Abstract
Negative symptoms are a core clinical feature of schizophrenia, but conceptual and methodological problems with current instruments can make their assessment challenging. One hypothesis is that current symptom assessments may be influenced by impairments in memory and may not be fully reflective of actual functioning outside of the laboratory. The present study sought to investigate the validity of assessing negative symptoms using ecological momentary assessment (EMA). Participants with schizophrenia (N=31) completed electronic questionnaires on smartphones four times a day for one week. Participants also completed Effort-Based Decision Making and Reinforcement Learning (RL) tasks to assess the relationship between EMA and laboratory measures, which tap into negative symptom relevant domains. Hierarchical linear modeling analyses revealed that clinician-rated and self-report measures of negative symptoms were significantly related to negative symptoms assessed via EMA. However, working memory moderated the relationship between EMA and retrospective measures of negative symptoms, such that there was a stronger relationship between EMA and retrospective negative symptom measures among individuals with better working memory. We also found that negative symptoms assessed via EMA were related to poor performance on the Effort task, while clinician-rated symptoms and self-reports were not. Further, we found that negative symptoms were related to poorer performance on learning reward contingencies. Our findings suggest that negative symptoms can be assessed through EMA and that working memory impairments frequently seen in schizophrenia may affect recall of symptoms. Moreover, these findings suggest the importance of examining the relationship between laboratory tasks and symptoms assessed during daily life.
Keywords: Negative symptoms, schizophrenia, Motivation, Pleasure, Ecological Momentary Assessment
Negative symptoms are a core clinical feature of schizophrenia (SZ) that include two dissociable facets of impairment: (A) disturbances in motivation and pleasure and (B) reductions in emotional expression (e.g., Horan, Kring, Gur, Reise, & Blanchard, 2011). These symptoms are independently associated with poor functional outcome, including both social and occupational impairments (Milev, Ho, Arndt, & Andreasen, 2005). Despite advances in pharmacotherapy for SZ, negative symptoms are only marginally responsive to currently available medications (Buchanan et al., 2007). One major hurdle in advancing treatment is the ability to reliably assess these symptoms utilizing measurement approaches that reflect our current understanding of negative symptoms in SZ (Kirkpatrick, Fenton, Carpenter, & Marder, 2006).
Two instruments have been developed in recent years to help fill this gap. The Clinical Assessment Interview for Negative Symptoms (CAINS; Kring, Gur, Blanchard, Horan, & Reise, 2013) and the Brief Negative Symptom Scale (Kirkpatrick et al., 2010) were designed to reliably assess our current conceptualization of negative symptoms. These clinician rated instruments show good convergent and discriminant validity and demonstrate good inter-rater reliability (Horan et al., 2011; Strauss et al., 2012). While these new instruments represent a major advancement, it is unclear whether the method of administering these instruments garners information that reflects the actual experience of negative symptoms as people go about their daily lives. For example, in these assessments, clinicians ask participants to reflect upon their experience of motivation and pleasure across previous week(s). It may be the case that participants have difficulty reflecting across an entire week to give accurate responses to questions. Moreover, participants may have difficulty recalling events and feelings from the beginning of the week, or may only recall large events, forgetting less salient moments of motivation or pleasure. Given that schizophrenia is associated with cognitive impairments, including impairments in working memory and episodic memory (e.g., Barch and Ceasar, 2012; Lee and Park, 2005), these interviews may be a challenge for patients to complete validly.
In addition to limitations based on cognitive demands, clinician rated interviews may also have additional biases. For example, symptom ratings are based on the clinician’s judgment from what is reported and observed during a single session, often conducted in an unfamiliar setting by an unfamiliar person outside of the subjects’ daily life. Given potential rater biases based on characteristics such as gender and race (e.g., Garb, 1997; Strauss & Culbreth, 2014), an instrument that does not require clinician judgment may provide an additional beneficial tool for assessment. Moreover, while rating scales are semi-structured, training and reliability between clinician raters may be limited and thus present additional confounds in symptom assessment.
Another issue with current clinical assessments is that they show variability in relationships with laboratory tasks thought to tap into relevant constructs. As one example, laboratory based tasks assessing effort-based decision-making are being developed to provide objective measures of motivation (e.g., Treadway, Buckholtz, Schwartzman, Lambert, & Zald, 2009). In these tasks, participants are presented with varying levels of reward and are asked to make a decision as to how much effort they are willing to exert to gain the reward. While these paradigms are designed to measure motivation, findings demonstrating a relationship with negative symptom measures of motivation have been inconsistent. Some studies assessing effort have demonstrated associations with negative symptoms (Barch, Treadway, & Schoen, 2014; Gold et al., 2013; Horan et al., 2015; Treadway, Peterman, Zald, & Park, 2015), while others show no relationship with negative symptoms (Docx et al., 2015; Fervaha et al., 2013; Gold et al., 2015; McCarthy, Treadway, Bennett, & Blanchard, 2016). Reinforcement learning (RL) is another domain conceptually linked to negative symptoms. Deficits in motivation and pleasure have been hypothesized to reflect impairments in reward learning, such that individuals with schizophrenia reporting anhedonia may have difficulty utilizing reward contingencies to guide future behavior (Waltz, Frank, Robinson, & Gold, 2007). While several studies have found that negative symptom measures relate to task performance on RL paradigms (Gold et al., 2012; Strauss et al., 2011), other studies have not (Culbreth, Gold, Cools, & Barch, 2015; Gradin et al., 2011; Strauss et al., 2015). It is possible that laboratory paradigms and symptom assessments provide distinct information, but it may also be the case that the types of cognitive impairments described above make it challenging to consistently link negative symptom assessments to laboratory based tasks, at least when the negative symptoms are assessed through typical clinical interviews. It may be that assessments of negative symptoms in daily life may be more consistently related to laboratory based paradigms assessing constructs thought to contribute to negative symptoms.
