most GK plans, we do prescribe to the 50% isodose. To contrast this, with linear accelerator plans, the prescription is usually to a higher isodose contour, which means that the dose in the center is not as high.
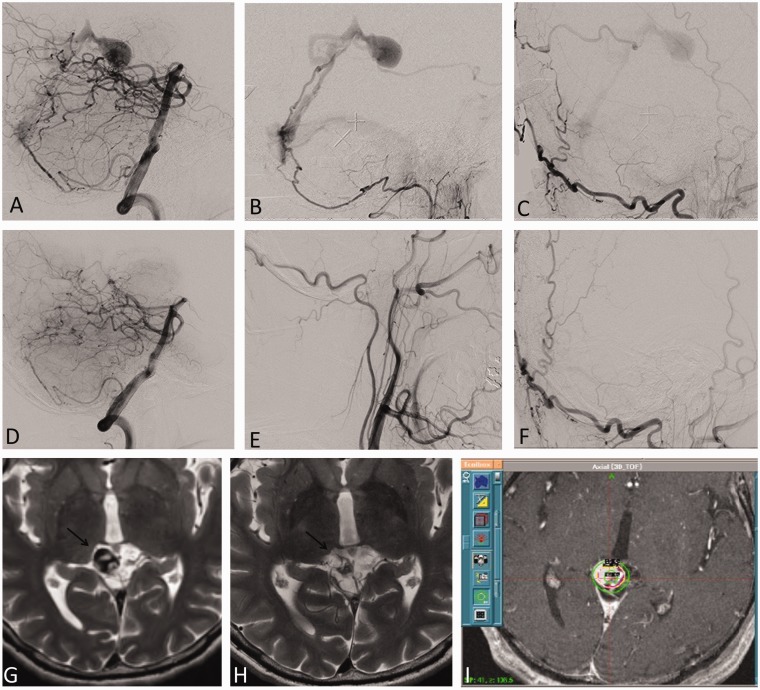
Figure 1.
A 51-year-old man presenting with Borden type 3 falco-tentorial DAVF at the vein of Galen. ((a)–(c)) Cerebral angiogram at the time of GK treatment planning. Lateral views of vertebral artery (a), ascending pharyngeal (b) and occipital artery (c) injections demonstrates the fistula drains via parafalacine veins into the vein of Galen. (i) At GK radiosurgery a volume of 1 cm3 was treated with a marginal dose of 25 Gy. Axial T2-weighted images of brain MRI at time of treatment (g), and three years after treatment (h) show the shrinking draining vein with loss of the original flow void (arrow), suggestive of cure. ((d)–(g)) Catheter cerebral angiogram three years after radiosurgery confirms cure as seen in the lateral projections of vertebral artery (d), ascending pharyngeal (e) and occipital artery (f) injections. DAVF: dural arteriovenous fistula; GK: Gamma Knife; MRI: magnetic resonance imaging.
