Abstract
CXC chemokines, macrophage inflammatory protein-2 (MIP-2) and KC, (a cloning designation based on ordinate and abscissa position) as well as the CXC chemokine receptor, CXCR2, are expressed in a variety of cells and tissues in adult mice. Targeted deletion of the gene encoding murine CXCR2 does not result in obvious changes in the development of the organ system of the mouse, though the CXCR2 −/− mouse is compromised with regard to its ability to resist infection, heal wounds, and maintain homeostasis when challenged with microbes and/or chemicals. In an attempt to develop insight into additional possible subtle roles of CXCR2 and its ligands in the development of the mouse, we examined the expression of MIP-2, KC, CXCR2, as well as the Duffy antigen binding protein for chemokines during embryonic (p.c.) days 11.5 through 14.5 in the mouse. We observed strong correlation between the expression of MIP-2 and CXCR2 in the developing brain, cardiovascular system and condensing mesenchyme between 11.5 and 13.5 days. Moreover, the expression of KC was parallel to the expression of the Duffy antigen binding protein for chemokines with regard to temporal pattern and tissue localization. MIP-2 and CXCR2 are highly expressed in the brain, first in the cerebellum and in the head mesenchyme, the meninges and the floor plate, and by 14.5 days are also present in the telencephalon, thalamus and hypothalamus. In the developing brain KC and Duffy were prominently expressed in the neuronal tracts, the forebrain, sympathetic ganglia, and along the periphery of the neural tube. However, KC and Duffy were less prevalent in the developing cardiovascular system, lung and other organs, muscle and bone, than are CXCR2 and MIP-2. These data suggest that the roles for these chemokines and their receptors during development may be more significant than was initially thought based upon the phenotype of the mice with targeted deletion of CXCR2 and Duffy.
Keywords: chemokines, CXCR2, development, Duffy antigen chemokine binding protein, KC, MIP-2
Chemokines are small molecules which function as chemoattractants to leukocytes while also exhibiting the ability to modulate angiogenesis, wound healing and tumorigenesis. Chemokines are grouped into four categories: CXC, CC, C and C3C chemokines based upon the presentation of conserved cysteine residues within the mature peptides. Chemokine receptors are classified as receptors for CXC, CC, C or CX3C chemokine ligands, though some of the receptors bind chemokines from more than one class.1 Intensive research into the physiological roles of chemokines and their receptors has led to extensive documentation of the role of these factors in disease and normal immune function. Much less is known about the role of chemokines during embryonic development. While the targeted gene deletion of chemokine receptors has been informative, multiple chemokines and chemokine receptors often address similar biological role—and this functional redundancy makes it difficult to address the biological role of chemokines.2 The CXC chemokines targeted to attract neutrophils are no exception. Thus far, only two receptors for these ligands have been identified: the murine homolog of human CXC chemokine receptor 2 (mCXCR2) and the murine homolog of the Duffy antigen binding protein for chemokines (mDuffy).3–5 In humans, CXCR1 binds interleukin (IL-)8 and granulocyte chemotactic protein-2 (GCP-2) with high affinity,6 but has a much lower affinity for the other ligands for CXCR2, including MGSA/GROα, β, γ, epithelial-cell-derived neutrophil activating peptide-78 (ENA-78), and NAP-27,8 CXCR2 is the only signaling receptor for the murine homologs of melanoma growth stimulatory activity/growth regulated protein (MGSA/GRO), ENA-78 and GCP-2.6,9,10 In the mouse, IL-8 has not been identified and no functional homolog of CXCR1 has been identified.
When mice, harboring a targeted deletion of mCXCR2, are maintained in microisolators in a specific pathogen-free (SPF) environment, the phenotype is characterized by excessive neutrophil and B lymphocyte accumulation. However, when these mice are maintained in a gnotobiotic facility where the mice are born under germ free conditions, housed in a sterile chamber where water, food and wood shavings are autoclaved, then later provided with a cocktail of known bacterial required to maintain the gut fluora, investigators found that the neutrophil and B lymphocyte numbers are within the normal range. mCXCR2 −/− mice exhibit a decreased recruitment of neutrophils into the peritoneal cavity in response to stimulus such as thioglycolate.3 In addition, these mice are often small, exhibit retardation in the wounding response, poor resistance to infection, and they do not respond well to environmental stress.11 These mice mate poorly, the males often die after mating and the litters born to −/− females are small (average 3–4 pups). These data suggest that the physiological role of chemokines which activate mCXCR2 may be more complex than was initially thought. In contrast, the mDuffy −/− mice exhibit a fairly normal phenotype.12 To obtain additional insight into the phenotypic properties of the mCXCR2 −/− mice, we examined the developmental expression of the murine CXC chemokines KC, (a cloning designation based on ordinate and abscissa position) macrophage inflammatory protein-2 (MIP-2), mCXCR2 and mDuffy between 11.5 and 13.5 days of development, using immunohistochemical staining, reverse transcriptase-polymerase chain reaction (RT-PCR) analyses, and Western blot analyses. The developmental expression of these chemokines and their receptors was not surprising, but several unexpected findings were uncovered which may provide insight into the failure of the mCXCR2 −/− mice to thrive in a non-infectious, but stressful environment.
RESULTS
Characterization of chemokine and chemokine receptor expression by RT-PCR
To verify the expression of chemokines and their receptors in the developing embryo, RT-PCR was performed using the primers and techniques described in the Methods section. We confirmed the expression of the mRNA for CXCR2 (Fig. 1A), KC (Fig. 1B), MIP-2 (Fig. 1C), and mDuffy (Fig. 1D). The control for these experiments was -actin, as represented by upper bands in each panel of the figures (Fig. 1).