Taken together, the existing research points to the importance of assessing different domains of motivation and pleasure while also taking into account impairments in memory that may make traditional clinical symptom interviews challenging. The existing work also raises the question as to whether laboratory paradigms assessing domains such as effortful decision-making and RL are related to negative symptoms as assessed in daily life. EMA is a method that can help address these challenges by allowing the assessment of experiences in daily life, outside of the laboratory, and putting less reliance on an individuals’ ability to remember experiences for prolonged periods of time. EMA also allows for multiple and repeated assessments over time to obtain a better understanding of experiences as they occur naturally during everyday life. A number of studies have utilized EMA in schizophrenia (e.g., Ben-Zeev et al., 2012; Gard et al., 2014; Granholm, Loh, & Swendsen, 2008; Myin-Germeys et al., 2003; Oorschot et al., 2013; Sanchez, Lavaysse, Starr, & Gard, 2014). Granholm and colleagues (2008) demonstrated that computerized EMA was a feasible and valid method of assessing emotional experience and symptoms of psychosis in people with schizophrenia. Further, Ben-Zeev and colleagues (2012) examined the relationship between daily reports of emotional experience and symptoms of psychosis assessed via EMA and retrospective reports. When comparing retrospective reports with EMA reported experience, they found that participants overestimated the intensity of their positive and negative affect in retrospective reports relative to their reports during daily life. Thus, there is evidence to suggest that retrospective reports may vary from affective ratings assessed in the moment. Other studies have assessed motivation and pleasure in schizophrenia utilizing EMA (e.g., Gard and Kring, 2006; Gard et al., 2014, Sanchez, Lavaysse, Starr & Gard, 2014). For example, Gard and colleagues (2007) found that people with schizophrenia showed a deficit in anticipatory pleasure for future motivated activities relative to controls. However, a follow up suggested that deficits may not be in ability to anticipate pleasure, but in ability to engage in goal directed behavior in daily life (Gard et al., 2014). Across these studies findings suggest that people with schizophrenia report engaging in less motivated behavior relative to healthy controls, and report similar levels of in the moment pleasure relative to controls. To our knowledge no study has utilized EMA to assess the motivation and pleasure aspects of negative symptoms to examine their relationship to laboratory tasks of motivated effort and RL, two domains thought to be linked to negative symptoms.
The current study was designed to examine the validity of EMA as an additional method for assessing motivation and pleasure in psychosis. By using EMA we may be able to overcome some of the potential limitations of clinician rated assessment scales, including (1) reducing the time frame of assessment and thus reducing memory demands, (2) removing clinician judgments, (3) asking subjects to report their experiences outside of the laboratory in their daily environment, (4) and collecting multiple measurements of symptoms across a weeks time. In addition, we sought to examine the relationship between symptom assessments and laboratory tasks thought to tap into relevant domains. As such, the current study had three aims. Our first aim was to test the hypothesis that impairments in motivation and pleasure measured in daily life would show good convergent validity with other methods of assessing negative symptoms, including a clinician-rated and self-report rating scale. In contrast, we hypothesized that EMA ratings of motivation and pleasure would not be related to psychosis. Our second aim was to test the hypothesis that working memory would moderate the relationship between momentary reports of motivation and pleasure and assessments that rely on retrospective reports of the prior weeks’ experiences. That is, we hypothesized that impairments in working memory would lessen the relationship between clinician-rated and EMA collected symptom interviews, suggesting that currently used negative symptom instruments may not always accurately reflect a person’s actual experiences throughout their day to day life. Finally, we aimed to examine the relationship between momentary assessments of motivation and pleasure with laboratory measures of effort and RL, two constructs thought to be tightly linked with motivation and pleasure.
Methods
Participants
Participants were 34 individuals meeting DSM-IV (American Psychiatric Association, 2000) criteria for SZ (n=27) or schizoaffective disorder (n=7). Notably, we chose not to recruit a control group because we did not think the questions being asked in the current study necessitated a control group, as the study’s focus was on individual differences among patients with SZ. Exclusion criteria included (1) DSM-IV diagnosis of substance abuse or dependence in the past 6 months; (2) DSM-IV diagnosis of a current mood disorder; (3) changes in medication 2 weeks prior to consent; (4) IQ less than 70 as measured by the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001); and (5) history of severe head trauma and/or loss of consciousness. Three participants were excluded due to less than 25% of EMA data being completed, yielding a final sample size of 31 SZ. All participants provided written informed consent to the protocol approved by the Washington University Institutional Review Board. Participant demographics are presented in Table 1.
Table 1.
Demographics, Clinical and Self-Report Measures
| Mean | SD | |
|---|---|---|
| Demographics | ||
| Age | 39.48 | 8.40 |
| Sex (% female) | 48% | |
| Education | 12.81 | 2.86 |
| Parental Education | 14.23 | 3.56 |
| Estimated IQ | 95.21 | 16.65 |
| Race (%) | ||
| African American | 67% | |
| Caucasian | 33% | |
| Clinical Ratings | ||
| BPRS Psychosis | 8.52 | 5.18 |
| CAINS MAP | 18.23 | 6.22 |
| CAINS EXP | 5.85 | 3.59 |
| Self-Report Measures | ||
| MAP-SR | 35.83 | 10.48 |
Note: Estimate IQ = premorbid IQ based on Wechsler Test of Adult Reading; BPRS Psychosis = Psychosis Subscale of the Brief Psychiatric Rating Scale; CAINS MAP = Motivation and Pleasure Subscale of the Clinical Assessment Interview for Negative Symptoms; CAINS EXP = Expression Subscale of the Clinical Assessment Interview for Negative Symptoms; MAP-SR = Motivation and Pleasure – Self Report rating scale.
Procedure
Participants completed 2 visits to the laboratory and 7 days of EMA assessment. On the first visit, participants completed a diagnostic interview and were trained on using the smartphone. Training consisted of instructing participants on using the phone along with reviewing questions on the EMA survey to ensure comprehension and ability to answer questions. For the next 7 days, participants completed the EMA protocol of the study responding to beeps 4 times per day. Following completion of the EMA protocol, participants returned to the laboratory and completed clinical symptom interviews to assess symptoms over the prior week. Participants also completed computerized laboratory tasks.