Lesions and presentations
Our patients with benign fistulae presented with quality of life symptoms or incidentally, whereas those with malignant fistulae would present either with quality of life symptoms, hemorrhage, or non-hemorrhagic deficits. Of the former symptoms, this was most likely to be bruit and/or tinnitus. This presentation was usually not significantly affected by Borden type, but headache was encountered with Borden III lesions exclusively. All our patients with ocular symptoms possessed Borden II lesions, which by virtue of their cavernous sinus involvement drained partly into the ophthalmic vein, though this can also be seen with Borden I lesions.11 All of the lesions presenting with hemorrhage were Borden III lesions, which are known to be most likely to present in this manner secondary to drainage into thin-walled and fragile cortical veins.12–14
Secondary pial blood supply was observed in two lesions, both of which were of the dorsal epidural type. These two lesions had the largest number of arterial feeders, the minimum number being nine. Both and only the patients with multiple DAVF exhibited secondary pial blood supply, which is known to represent a fistula that has grown and recruited supply from parenchymal vessels.15,16 When these lesions hemorrhage, it is felt that the pial component is most likely the focus for this event. It is possible that a venous steal effect in the dural sinus secondary to the high-flow dural arteriovenous shunt induces the pial arteriovenous component (Figure 2).2
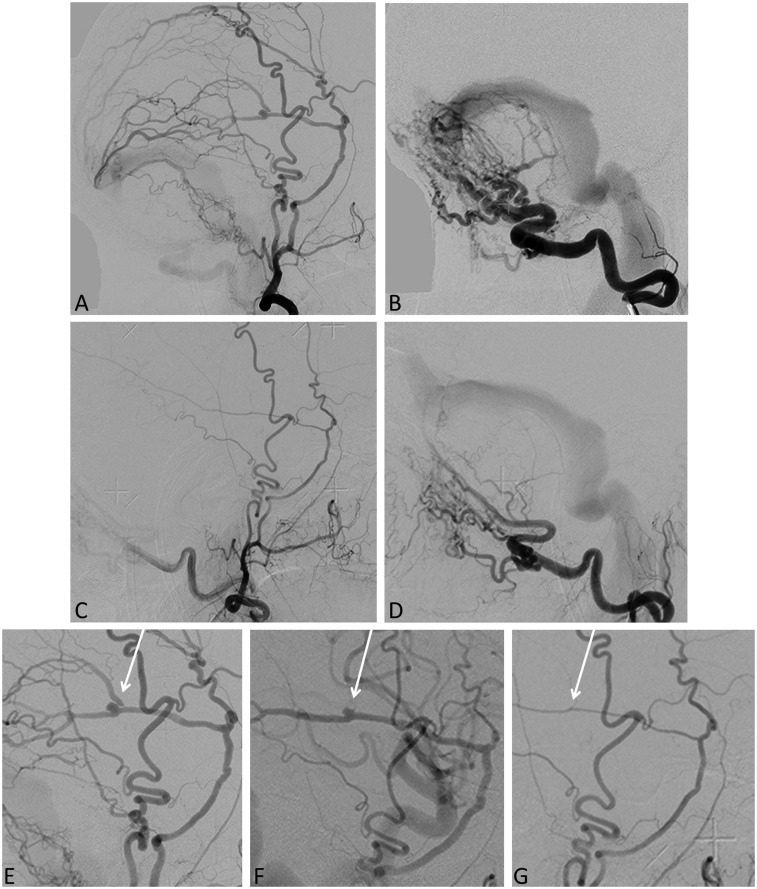
Figure 2.
Borden type I, torcular osteodural AV fistula. Lateral images of external carotid (a) and occipital artery (b) injections demonstrating the fistula draining into the region of the torcula and into the lateral dural sinus without cortical venous reflux. Cerebral angiogram of the external carotid (c) and occipital artery (d) three years after GK radiosurgery shows residual fistula with significant flow reduction. Patient had complete symptom resolution at one year post-GK treatment. Consecutive cerebral angiograms of the ECA at presentation (e), 1.5 years (f) and three years (g) after GK treatment show gradual caliber reduction of the middle meningeal artery and resolution of a flow-related aneurysm (arrow). AV: arteriovenous; GK: Gamma Knife; ECA: external carotid arteries.
All lesions that possessed venous pouches were Borden type III, and these were all of the lateral epidural type. They all had stenosis or outflow restriction. Given that lesions are significantly more likely to exhibit cortical venous reflux when stenosis/outflow restriction is present, it is perhaps not surprising that these lesions were Borden type III.17 Moreover, venous ectasia is associated with greater rates of hemorrhage.18
Treatment and outcome
Currently, outcome data suggest that endovascular treatment and microsurgery should be employed as first-line treatment with radiosurgery as salvage therapy for refractory cases.23 While patients with aggressive fistulae are clearly exposed to a risk of re-hemorrhage over the post-GK interval, there is no other for them. In our experience, GK radiosurgery also appears to be an effective option for patients in whom angioarchitecture, lesion depth, patient overall health, and technical factors render embolization risky. In addition, we found that in some cases patients who are unwilling to consider invasive treatment will still consider GK. Eight fistulae were angiographically cured (50%), whereas eight possessed persistent shunt (50%). Of these eight that persisted, four regressed to small residual lesions, and four were unchanged. Two of the four unchanged lesions have not yet reached three years, however. Our cure rate is concordant with most centers up to three years.19–23 Hanakita et al. noted a marked change from 51% at three years to 80% at five years.23 Söderman et al.20 were the first to report their experience, showing a 57% obliteration rate at two years, and the group also showed a 63%–70% obliteration of BAVMs at the same dose.