Figure 1. Expression of CXCR2, MIP-2 and KC mRNA in developing mouse embryo.
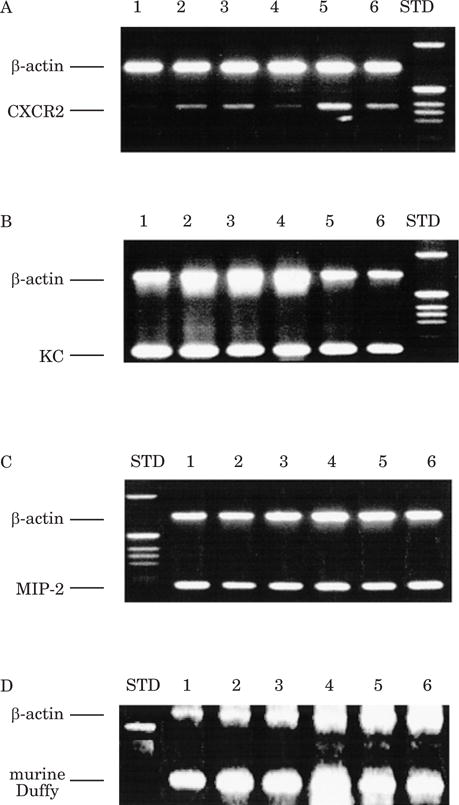
RT-PCR was performed on mouse embryos (9.5 to 17 days of development) according to the protocol as described in Materials and Methods. Lanes 1–6 represent cDNA from embryonic days 17.5, 15.5, 13.5, 11.5, 9.5, and ~8.5, respectively. The control for the PCR and RT reaction was simultaneous reaction with primers for β-actin. A: CXCR2 cDNA is detected in 15.5, 13.5, 9.5 and ~8.5 day embryos. B: KC cDNA is detected in ~8.5 through 17 day embryos. C: MIP-2 cDNA is detected in ~8.5 through 17 day embryos. D: mDuffy is detected in ~8.5 through 17 day embryos, with the strongest expression in 11.5 day embryos.
Expression of CXCR2, mDuffy, MIP-2 and KC during embryonic development
The expression of mCXCR2 and mDuffy, and MIP-2 and KC proteins was verified by immunostaining of 11.5, 12.5 and 13.5 day mouse embryos using antibodies specific for these ligands and their receptors. The presence of these chemokines and receptors in the various systems is detailed below.
Muscle and bone components
CXCR2
At 11.5 days, condensing mesenchyme which will give rise to cartilage and bone stains positive for CXCR2, as does the myotome plate which gives rise to the muscle (not shown). The developing limb bud is negative (not shown). By 13.5 days, the condensing vertebral cartilage is weakly positive and the notochord is strongly positive (not shown). The muscle of the diaphragm is strongly positive (not shown). The smooth muscle of the muscularis mucosa of the stomach is positive (not shown). In the body cavity, the muscle of the body wall is weakly positive, while the inner layer of the peritoneal lining is positive (not shown). The ectoderm of the limb bud is positive where differentiating keratinocytes are observed. Condensing cartilages in the limb bud are positive (not shown). The epidermis of the skin is positive (Fig. 2F). By 14.5 days the lining of epidermis of the mouth and the tongue stains positively for CXCR2 (not shown). Cartilaginous cells of the palate are also positive, and there are positive cells where the tongue and palate join (not shown). Along the vertebral column, vertebral discs are positive (not shown).
Figure 2. Immunolocalization of MIP-2 and CXCR2 in 13.5 day embryos.
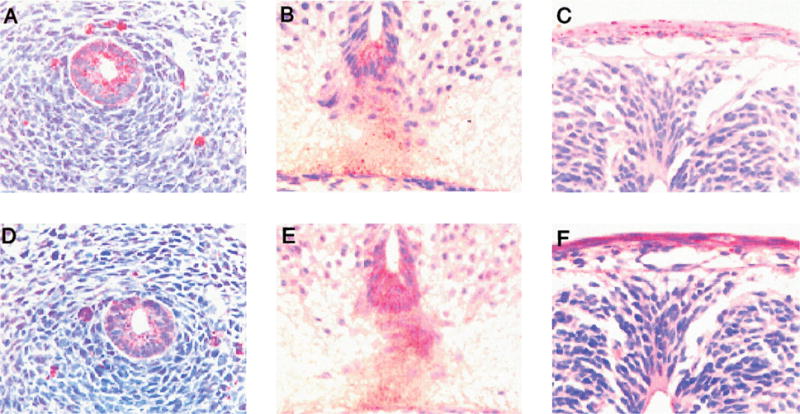
The esophagus stained positive for MIP-2 (A) and CXCR2 (D). The floor plate of the neural tube was positive for MIP-2 (B) and CXCR2 (E). C and F show the immunostaining for MIP-2 (C) and CXCR2 (F) in the ectoderm of the back skin.
mDuffy
At 11.5 days of development, the limb is negative, but by 13.5 days there is spotty positivity, probably in the neuronal tracts. By 14.5 days immunoreactive mDuffy is present in the dermis of the skin. In the inner ear, mDuffy expression is observed in the mesenchyme (not shown).
KC
KC immunostaining is spotty, and mimics that observed with the mDuffy antibody in the developing muscle, bone, and connective tissue.
MIP-2
At 13.5 days, the diaphragm muscle stains strongly positive for MIP-2 (not shown). The upper portion of the stomach has a population of MIP-2-positive cells. In the body wall the ectoderm is positive, the muscle layer is slightly positive, as are the notochord and surrounding condensing cartilage. The limb bud mesenchyme and peripheral condensing cartilage are positive (not shown). By 14.5 days of development, MIP-2 immunoreactivity is revealed in the hair follicles and the keratinocytes of the skin. Calcifying bone and cartilage cells are positive (not shown). The cartilage components of the skull are positive. The ear cartilage is positive and other areas of cartilaginous tissue are strongly positive (not shown). The muscle is strongly positive (not shown).