Clinical Assessment
Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV Axis I disorders. General psychiatric symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962). Negative symptoms were assessed using the CAINS (Kring et al., 2013) which includes a Motivation and Pleasure (MAP) and Expression (EXP) subscale, with higher scores indicating impairment. Participants also completed a self-report measure of negative symptoms, the Motivation and Pleasure Scale – Self-Report (MAP-SR; Llerena et al., 2013), an instrument modeled after the CAINS. Lower scores on the MAP-SR represent experiencing less motivation and pleasure throughout the week. Clinical assessments were completed either by a Ph.D.-level clinical psychologist or a master’s level psychologist. Clinical raters (EKM and AJC) rated a subset of CAINS and BPRS interviews (n=8) and showed high inter-rater reliability (CAINS ICC = .94; BPRS ICC = .90).
Ecological Momentary Assessment (EMA) Protocol
Participants were provided an Android-enabled smartphone to use during the EMA portion of the study. Phones were pre-loaded with the EasyM app, an application developed by Lathia and colleagues (Lathia, Rachuri, Mascolo, & Roussos, 2013), which prompted participants to complete the EMA questionnaire 4 times per day for 7 days between the hours of 10:00 AM and 7:00 PM. The questionnaires occurred pseudorandomly approximately every 3 hours. Participants were allotted 15 minutes to begin the survey, after which time their responses would not be counted. Participants who responded to less than 25% of interview prompts were excluded from the current analyses (n=3). Participants were paid $1.75 for each EMA questionnaire they completed within 15 minutes of beep and $40 per study visit.
EMA Questionnaire
The EMA questionnaire (shown in Table 2) was modeled after the CAINS-MAP subscale to assess motivation and pleasure across a variety of domains and time points. We chose to largely model our interview after existing negative symptom interviews such as the CAINS because the purpose of the study was to evaluate methods of administering negative symptom interviews rather than developing novel interview items. Participants were asked to indicate their current activities (i.e., work/school; TV/music/reading/exercise; chores; social activity; sleeping; eating; nothing in particular). Next, they indicated their (1) motivation and (2) pleasure in these activities on a 5-point scale from “not at all” to “extremely.” Participants were also asked to record their activities, motivation and pleasure (1) since the last beep (i.e., within the past 2 to 3 hours) and (2) in the upcoming 2 to 3 hours. Consistent with the CAINS-MAP and MAP-SR, we created a composite measure, summing motivation and pleasure ratings at each of the assessed time points (i.e., current, past, future), across domains of behavior to create an EMA-MAP score, with higher scores representing reduced motivation and pleasure. For the present study we chose not to present data separately by type of behavior or by time point, rather we wanted to obtain a global assessment of pleasure and motivation across time and behavior.1
Table 2.
EMA Interview
| EMA Questionnaire |
|---|
| What are you doing right now? |
| How much are you enjoying X? |
| How interested are you in X? |
| Since the last beep, which of the following activities have you done? |
| How much did you enjoy X? |
| How interested were you in X? |
| Which of the following activities do you anticipate doing within the next 2 to 3 hours? |
| How much do you think you will enjoy X? |
| How interested do you think you will be in X? |
Reinforcement Learning Task
We utilized a previously validated task, the Picture Incentive Learning Task (PILT; Gold et al., 2012), to assess RL. Stimuli consisted of 4 pairs of landscape pictures presented 1 pair at a time. Two pairs were associated with potential gain, while 2 pairs were associated with potential loss. Once presented with picture pairs, participants were instructed to select the picture that was most likely to either (1) earn money (Reward trials) or (2) avoid losing money (Loss trials). Correct responses for trials were reinforced on either 80% or 90% of trials. The task consisted of a total of 160 trials with 4 blocks of 40 trials and 40 trials per condition. We chose to look at the relationship of negative symptoms with a global measure of reward and loss learning thus, our outcome variables included accuracy in reward and loss trials at both the 80% and 90% reinforcement levels. Participants received between $1 and $4 from the task depending on task performance.
EEfRT Task
Participants performed a modified version of the Effort Expenditure for Rewards Task (EEfRT), described previously (Barch et al., 2014). This task, originally designed by Treadway and colleagues (2009), assesses participants’ willingness to complete easy or hard tasks for varying amounts of reward. The hard task involves using the nondominant pinkie finger to make approximately 100 button presses within 21-s for the chance to win a reward between $1.24 to $4.30. The easy task requires participants to use their dominant index finger to press a button approximately 20 times within a 7-s window for the opportunity to win a $1 reward. Prior to each trial, participants are told whether the trial has a 50% or 88% probability of being rewarded if completed successfully. Participants completed a total of 57 trials. To look at willingness to expend effort more globally, outcome measures included willingness to choose the hard task at the 50% and 88% probability levels. Participants were paid $9 for completion of the task.
Working Memory
Participants completed a Running Span task to assess working memory. Letters were presented on a computer screen one at a time, spaced 2-s apart. During a trial, an unpredictable number of letters was presented, and participants were asked to remember the last x number of letters from the list. The task began with participants recalling the last letter presented and went up to recalling the last 5 letters presented in an individual trial. Participants had to complete 2 out of 4 trials correctly to move on to the next block. The outcome measure was the total number of correct letters recalled across all trials.
Analyses
We used hierarchical linear modeling (HLM) in HLM 6.0 (Raudenbush, Bryk, Cheong, & Congdon, 2004) to investigate relationships between within-subject observations of EMA (Level 1) and between-subject observations including the CAINS, MAP-SR, and task performance variables (Level 2). We used cross-level interactions at Level 2 to investigate the hypothesized moderating effect of working memory on the relationship between EMA and retrospective reports of motivation and pleasure. We computed correlations between CAINS and MAP-SR and task performance. To analyze the incremental validity of EMA-MAP ratings we computed regression models entering CAINS at Step 1, MAP-SR at Step 2, and EMA-MAP at Step 3.
Results
EMA Feasibility Analyses
The mean response rate was 80% with 3 people falling below the 25% required completion rate. We first examined the feasibility of using EMA in a SZ population by conducting correlations between response rate of the entire sample (n=34) and demographic variables that may impact ability to respond to EMA questionnaires. As shown in Table 3, there was no relationship between response rates and demographic or symptom variables. Thus, findings suggest that the presence of psychiatric symptoms or demographic variables was not related to participants’ completing EMA questionnaires. All additional analyses included only participants with at least 25% completion rate (n=31).