One of our failed GK patients possessed a complex Borden III lesion and presented with pulsatile tinnitus. This same patient represented the sole patient of 16 lesions/sessions who was subject to complication. At six weeks following a 25 Gy treatment, this patient developed new severe headache. These headaches intensified up until one year post-GK, when they spontaneously resolved. On imaging at this time point, it was found that the lesion had changed from a Cognard IV type to Cognard III. There was no change in Borden type at this time. The patient eventually went on to cure with microsurgery since DAVF remained aggressive three years post-GK.
Although the number of treated patients is small, it is possible that embolization prior to radiosurgery makes successful obliteration less likely as is the case with attempted endovascular obliteration of BAVMs, and the majority of our angiographic failures had prior embolization.24,25
Clinical cure of symptoms was more prevalent than angiographic cure and was achieved in 13 lesions (86.0%). The remaining three lesions appeared to impart ongoing symptoms (14.0%), though they were reported to have lessened.
We noticed that angiographically persistent shunts were present in many symptomatically cured patients. Furthermore, the average time of reported symptomatic cure or palliation occurred at six months, even for lesions that would only later resolve angiographically. The average time for angiographic resolution was two years, and this time discrepancy has been seen in other studies.10,26 It is possible that the thickening effect of radiation on the vessel wall decreases the flow through the fistula and thus dampens the noise patients experience even prior to evident angiographic cure. This phenomenon of vessel wall thickening is well documented in the AVM literature.27
We found several imaging features were indicative of treatment response. In each lesion that exhibited partial or total involution, the first sign visible was a thickened lesion wall on MRI. This usually appeared between six months and one year after GK treatment. Between one and two years, the draining vein became smaller with later filling of the shunt (Figure 3). In one case, shrinking of the draining veins was seen at six months. When lesions possessed venous pouches, thrombosis of this element was noted between one and two years. In the case of a single Borden I occipital shunt, the entire lesion was noted to obliterate at one year with no preceding signs. No significant correlation between Borden type and cure was noted. Similarly, no Borden type reliably predicted treatment failure.
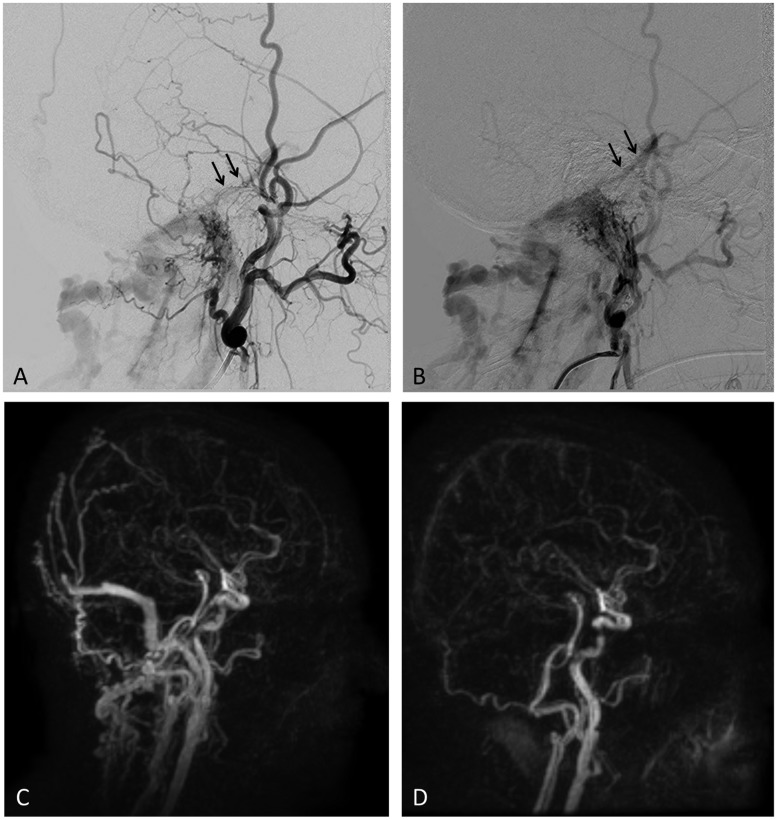
Figure 3.