Nervous system
The notochord is solely a derivative of the axial mesoderm and the floor plate is a part of the neuroectoderm. There are not multiple cell types within the notochord or the floor plate for the stages of embryonic development between E11.5 and E13.5. The staining of the neural tube and peripheral nervous system at E11.5–13.5 most probably represents axons, rather than oligodendrocytes or Schwann cells, since myelination of axons by the glial cells does not occur until much later stages of development.
MIP-2
At 11.5 days of development, the neural tube does not stain for MIP-2, but the head mesenchyme is positive (not shown). At 12.5 days of development, MIP-2 immunoreactivity is observed in the same regions as CXCR2 staining is observed, the head mesenchyme (not shown) and along the floor plate (not shown). This staining pattern for the floor plate persists through 13.5 days (Fig. 2B).
CXCR2
At 11.5 days of development, CXCR2 immunostaining was detected in the dorsal root ganglia and sympathetic neurons (data not shown). The staining is also strong in the notochord, which is derived from the paraxial mesoderm, and the floorplate (which is a part of the neuroectoderm) of the neural tube (not shown). Such staining patterns persist until later stages of development (see below). By 12.5 days of development, CXCR2 immunostaining is observed to be prevalent in the forebrain and in the head mesenchyme. The meninges of both brain and spinal cord are positive. The floor plate stains very strongly; staining is not present in the ventral midline of the forebrain, but from the midbrain extending toward the posterior spinal cord. At 13.5 days in the developing brain the floor plate continues to show immunoreactivity for CXCR2 (Fig. 2E). As the dorsal root ganglia differentiate, staining for immunoreactive mCXCR2 is no longer detected in these tissues by this stage.
By 13.5 days, sagittal sections of the developing brain reveal the hippocampus is positive, as are the telencephalon, thalamus and hypothalamus (not shown). The choroid plexus is strongly positive, especially around the 4th ventricle (not shown). In the developing pituitary gland there are some positive cells and the meningeal cells around the pituitary are strongly positive (not shown). The olfactory epithelium is also positive for mCXCR2 (not shown).
mDuffy
At 11.5 days of embryonic development, the floor plate stains strongly positive for immunoreactive mDuffy (not shown). The fibrous nerve tracts at the periphery of the differentiating neural tube also stain positively, as do axonal tracts (not shown). At 12.5 days of development, in the ventral forebrain immunostaining is detected in the olfactory bulb (Fig. 3A), and in the axonal fibres of the olfactory nerve (not shown). mDuffy immunoreactivity is also observed in the outer differentiated zone near the periphery of the neural tube, indicating mDuffy in axonal fibres of neurons (Fig. 3B). In the hindbrain the motor neurons are positive for mDuffy immunostaining. The sensory tracts extending from the spinal ganglia into the neural tube are positive (Fig. 3C). There is spotty positivity in the developing limb, probably in the neuronal tracts (Fig. 3D). At 13.5 days of development, the neuronal fibres both in the central and peripheral nervous system react strongly with antibodies to mDuffy; e.g., the enteric neurons around the gut are strongly positive (Fig. 4C), and the sympathetic ganglia are also highly positive in the non-cellular regions (Fig. 5E). By 14.5 days of development, the nerve tracts around the hair follicles were positive for mDuffy (not shown).
Figure 3. mDuffy and KC expression in the neuronal tissues in the mouse embryo.
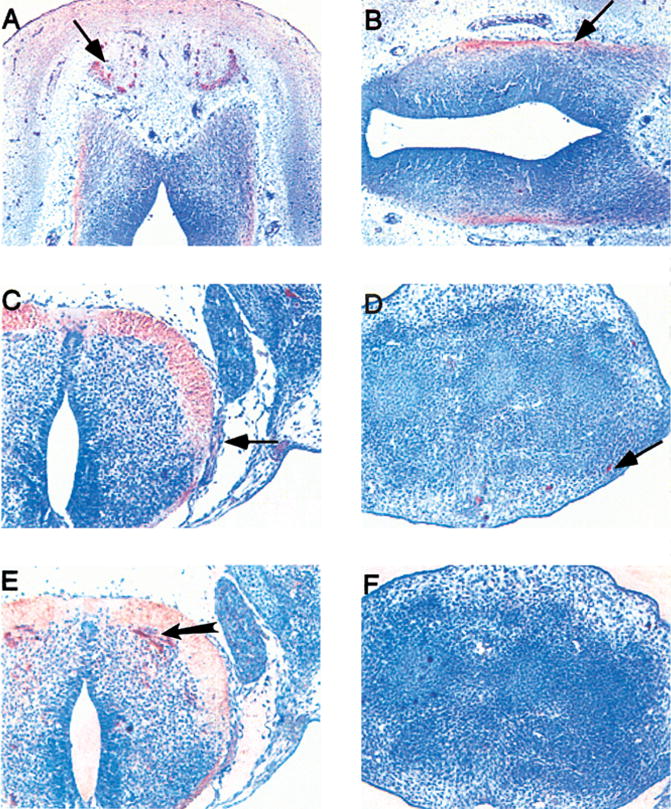
In panel A, the olfactory centre (arrow) in the 12.5 day embryo is positive for the mDuffy. B, C and D show neuronal tracts are positive for mDuffy in the forebrain (B), spinal cord and sensory neurons (C), and limbs (D). Neuronal tracts are positive for KC (E) around the neural tube, but the neuronal tracts in the limb bud are negative for KC (F).