Table 3.
Correlations Between EMA Response Rates and Demographic Variables
| Response Rate | ||
|---|---|---|
| r | p | |
| Age | −.28 | .18 |
| Education (Yrs) | .18 | .35 |
| Estimated IQ | .24 | .23 |
| Running Span | .19 | .33 |
| BPRS Psychosis Symptoms | .13 | .45 |
| CAINS MAP | −.11 | .58 |
| CAINS EXP | −.14 | .42 |
Convergent and Divergent Validity
We first examined whether the EMA questionnaire showed convergent validity with validated measures of negative symptoms (CAINS and MAP-SR). The CAINS-MAP significantly predicted EMA-MAP measure such that reduced motivation and pleasure on the CAINS predicted reduced motivation and pleasure assessed during daily life. Similarly, MAP-SR predicted EMA-MAP, suggesting that self-reported motivation and pleasure on the MAP-SR, predicted greater impairments outside the lab. This relationship was tempered by a significant cross-level interaction between running span and EMA, suggesting that running span moderated the relationship between MAP-SR and the EMA-MAP. Specifically, impaired working memory lessened the relationship between the two measures (b = −.27, p < .05). Further, we found that running span moderated the relationship between CAINS and EMA score (b = .31, p < .05). In contrast, our estimate of pre-morbid IQ, as measured by the WTAR, was not related to EMA-MAP and negative symptom ratings (p > .25). Finally, we examined the relationship between psychosis and EMA-MAP scores. As hypothesized, there was no significant relationship, providing evidence for divergent validity.
Relationship with Task Performance
Accuracy in reward and loss trials at both the 80% and 90% reinforcement levels are shown in supplement. Participants overall accuracy in learning reward across the 80% and 90% reinforcement rates was not significantly different (p = .12). Similarly, there was no significant difference in accuracy in learning to avoid loss between the 80% and 90% reinforcement conditions (p=.38). We next examined the relationship between RL and negative symptoms. As shown in Table 4, RL during reward trials of the PILT predicted EMA-MAP scores, such that ability to learn picture pairs associated with reward predicted greater motivation and pleasure with daily activities. The CAINS-MAP was significantly related to learning picture pairs associated with reward at the 90% contingency level, suggesting that high negative symptoms assessed via clinician ratings was related to poorer performance on reward learning when reinforcement contingencies are high (shown in Table 5). However, the MAP-SR was not related to RL reward trials on the PILT. When examining learning during potential loss conditions, accuracy was not predictive of motivation and pleasure on the EMA-MAP, CAINS-MAP, or MAP-SR.
Table 4.
Relationships Between EMA-MAP and Clinical Assessments and Task Behavior
| b | t | p | |
|---|---|---|---|
| BPRS Psychosis | −.04 | −1.25 | .21 |
| Negative Symptom Interview | |||
| CAINS MAP | .21 | 7.94 | .001 |
| MAP-SR | −.31 | −2.73 | .006 |
| Task Behavior | |||
| PILT Reward 90% | −.28 | −2.88 | .001 |
| PILT Reward 80% | −.31 | −2.95 | .006 |
| PILT Loss 90% | −.12 | −1.06 | .30 |
| PILT Loss 80% | −.10 | −.79 | .44 |
| EEfRT 50% Hard Choice | −7.88 | −2.44 | .02 |
| EEfRT 88% Hard Choice | −11.58 | −3.99 | .0005 |
Table 5.
Correlations between CAINS, MAP-SR and Task Behavior
| CAINS-MAP | MAP-SR | |
|---|---|---|
| Task Behavior | ||
| PILT Reward 90% | −.32* | .26 |
| PILT Reward 80% | −.24 | .20 |
| PILT Loss 90% | −.19 | .16 |
| PILT Loss 80% | −.29ˆ | .17 |
| EEfRT 50% Hard Choice | −.02 | −.14 |
| EEfRT 88% Hard Choice | −.12 | −.22 |
p < .05;
p = .08
In regards in the effort-based decision-making task, willingness to choose the hard task at the 50% and 88% probability levels on EEfRT is shown in the supplement. As previous studies have found, participants are significantly more likely to choose the hard task on the 88% probability level relative to the 50% probability level (t(30)=−3.12, p < .005). Further, we found that increased effort avoidance in the 50% and 88% probability conditions was predictive of reduced motivation and pleasure in daily life as indexed by EMA (Table 4). However, as shown in Table 5, there was no significant relationship between CAINS-MAP or MAP-SR and effort avoidance at the 50% or 88% probability conditions. Thus, our findings suggest a relationship between willingness to expend effort on our laboratory task and our EMA-MAP ratings of motivation and pleasure, but not on retrospective reported clinician ratings or self-report.
Finally, we sought to examine whether EMA predicted task performance above and beyond traditional symptom measures. To do this we calculated an average EMA-MAP score per participant. While this approach does not take full advantage of the repeated EMA measurements across time, this allowed us to take a conservative approach in comparing the magnitude of relationships between negative symptom measures (Meng, Rosenthal, & Rubin, 1992). We found that the relationship between EMA and EFfRT at 50% and 88% was significantly greater than it was with either CAINS (p < .01) or MAP-SR (p < .05). To further test the increment value of EMA and EEfRT, we conducted regressions predicting EEfRT using CAINS, MAP-SR and EMA-MAP rating. As shown in Table 6, willingness to exert effort at both the 50% and 88% probability conditions was significantly predicted by EMA-MAP while CAINS and MAP-SR were not significant predictors, indicating that EMA-MAP accounted for variance not accounted for by CAINS or MAP-SR. The relationship between EMA and PILT Reward at 90% and 80% was not significantly different from the relationship between PILT Reward and either CAINS or MAP-SR (ps > .17). Stepwise linear regressions predicting PILT Reward at 90% and 80% showed that EMA-MAP did not add incremental validity on top of CAINS scores.
Table 6.