Jugular bulb, Borden type I DAVF in a 63-year-old female presenting with tinnitus. Lateral images of external carotid artery (a) and ascending pharyngeal artery (b) injections show demonstrate a DAVF draining into the jugular vein and sub-occipital venous plexus and with reflux toward the superior petrosal sinus (arrow) and sigmoid sinus (as seen on (c)). There was no evidence of CVR. Time-resolved MRA (TRICKS) ((c) and (d)) shows the fistula at baseline similar to the findings on catheter angiogram. (c). One and a half years after GK the patient’s symptoms completely resolved and TRICKS MRA showed cure of the fistula (d). DAVF: dural arteriovenous fistula; GK: Gamma Knife; CVR: cortical venous reflux; MRA: magnetic resonance angiography; TRICKS: time-resolved imaging of kinetics.
All of the clinical failures were dural rather than osteodural fistulae. Conversely, however, only one osteodural lesion reached angiographic cure with GK. Osteodural lesions all symptomatically resolved at a minimum of one year, whereas many dural lesions clinically improved at three to six months. This may lend credence to the importance of thickening vessels, which may not be as readily permitted in the confines of an osteodural lesion (Figure 4). Osteodural lesions seem to respond more slowly but all eventually reach symptomatic cure unlike their dural counterparts, though our sample size is small.
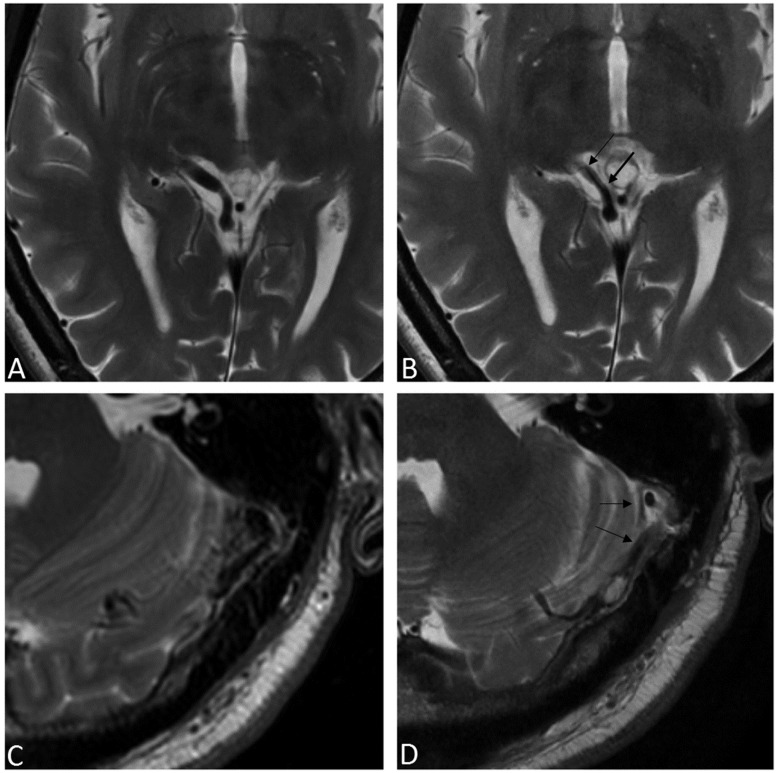
Figure 4.
T2W MRI of two cases showing venous wall thickening after GK radiosurgery. A right-sided petrous ridge DAVF pre- (a) and post- (b) GK demonstrating wall thickening of the single draining vein, the basal vein of Rosenthal (arrows in (b)). This fistula was reduced after GK but not cured and eventually disconnected by open surgery. Another patient with a left-sided sigmoid sinus DAVF pre- (c) and post- (d) GK exhibits the same response of venous wall thickening after treatment (arrows in (d)). This fistula was cured by GK. T2W: T2-weighted; MRI: magnetic resonance imaging; GK: gamma knife; DAVF: dural arteriovenous fistula.
