Abstract
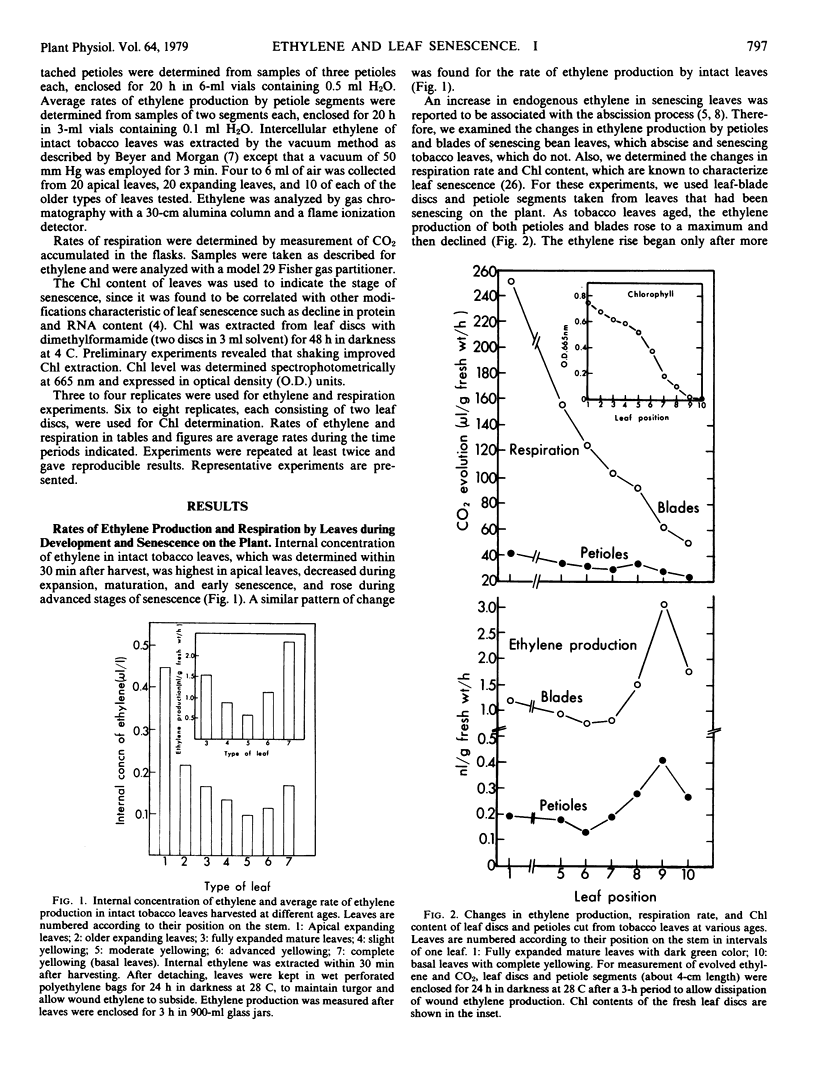
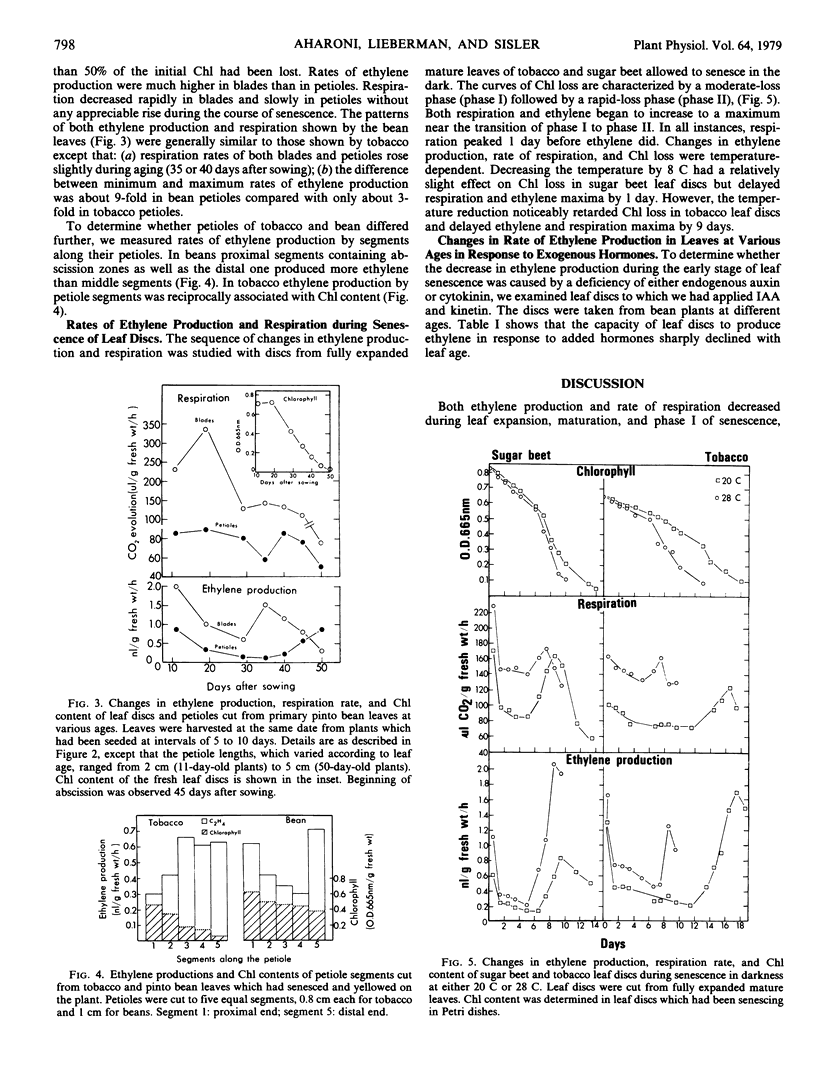
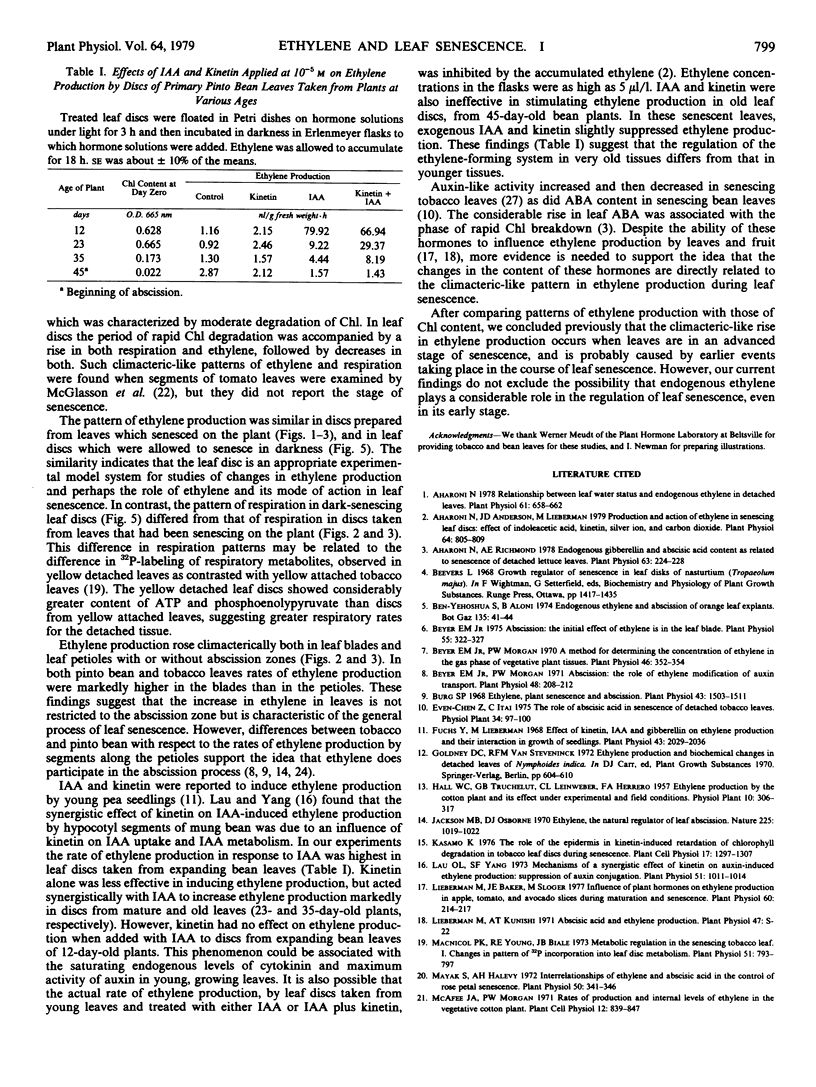
Changes in the patterns of ethylene production, chlorophyll content, and respiration were studied in relation to the senescence of intact leaves and leaf discs. The primary leaves of pinto bean, which abscise readily during natural senescence, and tobacco and sugar beet leaves, which do not abscise, were used. A decrease in the rate of ethylene production and respiration, during the slow phase of chlorophyll degradation, was observed in leaf-blade discs cut from mature leaves and aged in the dark. During rapid chlorophyll loss both ethylene production and respiration increased and then decreased. These climacteric-like patterns were shown by leaf discs of all three species. Discs taken from leaves that had been senescing on the plant also showed a climacteric-like rise in ethylene production but not in respiration, which decreased continuously with leaf age. Climacteric-like patterns in the rise of ethylene and respiration for leaf discs were also shown by the petioles of both bean and tobacco leaves. This indicates that the rise