Stepwise Regression Analyses
| Model Step | Predictor Variable | Beta | R2 | p |
|---|---|---|---|---|
| Dependent Variable: EEfRT 50% Hard Choice | ||||
| 1 | CAINS | −.09 | .01 | .63 |
| 2 | MAP-SR | .19 | .10 | .53 |
| 3 | EMA-MAP | −.41 | .23 | .02 |
| Dependent Variable: EEfRT 88% Hard Choice | ||||
| 1 | CAINS | −.14 | .06 | .59 |
| 2 | MAP-SR | .20 | .13 | .43 |
| 3 | EMA-MAP | −.49 | .25 | .009 |
| Dependent Variable: PILT Reward 90% | ||||
| 1 | CAINS | −.55 | .30 | .003 |
| 2 | MAP-SR | .23 | .33 | .33 |
| 3 | EMA-MAP | −.27 | .35 | .39 |
| Dependent Variable: PILT Reward 80% | ||||
| 1 | CAINS | −.46 | .21 | .01 |
| 2 | MAP-SR | .38 | .24 | .19 |
| 3 | EMA-MAP | −.37 | .24 | .20 |
Discussion
Prior to new measures such as the CAINS and the BNSS, assessments of negative symptoms were outdated representations of our current view of negative symptoms. The release of these new measures brought about an important step forward in our ability to characterize these devastating symptoms. However, there are still several limitations inherent in assessing symptoms through clinician interview (e.g. cognitive demand of retrospective reports, potential rater bias, limited data points). The goal of the current study was to utilize these new gold standard measures of negative symptoms and examine whether the method of assessment (e.g., retrospective clinician rated interviews vs. self reports in the moment) impacts our ability to characterize symptoms and relate them to laboratory task performance. We found convergent validity between validated measures of negative symptoms (clinician rated and self-report) and EMA, suggesting that both methods seem to be indexing similar constructs. However, this relationship was moderated by working memory, suggesting that impairments in memory lessened the relationship between clinician rated reports of symptoms and those reported during daily life via EMA. Further, we found that reports of motivation and pleasure during daily life were related to tasks assessing effort-based decision-making and RL. These results suggest important considerations for the assessment of negative symptoms in SZ and research examining the relationships between such assessments and experimental tasks.
First, critical to studying new methods of assessment was examining the feasibility of using EMA to measure motivation and pleasure in daily life. Our findings confirm previous literature suggesting that EMA is a feasible method in those with schizophrenia (e.g., Kimhy et al., 2006; Myin-Germeys, Birchwood & Kwapil, 2011). Results showed that participants with schizophrenia are able to report their current experiences of motivation and pleasure utilizing smartphone technology with a high completion rate (~80%). One concern with utilizing EMA was that it may be associated with IQ, age, or working memory thus limiting the ability to use this assessment method of assessment with certain people. However, variables such as age, pre-morbid IQ, working memory were not significantly related to completion rates suggesting that demographic and cognitive variables may not be deterrents to utilizing EMA. Another concern was that people with greater symptoms (either positive or negative symptoms) may be less likely to complete EMA surveys and thus skewing the data we collected. Neither positive nor negative symptoms were related to response rates, thus suggesting that severity of symptoms was not an important factor in ability to respond to EMA surveys and suggesting feasibility in utilizing EMA in a population with a range of symptoms. While participants demonstrated a high response rate to EMA surveys, consistent with prior EMA research, we removed a small number of participants (9%) from the sample due to an insufficient number of EMA responses. Given the small number excluded, we were unable to better characterize these non-responders. It may be the case that the few non-responders in the present study were less comfortable using technology and thus better efforts could be made to train participants prior to beginning the EMA protocol. One factor that may have contributed to our relatively high response rate was the payment participants received per survey completed. In the present study participants were paid $1.75 per survey completed within 15 minutes of the beep, which was chosen based on recent work in a similar population who found similar response rates (Gard et al., 2014). Other potential factors that may be important to response rate include length of questionnaire; number of questionnaires per day; and the time of day surveys are administered (e.g., scheduling beeps around individuals’ sleep/wake times rather than set times). Getting a better sense of who may or may not respond to EMA surveys will be important should this technology be used in treatment trials. Future studies including a larger sample should collect information related to prior telephone usage, ease of use, and other demographic variables that may be related to response rates to better characterize qualities that may impact EMA response rates.
Crucial to the development of treatments for negative symptoms is the ability to reliably and accurately assess negative symptoms in daily life. New measures of negative symptoms, such as the CAINS, have demonstrated good reliability and validity. However, one lingering question about these new symptom measures is whether they are impacted by the cognitive demand inherent in recalling events and feelings over the prior week. Further, it is unclear whether clinician interviews are an accurate representation of how people feel when going about their daily life outside of the laboratory setting. The present findings suggest that retrospective clinician ratings and self-report ratings are highly related to those assessed outside the laboratory. However, working memory performance did moderate the relationship between retrospective reports and reports assessed via EMA, while premorbid estimate of IQ did not. Thus our findings suggest that individuals with lower working memory capacity may show a reduced relationship between current emotional and motivational experience (measured via EMA) and retrospective negative symptom measures. This finding is consistent with theories suggesting that memory plays an important role in recounting emotional experiences and anticipating future experiences (Schacter, Addis, & Buckner, 2007). It also raises important concerns given that memory impairments are a core feature of SZ. Should retrospective reports contributing to negative symptom assessments be unreliable due to working memory, treatments geared at targeting these symptoms may not pick up on subtle changes in symptoms. As such, clinical trials may wish to incorporate EMA assessment of symptoms to allow researchers to more precisely pinpoint changes in treatment while removing the confound of working memory (Velligan et al., 2015). It may be that those with greater impairments in working memory would be better suited towards EMA assessment techniques, while those without impairments can utilize retrospective ratings. It could also be the case that retrospective reports pull for a more global impression across the previous week(s) time which could be more to functional outcomes and treatment goals. Future studies should investigate whether retrospective reports or in the moment EMA ratings of negative symptoms are better predictors of success. While the present study focused on examining working memory as a moderator, it may be the case that other cognitive impairments may also play an important role. For example, it may be the case that emotional memory in particular is especially relevant in moderating retrospective reports of motivation and pleasure from in the moment reports. Other cognitive domains such as executive functioning, cognitive control, attention may also be relevant in moderating this relationship and should be considered in future studies.