Figure 4. Chemokines and chemokine receptors expression in the developing gut.
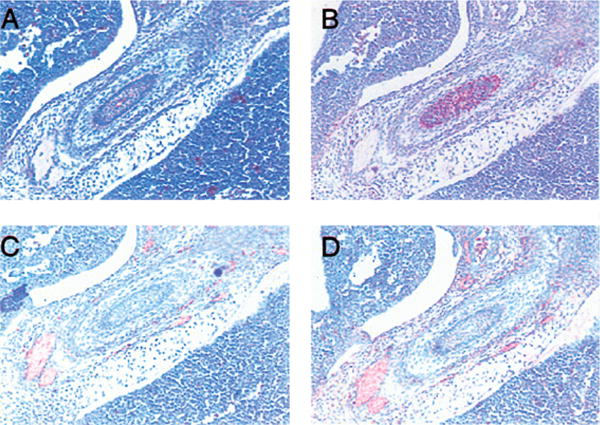
By 13.5 days of development, the muscularis mucosa of the gut is positive for expression of CXCR2 (A) and MIP-2 (B), while the neuronal tracts among the intestinal muscles are positive for mDuffy (C) and KC (D).
Figure 5. The expression of CXC chemokines and receptors in and around the blood vessels.
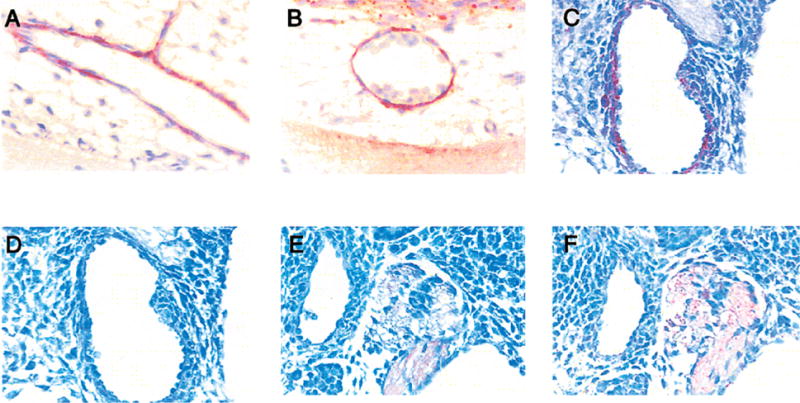
CXCR2 immunoreactivity is observed in the endothelial cells lining the blood vessels (A,B) and the dorsal aorta (C) in 12.5 day embryos. Immunoreactivity for MIP-2 (D), mDuffy (E) and KC (F) are not as evident in the blood vessels. The sympathetic ganglia are also positive for mDuffy (E) and KC (F).
KC
Specific staining for KC is not detected at 11.5 or 12.5 days of embryonic development. KC is expressed in the neuronal tracts and exhibits a pattern of staining similar to that of mDuffy at 13.5 days. The tracts of motor neurons and sensory neurons are positive in and around the neural tube (Fig. 3E). The ganglia and neuronal fibre tracts in the wall of the gut are positive (Fig. 4D). At 13.5 days, KC positive cells in the ganglia are first observed in the non-cellular regions of the sympathetic ganglia (Fig. 5F).
Cardiovascular system
CXCR2
The endothelial cells lining the blood vessels of the developing heart stain strongly positive for immunoreactive CXCR2 (Fig. 5A,B), as do the endothelial cells of the dorsal aorta (not shown) and blood vessels in the developing brain (not shown). At 12.5 days, staining in the endothelial cells of the dorsal aorta is more prominent (Fig. 5C). The lining of the central veins in the liver is also positive for CXCR2 by 13.5 days (not shown). In the developing cardiovascular system, the most striking expression of CXCR2 in the 11.5 day mouse embryo is seen in the heart, where the atrium and ventricles are strongly positive (Fig. 6A). The endocardium is weakly positive, while the mesocardium reacts very strongly with antibodies to CXCR2 (Fig. 6B). Such a staining pattern for CXCR2 persists through 14.5 days (not shown).
Figure 6. Chemokines and chemokine receptor expression in the heart.
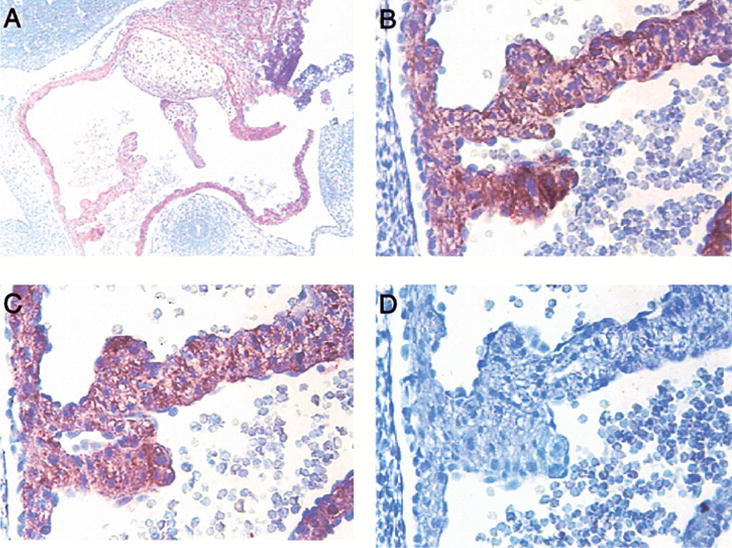
In the 11.5 mouse embryo, the mesocardium and endocardium in the wall of the developing heart were positive for CXCR2 (A). The MIP-2 (C) has a similar pattern as CXCR2 (see, panel B). Expression of mDuffy (D) and KC (data not shown) was not detected in the heart.
mDuffy
At 11.5, 12.5, and 13.5 days of development most of the blood vessels are negative (Fig. 5E), with occasional ones in the brain showing weak positivity for mDuffy. The heart is mDuffy-negative (Fig. 6D). In the liver, the only mDuffy positive cells appear to be red blood cells (not shown).