The two lesions with secondary pial blood supply both did not cure angiographically after GK. They were both failed endovascular cases, and one of these also failed surgery. Both lesions, however, reached symptomatic cure with GK. It is accepted that pial supply permits significant arterial supply to a shunt. It stands to reason that this would make for a more recalcitrant lesion.8 It also appears in our experience that lesions with many arterial feeders are less likely to obliterate with GK. All but one failure possessed three or more feeders, and the majority possessed five or more.
A reliable relationship between dose and effective cure was difficult to establish since our sample size is small. Neither 15 Gy nor 20 Gy plans experienced any failures. However, the most common treatment plan involved using a 25 Gy dose, which produced six successes and three failures. Our doses are inversely increased according to lesion size and adjusted in relation to location proximity to eloquent regions of the brain. The rationale for this was first defined in the AVM literature, and it is felt that similar caution is likely needed for the treatment of DAVF.28 All three failures were small targets of 1.17 cm3 or less. These targets’ characteristics were all different (i.e. pial vein, sinus wall, arterial network). We encountered no latency period hemorrhage in our patients out to three years. The major problem with treating AVMs in general and DAVF in particular is defining the target. It is often unclear whether abnormal vessels in the vicinity of the nidus are part of the nidus that should be radiated or are angiomatized vessels that should be avoided. To the best of our ability, we have defined the region of shunting of each AVF and included at least 98% of it in the prescription volume. The prescription volume is always slightly larger than the defined target because conformality is never perfect.
We do not believe that the failures were undertreated as we deliberately left parts of the AVF out or because we used too low a radiation dose. The failures are due either to our failure to recognize parts of the AVF, or to inherent increased resistance to radiation. We know that susceptibility to radiation varies from individual to individual and that even when the target is obvious, obliteration after radiosurgery is not 100%.
Conclusions
GK treatment is a safe treatment for cranial DAVF. The technique is very effective in palliating symptoms (86%), and therefore has a particular role in symptomatic benign fistulae. In our experience it is only somewhat effective in achieving angiographic cure (50% at three years and 75% at five years), and latency periods are significant.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Miller TR, Gandhi D. Intracranial dural arteriovenous fistulae: Clinical presentation and management strategies. Stroke 2015; 46: 2017–2025. [DOI] [PubMed] [Google Scholar]
- 2.Lai CW, Agid R, van den Berg R, et al. Cerebral arteriovenous fistulas induced by dural arteriovenous shunts. AJNR Am J Neuroradiol 2005; 26: 1259–1262. [PMC free article] [PubMed] [Google Scholar]
- 3.Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol 2003; 46: 206–220. [DOI] [PubMed] [Google Scholar]
- 4.Söderman M, Pavic L, Edner G, et al. Natural history of dural arteriovenous shunts. Stroke 2008; 39: 1735–1739. [DOI] [PubMed] [Google Scholar]
- 5.Van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multidisciplinary management of spinal dural arteriovenous fistulas: Clinical presentation and long-term follow-up in 49 patients. Stroke 2002; 33: 1578–1583. [DOI] [PubMed] [Google Scholar]
- 6.Cruz JP, van Dijk R, Krings T, et al. Ophthalmic vein compression for selected benign low-flow cavernous sinus dural arteriovenous fistulas. J Neurosurg 2013; 119: 239–242. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001; 32: 1430–1442. [DOI] [PubMed] [Google Scholar]