In regards to experimental task relationships, our research also replicates and extends previous research investigating effort-based decision-making and RL, two constructs thought to be important to understanding negative symptoms. Consistent with some prior research, we did not find a relationship between effort-based decision-making and clinician-rated negative symptoms (Gold et al., 2015; McCarthy et al., 2016). However, we did find that patients who demonstrated increased effort avoidance showed greater impairments in motivation and pleasure in daily life. These findings suggest that willingness to allocate effort to gain reward on a laboratory task is an important predictor of daily reports of motivation and pleasure. This extends previous research by demonstrating a link to ecologically valid assessments of motivation and effort-based decision-making. As noted in the introduction, research examining the relationship between clinician-rated negative symptoms and willingness to choose the hard task has been mixed (Horan et al., 2015). We contend that the EMA-MAP score, comprised of multiple measurements over multiple days while going about daily life, potentially represents a more sensitive measurement of motivation and pleasure among SZ patients. Indeed, when adding CAINS, MAP-SR, and EMA-MAP to a model predicting effort expenditure, we found that our EMA-MAP ratings added significant incremental validity beyond the two other retrospective negative symptom ratings. Future research should replicate these findings and extend them by investigating other paradigms assessing effort-based decision-making and its relationship to daily experiences of motivation and pleasure, as well as to function in everyday life.
Looking at RL in response to reward, we found that lower levels of motivation and pleasure during daily activities, and on a clinician-rated interview, was related to a diminished ability to use reward history to adaptively guide behavior. These findings replicate previous research demonstrating a link between reward-learning tasks and motivation and pleasure assessed via clinician-ratings and self-report (Gold et al., 2012; Strauss et al., 2011). This work extends previous research by demonstrating that the ability to use reward history to guide decision-making is also predictive of motivation and pleasure in daily life. While our EMA ratings did not provide incremental validity above and beyond the retrospective ratings of the CAINS, these findings are important in that they suggest a tight pairing between negative symptoms and reward-learning in the 90% reinforcement condition regardless of how symptoms are assessed. Consistent with Gold and colleagues (Gold et al., 2012), we did not find a relationship between retrospective reports of negative symptoms and reward learning when reinforced at an 80% contingency rate. However, we did find that reward learning in this condition was related to reports of motivation and pleasure assessed during daily life. Thus it may be the case that when reinforcement contingency rates are less certain, the relationship between negative symptoms and reward learning is less robust. This finding supports our contention that EMA may be a more sensitive measure of negative symptoms, which reduces cognitive demands and assesses experiences and feelings as they are happening. It also lends support to the theory that reward learning, and the ability to use that information to guide future decisions, may be an important mechanism for maintaining impairments in motivation.
The present findings also support and extend research suggesting that learning to avoid loss is unrelated to motivation or pleasure as assessed via clinical interview, self-report or in daily life (Gold et al., 2012; Waltz et al., 2007). This provides strong confirmatory evidence that negative symptoms is not related to ability to learn how to avoid loss, instead it points to the specificity of RL in response to reward as key to understanding motivational impairments. This level of specificity is important in identifying mechanisms for understanding negative symptoms and for developing treatments for such impairments.
The aim of the present study was to assess motivational and pleasure impairments in schizophrenia utilizing EMA and to relate these symptoms to laboratory tasks. Because the focus of the study was on the assessment of negative symptoms in schizophrenia, we did not include a control group. Thus we can make no claims as to the generalizability of our findings to healthy controls or other patient groups. This represents a limitation to the present study in that we are unable to make claims as to the validity of assessing negative symptoms via EMA in other populations. Moreover, we cannot demonstrate deficits in task performance in the patient group, relative to healthy controls. While reward learning and effort avoidance have fairly consistently been shown to be impaired in people with schizophrenia, we are unable to say whether our population is similarly impaired relative to a matched sample of healthy controls. Should our population show unexpectedly intact reward learning or effort, it might impact our ability to draw conclusions related to its link with negative symptoms. For example, should participants in the current study show an uncharacteristic pattern of willingness to expend effort, they may also show an unexpected pattern of negative symptoms or clarity about their symptoms. This may in turn impact the relationship between symptoms and task performance making task and symptom assessment be more or less related relative to other samples. While we acknowledge this possibility, we would argue that this issue would likely impact all three negative symptom measures and thus may lend itself to consistent findings across the three measures, rather than the differential effects that we saw for some measures. Further, the present findings are consistent with previous findings and are consistent with our a priori hypotheses. Future studies should investigate the relationship between motivation and pleasure in daily life with laboratory tasks assessing effort and RL to clarify whether these constructs are related in a broader range of people with and without psychiatric diagnoses.
The present study had several additional limitations. First, all but two of our participants were taking antipsychotic medications. While this is common in SZ, it is difficult to determine the impact of medication on emotional responses and task behavior. Nonetheless, individuals with SZ taking medications often continue to show negative symptoms and thus it is important to understand the most sensitive ways to measure this symptom domain in medicated patients. Second, while we had the power to detect significant relationships between task data and EMA, our sample size was modest and would benefit from additional participants. It will be important for future studies to replicate these findings in a larger sample. Finally, participants completed one week of EMA assessment, then returned to the lab to complete a clinician rated CAINS and laboratory tasks. It may be the case that completing EMA made participants more aware of their motivation and pleasure throughout the week and thus improved their recall assessed via CAINS, which may have improved the coherence between EMA and retrospective reports. Moreover, the relationship between CAINS and task performance in the present sample might be somewhat inflated relative to other studies where EMA prior to CAINS assessment was not involved. Thus the generalizability of the current findings are constrained by the study design which prompts people to be more aware of their motivation and pleasure throughout the week.