KC
The developing heart and blood vessels stain negative for KC.
MIP-2
There is no staining in the dorsal aorta or umbilical cord at 11.5 days of development. The developing heart reacts very strongly with antibody to MIP-2, in the mesocardial cells, and to a lesser extent, in the endocardium (Fig. 6B).
Lung and other internal organs
CXCR2
At 11.5–13.5 days, there is patchy staining in the endoderm of the trachea (not shown). The foregut epithelium is slightly positive, with a patchy staining pattern, as is represented in the esophagus (Fig. 2A). A few positive cells are present in the liver, and these cells appear to be rounded, giving the impression that they are a sub-population of haematopoietic cells (Fig. 4A). The epithelium of the oesophagus is positive (Fig. 2A), while the intestine and pancreas (not shown) are negative. By 13.5 days the developing thyroid gland has some CXCR2-positive cells (Fig. 7D). At 13.5–14.5 days, the lung epithelium is positive with patchy staining in some of the lung buds (Fig. 7B), but not all.
Figure 7. Chemokines and chemokine receptor expression in lung and thyroid gland in the 13.5 day mouse embryo.
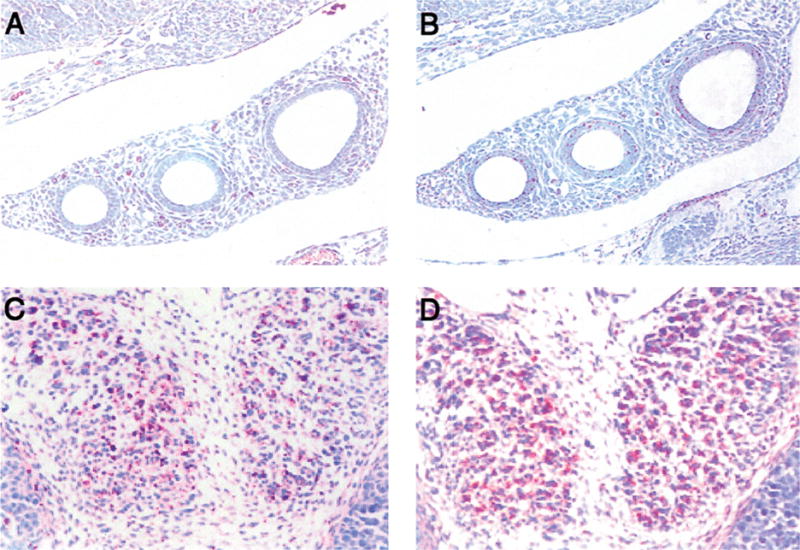
The epithelial cells of the bronchi have patched immunoactivity for MIP-2 (A), but are almost negative for CXCR2 (B). Both MIP-2 (C) and CXCR2 (D) positive cells are observed in the developing thyroid gland.
mDuffy
At 11.5 days, non-cellular neuronal fibre tracts around the gut react strongly with antibody to mDuffy (see above), though the gut mesenchyme and epithelium are negative (Fig. 4C). By 14.5 days in the lung, the red blood cells are positive and the bronchial mesenchyme is positive (not shown).
KC
Significant immunostaining for KC is not observed in the developing internal organs at 11.5–13.5 days.
MIP-2
At 11.5 days, the gut endoderm-derived epithelium stains positive for MIP-2 at the junction of the oesophagus and trachea (not shown). At 13.5 days of development, the epithelium of the gut is strongly positive for MIP-2 (Fig. 4B). The lung is positive with the bronchi (not shown) and mesodermal derivatives exhibiting patchy immunoreactivity for MIP-2 (Fig. 7A). The developing thyroid gland stains positively for MIP-2 immunoreactivity (Fig. 7C). The liver exhibits patchy immunoreactivity, possibly a sub-population of haematopoietic cells. By 14.5 days of development, endodermal structures in the gut are strongly positive (not shown). There is spotty positive staining in salivary glands (not shown). The epithelial cells of the lung continued to show spotty but weak staining for MIP-2 (Fig. 7A).
DISCUSSION
This report describes the first characterization of the embryonic expression of CXCR2, mDuffy, and some of the ligands which interact with these receptors. There was a general trend of correlation of MIP-2 and mCXCR2 expression in tissues. KC expression appeared to coincide with mDuffy expression. We were not surprised to observe strong expression of CXCR2 and mDuffy in the brain of the developing mouse, based upon the prior demonstration that mDuffy is highly expressed in cerebellar Purkinje cell bodies and processes and that CXCR2 is expressed in the hippocampus in the pyramidal cells, the dentate nucleus of the cerebellum, paraventricular nucleus, locus coeruleus, anterior pontine nuclei, anterior horn, intermediolateral cell column, and dorsal nucleus of Clarke of the spinal cord, the motor neurons in astrocytes, microglia, and in Alzheimer’s plaques.14–18 We observed strong CXCR2 expression in the floor plate prior to differentiation, but not after differentiation.