- 8.Geibprasert S, Pereira V, Krings T, et al. Dural arteriovenous shunts: A new classification of craniospinal epidural venous anatomical bases and clinical correlations. Stroke 2008; 39: 2783–2794. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk JM, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002; 33: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 10.Wu HM, Pan DH, Chung WY, et al. Gamma Knife surgery for the management of intracranial dural arteriovenous fistulas. J Neurosurg 2006; 105(Suppl): 43–51. [DOI] [PubMed] [Google Scholar]
- 11.Piippo A, Niemela M, van Popta J, et al. Characteristics and long-term outcome of 251 patients with dural arteriovenous fistulas in a defined population. J Neurosurg 2013; 118: 923–934. [DOI] [PubMed] [Google Scholar]
- 12.Gross BA, Du R. The natural history of cerebral dural arteriovenous fistulae. Neurosurgery 2012; 71: 594–602. discussion 602–603. [DOI] [PubMed] [Google Scholar]
- 13.Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg 1990; 72: 839–850. [DOI] [PubMed] [Google Scholar]
- 14.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995; 82: 166–179. [DOI] [PubMed] [Google Scholar]
- 15.Pelz DM, Lownie SP, Fox AJ, et al. Intracranial dural arteriovenous fistulae with pial venous drainage: Combined endovascular-neurosurgical therapy. Can J Neurol Sci 1997; 24: 210–218. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Periakaruppan A. Intracranial dural arteriovenous fistulas: A review. Indian J Radiol Imaging 2009; 19: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mironov A. Cranial dural arteriovenous fistulas: Clinical findings and radiologic diagnostics. Klin Neuroradiol 2005; 15: 32–39. [Google Scholar]
- 18.Bulters DO, Mathad N, Culliford D, et al. The natural history of cranial dural arteriovenous fistulae with cortical venous reflux—the significance of venous ectasia. Neurosurgery 2012; 70: 312–318. discussion 318–319. [DOI] [PubMed] [Google Scholar]
- 19.Cognard C, Januel AC, Silva NA, Jr, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. AJNR Am J Neuroradiol 2008; 29: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Söderman M, Dodoo E, Karlsson B. Dural arteriovenous fistulas and the role of gamma knife stereotactic radiosurgery: The Stockholm experience. Prog Neurol Surg 2013; 27: 205–217. [DOI] [PubMed] [Google Scholar]
- 21.Cifarelli CP, Kaptain G, Yen CP, et al. Gamma knife radiosurgery for dural arteriovenous fistulas. Neurosurgery 2010; 67: 1230–1235. discussion 1235. [DOI] [PubMed] [Google Scholar]
- 22.Chen CJ, Lee CC, Ding D, et al. Stereotactic radiosurgery for intracranial dural arteriovenous fistulas: A systematic review. J Neurosurg 2015; 122: 353–362. [DOI] [PubMed] [Google Scholar]
- 23.Hanakita S, Koga T, Shin M, et al. Role of Gamma Knife surgery in the treatment of intracranial dural arteriovenous fistulas. J Neurosurg 2012; 117(Suppl): 158–163. [DOI] [PubMed] [Google Scholar]
- 24.Andrade-Souza YM, Ramani M, Scora D, et al. Embolization before radiosurgery reduces the obliteration rate of arteriovenous malformations. Neurosurgery 2007; 60: 443–451. discussion 451–452. [DOI] [PubMed] [Google Scholar]
- 25.Andrade-Souza YM, Ramani M, Beachey DJ, et al. Liquid embolisation material reduces the delivered radiation dose: A physical experiment. Acta Neurochir (Wien) 2008; 150: 161–164. discussion 164. [DOI] [PubMed] [Google Scholar]
- 26.Söderman M, Edner G, Ericson K, et al. Gamma knife surgery for dural arteriovenous shunts: 25 years of experience. J Neurosurg 2006; 104: 867–875. [DOI] [PubMed] [Google Scholar]
- 27.Kashba SR, Patel NJ, Grace M, et al. Angiographic, hemodynamic, and histological changes in an animal model of brain arteriovenous malformations treated with Gamma Knife radiosurgery. J Neurosurg 2015; 123: 954–960. [DOI] [PubMed] [Google Scholar]
- 28.Pan DH, Wu HM, Kuo YH, et al. Intracranial dural arteriovenous fistulas: Natural history and rationale for treatment with stereotactic radiosurgery. Prog Neurol Surg 2013; 27: 176–194. [DOI] [PubMed] [Google Scholar]