In summary, the current study is an important addition to the literature investigating negative symptoms in SZ by utilizing an ecologically valid method of assessment and relating these to previously validated retrospective measures of negative symptoms. Our findings demonstrate that working memory, but not pre-morbid IQ, impacts the validity of retrospective reports of motivation and pleasure deficits collected one week later. Our findings also suggest that laboratory tasks assessing effort-based decision-making and RL are reliable predictors of motivation and pleasure in daily life, providing validation that these tasks tap into constructs meaningful to everyday life. Future research should utilize this method of assessment to gain further insight into the daily experience of negative symptoms take advantage of the mobile technology which allows for assessment of a number of things such as mobility; audio recordings to assess variables related to expression; and measures of psychophysiology such as heart rate. In addition, future studies should track negative symptoms assessed via EMA and their relationship with functional outcomes, and as a method of assessing change in response to treatments.
Supplementary Material
Figure 1.
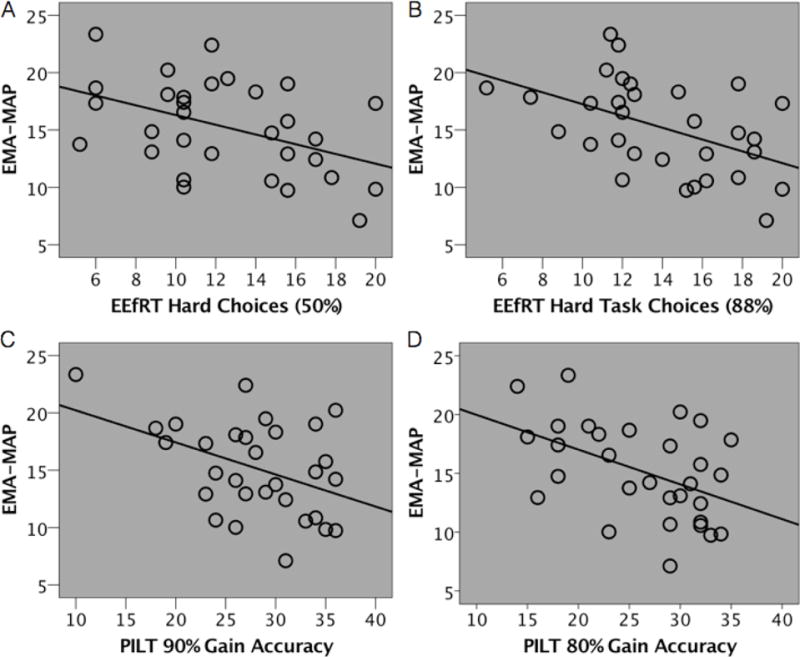
Average EMA-MAP score correlated with task performance.
Recent advances have been made in the assessment of negative symptoms, however, questions remain regarding whether current assessment techniques reflect how people actually feel as they go about their daily life. In the current study, we found that ecological momentary assessment is a feasible method for assessing impairments in motivation and pleasure. Moreover, we found that impairments in working memory may impact participants’ ability to report their negative symptoms via currently used assessment strategies.
Footnotes
Dr Moran and Mr. Culbreth report no conflicts of interest. Dr. Barch has consulted for Pfizer, Amgen, Takeda, and Upsher-Smith and has a contract to analyze imaging data for Pfizer.
Portions of this data were presented at the Society of Affective Science 2016 annual meeting and at the Society of Biological Psychiatry 2016 annual conference.
For exploratory purposes, post-hoc analyses were conducted looking at EMA motivation and pleasure ratings independently at each time point (i.e., current, past, future). All findings remained the same regardless of time point, thus presented findings represent an EMA composite across time point to mirror the composite score on the CAINS.
References
- American Psychiatric Association. DSM-IV. Diagnostic and Statistical Manual of Mental Disorders 4th edition TR 2000 [Google Scholar]
- Barch DM, Ceasar A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in Cogntive Science. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Treadway MT, Schoen N. Effort, anhedonia, and function in schizophrenia: reduced effort allocation predicts amotivation and functional impairment. Journal of Abnormal Psychology. 2014;123(2):387–97. doi: 10.1037/a0036299. http://doi.org/10.1037/a0036299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, McHugo GJ, Xie H, Dobbins K, Young MA. Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and nonclinical comparison group. Schizophrenia Bulletin. 2012;38(3):396–404. doi: 10.1093/schbul/sbr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Carpenter WT. The cognitive and negative symptoms in schizophrenia trial (CONSIST): The efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Culbreth AJ, Gold JM, Cools R, Barch DM. Impaired Activation in Cognitive Control Regions Predicts Reversal Learning in Schizophrenia. Schizophrenia Bulletin. 2015 doi: 10.1093/schbul/sbv075. sbv075. http://doi.org/10.1093/schbul/sbv075. [DOI] [PMC free article] [PubMed]
- Docx L, de la Asuncion J, Sabbe B, Hoste L, Baeten R, Warnaerts N, Morrens M. Effort discounting and its association with negative symptoms in schizophrenia. Cognitive Neuropsychiatry. 2015;20(2):172–185. doi: 10.1080/13546805.2014.993463. [DOI] [PubMed] [Google Scholar]
- Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. Journal of Psychiatric Research. 2013;47(11):1590–6. doi: 10.1016/j.jpsychires.2013.08.003. http://doi.org/10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. http://doi.org/10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Sanchez AH, Cooper K, Fisher M, Garrett C, Vinogradov S. Do people with schizophrenia have difficulty anticipating pleasure, engaging in effortful behavior, or both? Journal of Abnormal Psychology. 2014;123(4):771–82. doi: 10.1037/abn0000005. http://doi.org/10.1037/abn0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Kool W, Botvinick MM, Hubzin L, August S, Waltz JA. Cognitive effort avoidance and detection in people with schizophrenia. Cognitive, Affective & Behavioral Neuroscience. 2015;15(1):145–54. doi: 10.3758/s13415-014-0308-5. http://doi.org/10.3758/s13415-014-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry. 2013;74(2):130–6. doi: 10.1016/j.biopsych.2012.12.022. http://doi.org/10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Matveeva TM, Kasanova Z, Strauss GP, Herbener ES, Frank MJ. Negative Symptoms and the Failure to Represent the Expected Reward Value of Actions: Behavioral and Computational Modeling Evidence. Archives of General Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. http://doi.org/10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain: A Journal of Neurology. 2011;134(Pt 6):1751–64. doi: 10.1093/brain/awr059. http://doi.org/10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophrenia Bulletin. 2008;34(3):504–514. doi: 10.1093/schbul/sbm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophrenia Research. 2011;132(2–3):140–145. doi: 10.1016/j.schres.2011.06.030. http://doi.org/10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Reddy LF, Barch DM, Buchanan RW, Dunayevich E, Gold JM, Green MF. Effort-Based Decision-Making Paradigms for Clinical Trials in Schizophrenia: Part 2—External Validity and Correlates. Schizophrenia Bulletin. 