These data suggest that CXCR2 may be involved in the trafficking of neuronal processes to the correct site. However, in spite of the widespread expression of CXCR2 during neuronal development, the CXCR2 −/− mouse has a normal phenotype at birth, and while the size of the mice remains small, the organs develop appropriately. The main phenotype of the mouse is that it is compromised immunologically and does not respond well to stress. There is no evidence of neurological problems in the CXCR2 −/− mouse, though the mouse has not been studied after CNS trauma such as stab wounding, cerebral ischaemia or in chronic experimental allergic encephalomyelitis (EAE). There are reports that the ligand for this receptor plays an important role in the repair of damaged neurons in the EAE model19 and that the ligand can modulate neurotransmitter release in rat cerebellar neurons.20 Thus, with the appropriate stress situation, more may be learned about the function of CXCR2 in repair of neuronal damage and in the regulation or neurotransmitter release.
The mDuffy −/− mouse has recently been described as also having a normal phenotype.12 Again, the role for mDuffy in neuronal development and neurological functioning is not at all clear. There is no evidence that this receptor transduces an intracellular signal once ligand binds, though it is reported to serve as a clearance receptor to remove excess chemokine after an inflammatory response. Alternatively, mDuffy may be involved in the presentation of ligand to other functional chemokine receptors. Since loss of mDuffy was without consequence in that no neurological deficiencies were observed,12 it has been proposed that if this receptor has a function, there is redundancy such that loss of mDuffy has no phenotypic consequence.
KC exhibits an expression pattern similar to that of mDuffy in the neuronal tracts. A KC transgenic mouse has been developed where the expression of the transgene was directed through the myelin basic protein promoter enhancer which drives expression of the transgene to the oligodendrocytes.21 Transgenic mice from one of six founder lines, (high expressor line 17), exhibited a grossly decreased line span. Roughly one third of the pups born were dead prior to 1 year of age and of those that died within the span of the first year, one third died within 100–150 days. For those found dead within the first year, 38% exhibited a syndrome of progressive neurological dysfunction. This was usually evident by 40 days, considerably after the peak of chemokine expression. Problems noted were slowing of the righting reflex, circling behaviour and clumsiness in the absence of prominent weakness, followed by rigidity of the hind limbs and tail and profound truncal instability, such that maintenance of posture for taking in food and water was a problem. Wasting became a prominent feature in the affected mice. There was widespread neutrophil infiltration in the brain. There was little evidence of demyelination, though astrocytosis followed the pattern of neutrophil infiltration. Infiltrated neutrophils appeared to die by apoptosis, without degranulation.21 The activation of microglia and astroglia may have played a dominant role in the development of the neurological symptoms. Thus, continuous exposure of neurons to KC leading to either downregulation of CXCR2 or chronic stimulation of neurons could be part of the mechanism for the abnormality.
The strong expression of CXCR2 and its ligand, MIP-2, in the cardiovascular system lead us to expect cardiovascular defects in the CXCR2 −/− mouse. While the heart develops appropriately in these mice, CXCR2 −/− mice are somewhat resistant to development of atherosclerotic lesions when bred on a low density lipoprotein (LDL) receptor-deficient background due the failure of macrophage accumulation in the atherosclerotic plaque.22
The relatively low expression of CXCR2 and mDuffy in the lung and gut are predictive of the failure to see abnormalities in the development of these organs in the CXCR2 −/− and mDuffy −/− mice. However, MIP-2 is strongly expressed in the gut endoderm and the lung between 11.5 and 14.5 days of development. Salivary glands are also strongly positive for MIP-2. The expected role for expression of these ligands is in the recruitment of leukocytes to sites needed to fight infection and in the homeostasis of the gut epithelium.23 However, CXCR2 −/− mice remain quite small and do not grow as well as the heterozygous and wild-type mice. A role for CXCR2 in absorption or homeostasis of the gut has not yet been explored.
CXCR2 is expressed widely in the condensing mesenchyme which gives rise to cartilage and bone. Also the notochord, the smooth muscle of the muscularis mucosa of the stomach, the liver, developing limbs, and differentiating keratinocytes are strongly positive for CXCR2 as early as 13.5 days of development. mDuffy is not so abundantly expressed in these tissues. The pattern of MIP-2 expression follows that of CXCR2, suggesting that this ligand/receptor interaction is important for development of cartilage and bone. Again, the normal development of the skeletal system of the CXCR2 −/− mouse suggests that the functions of CXCR2 in skeletal development and development of the digestive system and skin must be redundant.
In conclusion, we demonstrate here that the widespread expression of chemokines and chemokine receptors during development provides important information on the potential roles of these chemokine/chemokine receptor interactions in vivo. Targeted deletion of the receptors or ligands may not cause an obvious developmental defect, but may point to tissues susceptible to abnormalities with specific environmental stresses. Moreover, combined deletion of both CXCR2 and other chemokine receptors may be especially informative.
MATERIALS AND METHODS
Materials
Antibodies to mCXCR2 were obtained from Santa Cruz Biotechnology, Santa Cruz, California, USA. Rabbit antibodies to murine MIP-2 and KC were from R & D Systems, Minneapolis, Minnesota, USA. Sheep antibody to mDuffy was developed in collaboration with Bionostics Incorporated, North York, Ontario, Canada (Du et al., submitted). Embryos were collected from C57Bl mice at the appropriate developmental stage, fixed in paraformaldehyde, embedded in paraffin, sectioned and stained using previously described procedures.13
Immunohistochemistry
Embryos were fixed in paraformaldehyde, embedded in paraffin, sectioned and processed for immunohistochemistry. Staining was performed using the ABC kit from Vector Laboratories, Burlingame, California, USA. The substrate for the peroxidase was aminoethyl carbazole, which produces a red immunoprecipitate as an end-product of the peroxidase activity. Negative controls for the immunostaining included inclusion of a non-specific IgG and/or elimination of the primary antibody with the remainder of the Vectastain protocol intact.