of ethylene and respiration is characteristic of the general process of senescence in leaves and is not restricted to the abscission process. In contrast to the ethylene-forming systems in climacteric fruits and many flowers, the one in leaves declines sharply in the early stages of senescence. The subsequent rise of ethylene production appears to be associated with the rapid phase of chlorophyll breakdown, and may indicate the final stage of the senescence process during which ethylene could be actively involved in inducing leaf abscission.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N. Relationship between Leaf Water Status and Endogenous Ethylene in Detached Leaves. Plant Physiol. 1978 Apr;61(4):658–662. doi: 10.1104/pp.61.4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Abscission: the initial effect of ethylene is in the leaf blade. Plant Physiol. 1975 Feb;55(2):322–327. doi: 10.1104/pp.55.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. A method for determining the concentration of ethylene in the gas phase of vegetative plant tissues. Plant Physiol. 1970 Aug;46(2):352–354. doi: 10.1104/pp.46.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene, plant senescence and abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1503–1511. [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Baker J. E., Sloger M. Influence of Plant Hormones on Ethylene Production in Apple, Tomato, and Avocado Slices during Maturation and Senescence. Plant Physiol. 1977 Aug;60(2):214–217. doi: 10.1104/pp.60.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol P. K., Young R. E., Biale J. B. Metabolic regulation in the senescing tobacco leaf: I. Changes in pattern of p incorporation into leaf disc metabolites. Plant Physiol. 1973 Apr;51(4):793–797. doi: 10.1104/pp.51.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S., Halevy A. H. Interrelationships of ethylene and abscisic Acid in the control of rose petal senescence. Plant Physiol. 1972 Sep;50(3):341–346. doi: 10.1104/pp.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlasson W. B., Poovaiah B. W., Dostal H. C. Ethylene production and respiration in aging leaf segments and in disks of fruit tissue of normal and mutant tomatoes. Plant Physiol. 1975 Oct;56(4):547–549. doi: 10.1104/pp.56.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]