2015;41(5):1055–65. doi: 10.1093/schbul/sbv090. http://doi.org/10.1093/schbul/sbv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Delespaul P, Corcoran C, Ahn H, Yale S, Malaspina D. Computerized experience sampling method (ESMc): Assessing feasibility and validity among individuals with schizophrenia. Journal of Psychiatric Research. 2006;40(3):221–230. doi: 10.1016/j.jpsychires.2005.09.007. http://dx.doi.org/10/1016/j.jpsychires.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bulletin. 2006;32(2):214–9. doi: 10.1093/schbul/sbj053. http://doi.org/10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, Marder SR. The Brief Negative Symptom Scale: Psychometric Properties. Schizophrenia Bulletin. 2010;37(2):300–305. doi: 10.1093/schbul/sbq059. http://doi.org/10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The Clinical Assessment Interview for Negative Symptoms (CAINS): Final development and validation. American Journal of Psychiatry. 2013;170(2):165–172. doi: 10.1176/appi.ajp.2012.12010109. http://doi.org/10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia N, Rachuri K, Mascolo C, Roussos G. Proceedings of the 2013 ACM conference on Pervasive and ubiquitous computing adjunct publication - UbiComp ’13 Adjunct. New York, USA: ACM Press; 2013. Open source smartphone libraries for computational social science; pp. 911–920. http://doi.org/10.1145/2494091.2497345. [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. http://dx.doi.org/10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Llerena K, Park SG, McCarthy JM, Couture SM, Bennett ME, Blanchard JJ. The Motivation and Pleasure Scale-Self-Report (MAP-SR): reliability and validity of a self-report measure of negative symptoms. Comprehensive Psychiatry. 2013;54(5):568–74. doi: 10.1016/j.comppsych.2012.12.001. http://doi.org/10.1016/j.comppsych.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophrenia Research. 2016 doi: 10.1016/j.schres.2015.12.017. http://doi.org/10.1016/j.schres.2015.12.017. [DOI] [PMC free article] [PubMed]
- Meng X, Rosenthal R, Rubin DB. Comparing correlated correlation coefficients. Psychological Bulletin. 1992;111(1):172–75. http://doi.org/10.1037/0033-2909.111.1.172. [Google Scholar]
- Milev P, Ho BC, Arndt S, Andreasen NC. Predictive Values of Neurocognition and Negative Symptoms on Functional Outcome in Schizophrenia: A Longitudinal First-Episode Study With 7-Year Follow-Up. American Journal of Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. http://doi.org/10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Birchwood M, Kwapil T. From environment to therapy in psychosis: A real-worl momentary assessment approach. Schizophrenia Bulletin. 2011;37:244–247. doi: 10.1093/schbul/sbq164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Peeters F, Havermans R, Nicolson NA, DeVries MW, Delespaul P, van Os J. Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatrica Scandinavica. 2003;107(2):124–131. doi: 10.1034/j.1600-0447.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- Oorschot M, Lataster T, Thewissen V, Lardinois M, Wichers M, Van Os J, Myin-Germeys I. Emotional experience in negative symptoms of schizophrenia-no evidence for a generalized hedonic deficit. Schizophrenia Bulletin. 2013;39(1):217–225. doi: 10.1093/schbul/sbr137. http://doi.org/10.1093/schbul/sbr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10(3):799–812. http://doi.org/10.2466/pr0.1962.10.3.799. [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon RT. HLM 6: Hierarchical linear and nonlinear modeling. 2008 2004 [Google Scholar]
- Sanchez AH, Lavaysse LM, Starr JN, Gard DE. Daily life evidence of environment-incongruent emotion in schizophrenia. Psychiatry Research. 2014;220(1–2):89–95. doi: 10.1016/j.psychres.2014.07.041. http://doi.org/10.1016/j.psychres.2014.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. http://doi.org/10.1080/08995600802554748. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biological Psychiatry. 2011;69(5):424–31. doi: 10.1016/j.biopsych.2010.10.015. http://doi.org/10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, Kirkpatrick B. Next-generation negative symptom assessment for clinical trials: Validation of the Brief Negative Symptom Scale. Schizophrenia Research. 2012;142(1):88–92. doi: 10.1016/j.schres.2012.10.012. http://dx.doi.org/10.1016/j.schres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Thaler NS, Matveeva TM, Vogel SJ, Sutton GP, Lee BG, Allen DN. Predicting psychosis across diagnostic boundaries: Behavioral and computational modeling evidence for impaired reinforcement learning in schizophrenia and bipolar disorder with a history of psychosis. Journal of Abnormal Psychology. 2015;124(3):697–708. doi: 10.1037/abn0000039. http://doi.org/10.1037/abn0000039. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS One. 2009;4(8):e6598. doi: 10.1371/journal.pone.0006598. http://doi.org/10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Peterman JS, Zald DH, Park S. Impaired effort allocation in patients with schizophrenia. Schizophrenia Research. 2015;161(2–3):382–5. doi: 10.1016/j.schres.2014.11.024. http://doi.org/10.1016/j.schres.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Mintz J, Sierra C, Martin ML, Fredrick M, Maglinte GA, Corey-Lisle PK. The Daily Activity Report (DAR) a Novel Measure of Functional Outcome for Serious Mental Illness. Schizophrenia Bulletin. 2015 doi: 10.1093/schbul/sbv185. sbv185. http://doi.org/10.1093/schbul/sbv185. [DOI] [PMC free article] [PubMed]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological Psychiatry. 2007;62(7):756–64. doi: 10.1016/j.biopsych.2006.09.042. http://doi.org/10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler: Wechsler Test of Adult Reading: WTAR. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