RT-PCR
RNA extracted from mouse embryos (day 9.5 to 17 of fetal development) was treated with DNAse 1 using the Message RClean kit (Gen Hunter Corporation, Nashville, Tennessee, USA). The DNA-free RNA was used for RT-PCR. The primers were as follows: MIP-2: 5′ primer: 5′-CCACTCTCAAGGGCGGTCAAA-3′, 3′ primer: 5′-TACGATCCAGGCTTCCCGGGT-3′; KC: 5′-primer: 5′-CGCTGCTGCTGCTGGCCACCA-3′, 3′-primer: 5′-GGCTATGACTTCGGTTTGGGTGCAG-3′; mCXCR2: 5′-primer: 5′-GGCGGGGTAAGACAAGAA TC-3′; 3′-primer: 5′-GGCAAGGTCAGGGCAAAGAA-3′; mDuffy: 5′-primer: 5′-GGCACTTATCTTGGAGCCAC-3′, 3′-primer: 5′-GTCACTCGAGAGTTCATAGG-3′; β-actin: 5′-primer: 5′-CCACCAGACAACACTGTGTTG-3′, 3′ -primer: 5′-AGAGGTATCCTGACCCTGAAG-3′.
Typical RT-PCR conditions were as follows: after 5 min of initial denaturation, 35 cycles of denaturing at 94°C for 1 min, annealing at 57°C for 1 min and extension at 72°C for 1.5 min, followed by 5 min of final extension at 72°C. Ten microlitres of each PCR product were analysed by 1.2% agarose gel electrophoresis with ethidium bromide staining.
Verification of antibody specificity
mDuffy antibody
Polyclonal antibodies to a peptide from the amino terminus of mDuffy [NYFEDNYSYELSSDC-amide] conjugated to KLH were developed in sheep in collaboration with Bionostics (North York, Ontario, Canada) following standard protocols. The serum from immunized sheep was purified over a Glutathione S-Transferase (GST)–mDuffy fusion protein affinity column and the specificity of binding to the GST–mDuffy was confirmed against GST alone and by blocking the immunostaining with the peptide to which the antibody was developed. Specificity of the antibody was determined by Western blot analysis of lysates from Escherichia coli expressing the mDuffy–GST fusion protein encoding the full amino terminus of mDuffy fused to the C-terminus of glutathione-S-transferase. Lysates were prepared for electrophoresis by solublization in sodium dodecyl sulfate (SDS) electrophoresis loading buffer (0.125 M Tris-Cl, pH 6.8, 1% SDS, 2.5% β-mercaptoethanol, 10% glycerol and 0.1% bromophenol blue) and electrophoresed on a 10% polyacrylamide gel with SDS and reducing agents. The samples were then transferred to nitrocellulose (Bio-Rad, Hercules, California, USA), blocked with a 5% solution of Carnation dry milk in Tris-buffered saline (TBS) with 0.05% TWEEN-20, incubated with a 1:500 dilution of sheep polyclonal antiserum to mDuffy in the same buffer at room temperature for 2 h, washed three times in TBS with 0.05% TWEEN-20, then incubated with a horseradish peroxidase-conjugated chicken anti-sheep antibody (1:5000) for 1 h at room temperature then washed three times with TBS prior to development with the electro chemilluminescence (ECL) assay system (Amersham, Piscataway, New Jersey, USA). Controls were lysates from E.coli expressing human CCR1-GST fusion protein.
A second test of specificity of the mDuffy antiserum was immunodetection by dot blot analysis of the peptide to which the antiserum was raised, coupled to bovine serum albumin (BSA). The mDuffy antiserum (1:1500 dilution) specifically bound the mDuffy peptide–BSA conjugate, but not bovine serum albumin alone. Based upon these positive results we affinity-purified the sheep mDuffy antiserum over a GST– mDuffy (amino-terminus) affinity column. The affinity-purified antibody was used for immunostaining and Western blot at a 1:250 dilution. The Vectastain ABC kit was used to detect the antigen and the substrate for the peroxidase was amino-ethyl-carbazole. For Western blots, membrane preparations from liver, brain and spleen of mDuffy transgenic mice and wild-type mice were subjected to reducing SDS polyacrylamide gel electrophoresis (SDS-PAGE), trans-blotted to nitrocellulose, blocked and examined for immunodetection of the mDuffy receptor using the GST fusion protein for mDuffy as a positive control. The second antibody (goat anti-sheep IgG) was used at a 1:2500 dilution. Membranes from mDuffy transgenic mice exhibited an enhanced band for mDuffy around 38 kDa as compared to membranes from wild-type mice.
mCXCR2 antibody
The specificity of the mCXCR2 antibody (Santa Cruz, rabbit IgG) was confirmed by immunostaining sections of skin from CXCR2 −/− and CXCR2+/+ mice using a 1:250 dilution of the CXCR2 antibody, followed by the Vectastain ABC enhancement and amino-ethyl-carbazole as the substrate for the peroxidase. Under these conditions, the keratinocytes from CXCR2 +/+ mice, but not CXCR2 −/− mice stained positively for mCXCR2.
MIP-2 antibody
The specificity of the MIP-2 antibody (Santa Cruz Biotechnology, goat IgG, 1:250 dilution) was confirmed by Western blot where melanocytes over-expressing MIP-2 under the control of the tyrosinase promoter/enhancer were compared with normal melanocytes. For a positive control, recombinant MIP-2 was used. A ~ 7.9 kDa band migrated at the same position as the recombinant MIP-2 in melanocytes from MIP-2 over expressing melanocytes and the band was only faintly observed in the normal melanocytes.
KC antibody
Specificity of the KC antibody from R & D Systems was verified by Western blot as described above, using recombinant KC as a positive control and with KC enriched lysates as compared to lysates poor in KC. Primary antibodies were used at a 1:250 dilution and secondary antibodies at a 1:2500 dilution.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (AR), from the NCI :CA56704 (AR) and CA34590 (AR). We appreciate the help from the laboratory of Brigid Hogan (Howard Hughes Medical Institute, Nashville, Tennessee, USA) and the expert technical assistance of Yingchun Yu and Snjezana Milatovic.
References
- 1.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 2.Devalaraja M, Richmond A. Multiple chemotactic factors: Fine control or redundancy? Trends Pharm Sci. 1999;236:151–156. doi: 10.1016/s0165-6147(99)01342-5. [DOI] [PubMed] [Google Scholar]
- 3.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B-cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 4.Luo H, Chaudhuri A, Johnson KR, Neote K, Zbrzezna V, He Y, Pogo AO. Cloning, characterization, and mapping of a murine promiscuous chemokine receptor gene-homolog of the human Duffy gene. Genome Res. 1997;7:932–941. doi: 10.1101/gr.7.9.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang T, Owen JD, Du J, Walker CL, Richmond A. Molecular cloning and characterization of a mouse gene with homology to the Duffy-antigen receptor for chemokines. DNA Sequence. 1998;9:129–143. doi: 10.3109/10425179809072188. [DOI] [PubMed] [Google Scholar]
- 6.Wuyts A, Van Osselaer N, Haelens A, Samson I, Herdewijn P, Ben-Baruch A, Oppenheim JJ, Proost P, Van Damme J. Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochem. 1997;36:2716–2723. doi: 10.1021/bi961999z. [DOI] [PubMed] [Google Scholar]
- 7.Moser B, Schumacher C, von Tscharner V. Clark-Lewis I and Baggiolini M, Neutrophil-activating peptide 2 and gro/melanoma growth-stimulatory activity interact with neutrophil-activating peptide 1/interleukin 8 receptors on human neutrophils. J Biol Chem. 1991;266:10666–10671. [PubMed] [Google Scholar]
- 8.Ahuja SK, Murphy PM. The CXC chemokines growth-regulated oncogene (GRO)alpha, GRO beta, GRO gamma, neutrophil-activating peptide-2, and epithelial cell-derived neutrophil-activating peptide-78 are potent agonists for the type B, but not the type A, human interleukin-8 receptor. J Biol Chem. 1996;271:20545–20550. doi: 10.1074/jbc.271.34.20545. [DOI] [PubMed] [Google Scholar]
- 9.Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski SR, Jr, Conklyn MJ, Breslow R, Showell HJ, Gerard C. The murine interleukin-8 type B receptor homologue and its ligands. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 10.Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptors. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- 11.Devalaraja R, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knock-out mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo H, Chaudhuri A, Zbrzezna V, He Y, Pogo AO. Deletion of the murine duffy gene (Dfy) reveals that the Duffy receptor is functionally redundant. Mol Cell Biol. 2000;20:3097–3101. doi: 10.1128/mcb.20.9.3097-3101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuta Y, Hogan BLM. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy M, Jones J, Whittemore SR, Haviland DL, Wetsel RA, Barnum SR. Expression of the receptors for the C5a anaphylatoxin interleukin-8 and FMLP by human astrocytes and microglia. J Neuroimmunol. 1995;61:71–78. doi: 10.1016/0165-5728(95)00075-d. [DOI] [PubMed] [Google Scholar]
- 15.Halks-Miller M, Hesselgesser J, Miko IJ, Horuk R. Chemokine receptors in developing human brain. Methods Enzymol. 1997;288:27–38q. doi: 10.1016/s0076-6879(97)88005-6. [DOI] [PubMed] [Google Scholar]
- 16.Horuk R, Martin AW, Wang Z-X, Scweitzer L, Gerassimides A, Lu Z-H, Hesselgesser J, Kim J, Parker J, Hadley TJ, Perez HD, Peiper SC. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997;158:2882–2890. [PubMed] [Google Scholar]
- 17.Peiper SC, Horuk R. Chemokine receptors in the brain: hierarchical expression by subsets of neurons. In: Horuk, editor. Chemoattractant Ligands and Their Receptors. Vol. 15. CRC Press; New York: 1996. pp. 353–366. [Google Scholar]
- 18.Xia MQ, Qin SX, McNamara M, Mackay CR, Hyman BT. Interleukin-8 receptor B immunoreactivity in brain and neuritic plaques of Alzheimers disease. Am J Pathol. 1997;150:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 19.Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7, -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 20.Ragozzino D, Giovannelli A, Mileo AM, Limatola C, Santoni A, Eusebi F. Modulation of the neurotransmitter release in rat cerebellar neurons by GROβ. NeuroReport. 1998;9:3601–3606. doi: 10.1097/00001756-199811160-00011. [DOI] [PubMed] [Google Scholar]
- 21.Tani M, Fuentes ME, Peterson JW, Trapp BD, Durham SK, Loy JK, Bravo R, Ransohoff RM, Lira SA. Neutrophil infiltration, glial reaction, and neurological disease in transgenic mice expressing the chemokine N51/KC in oligodendrocytes. J Clin Invest. 1996;98:529–539. doi: 10.1172/JCI118821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Mori M, Seto K, Shibata F, Nagahashi S, Kawaguchi C, Suzuki M, Matsui H, Watanage K, Miura S, Ishii H. Rat CXC chemokine GRO/CINC-1 paradoxically stimulates the growth of gastric epithelial cells. Aliment Pharmacol Ther, Supplement. 2000;14:94–100. doi: 10.1046/j.1365-2036.2000.014s1094.x. [DOI] [PubMed] [Google Scholar]


