Abstract
The clinical management of large-size cartilage lesions is difficult due to the limited regenerative ability of the cartilage. Different biomaterials have been used to develop tissue engineering substitutes for cartilage repair, including chitosan alone or in combination with growth factors to improve its chondrogenic properties. The main objective of this investigation was to evaluate the benefits of combining activated platelet-rich plasma with a stabilized porous chitosan scaffold for cartilage regeneration. To achieve this purpose, stabilized porous chitosan scaffolds were prepared using freeze gelation and combined with activated platelet-rich plasma. Human primary articular chondrocytes were isolated and cultured in stabilized porous chitosan scaffolds with and without combination to activated platelet-rich plasma. Scanning electron microscopy was used for the morphological characterization of the resulting scaffolds. Cell counts were performed in hematoxylin and eosin–stained sections, and type I and II collagen expression was evaluated using immunohistochemistry. Significant increase in cell number in activated platelet-rich plasma/stabilized porous chitosan was found compared with stabilized porous chitosan scaffolds. Chondrocytes grown on stabilized porous chitosan expressed high levels of type I collagen but type II was not detectable, whereas cells grown on activated platelet rich plasma/stabilized porous chitosan scaffolds expressed high levels of type II collagen and type I was almost undetectable. In summary, activated platelet-rich plasma increases nesting and induces the differentiation of chondrocytes cultured on stabilized porous chitosan scaffolds.
Keywords: Cartilage tissue engineering, stabilized porous chitosan, activated platelet-rich plasma
Introduction
Osteoarthritis is a complex disease characterized by the progressive loss of articular cartilage and chondrocytes within synovial joints. This clinical syndrome affects not only the elderly but also young people and occurs because of different causative agents including sport-related injury, trauma, and tumors. The management of cartilage lesions is problematic for surgeons due to the limited regenerative ability of cartilage, which is generally attributed to its avascularity and hypocellularity. Generally, smaller defects are treated with arthroscopic bone marrow stimulation (microfracture and drilling), whereas replacement procedures including osteochondral autografts, allografts, or cell implantation are used for larger lesions. Unfortunately, the cartilage resulting from these procedures is frequently fibrocartilage instead of functional hyaline cartilage.1,2
The need for suitable methodologies for cartilage regeneration has encouraged tissue engineering studies centered on the development of substitutes for the clinical repair of severe cartilage defects. For example, different biomaterials have been tested as scaffolds for cartilage repair, including chitosan. Chitosan [poly-(β-1/4)-2-amino-2-deoxy-d-glucopyranose] is a copolymer composed of d-glucosamine and N-acetyl-d-glucosamine bonds and β-bonds derived from the alkaline deacetylation of chitin. Chitosan shares some structural characteristics with the glycosaminoglycans present in hyaline cartilage. Stabilized porous chitosan (SPCHT) scaffolds have been used to generate functionalized tissue substitutes, either alone or in combination with other biomaterials such as polycaprolactone, silk fibrils, genipin, and chondroitin sulfate or polyester, among others.3 Different strategies can improve the chondrogenic ability of chitosan, such as its conjugation to transforming growth factor-beta (TGF-β; alone or encapsulated in microparticles), association with hydrogels including type II collagen or platelet-rich plasma (PRP), or the use of specific bioreactors.4–6
PRP contains higher levels of platelets than whole plasma. Once activated (αP-PRP) by thrombin or CaCl2, platelets release α-granules and a hydrogel containing high levels of growth factors including TGF-β, insulin-like growth factor (IGF), or vascular endothelial growth factor (VEGF) is formed. Several studies demonstrated that PRP increases chondrocyte cell growth and differentiation, as well as the synthesis of chondral matrix in vitro and in vivo.7 Thus, αP-PRP is a powerful tool for cartilage regeneration because it provides an autologous source of growth factors and can be associated with other biomaterials to improve their chondral regeneration potential.8
Therefore, the aim of this study was to analyze the effects of αP-PRP on the nesting and differentiation of human chondrocytes cultured on SPCHT scaffolds.
Materials and methods
Preparation and description of SPCHT scaffolds
Chitosan was purchased from Sigma Aldrich Chemical (Madrid, Spain). The deacetylation degree of chitosan (DD) was 85%, as determined by nuclear magnetic resonance (1H-NMR), and its mean molecular weight (Mw) was 315 kDa, as measured by gel permeation chromatography. Three-dimensional (3D) hierarchical pore structure scaffolds were prepared by freeze gelation. Briefly, 1, 2, or 3% (wt) chitosan was dissolved in 2% (w/w) acetic acid with stirring overnight. The solutions were poured into a cylindrical mold (7 mm diameter and 25 mm thickness) and frozen in liquid nitrogen. Then, they were soaked in a sodium hydroxide and ethanol mixture at −20°C for 3 days. Afterward, the scaffolds were washed with distilled water until they reached pH 7 to remove the excess NaOH. Finally, the porous samples were transferred into a freeze drier (Telstar) and lyophilized at −80°C for 3 days to ensure that they were completely dried. Disks 3 mm in diameter and 1 mm in thickness were cut from the produced porous sheets for use in cell culture. The non-cytotoxicity of the resulting scaffolds was assessed using MTT assays, and the results were consistent with previously published results.5
αP-PRP preparation
PRP was obtained from 4.8 mL peripheral human blood by centrifugation at 1800 r/min at room temperature and prepared as previously reported.9 The plasma was divided into four fractions according to the platelet content. The fourth fraction, containing the highest density of platelets (four times higher than normal blood), was used and injected into SPCHT scaffolds (described below). To activate the PRP, CaCl2 was added to a final concentration of 0.45 mM.
Chondrocyte isolation and cell culture
Human primary chondrocytes were isolated from the knee articular cartilage of donor patients undergoing total knee arthroplasty using standard protocols. All individuals provided informed consent. The study was conducted in accordance with the Declaration of Helsinki and applicable local regulatory requirements and laws. All procedures were approved by the Ethics Committee of the University Clinical Hospital of Valencia (Spain). Chondrocytes were isolated using hyaluronidase, pronase, and collagenase as previously reported.10 The resulting chondrocytes were cultured in dulbecco’s Modified Eagle’s Medium (DMEM) basal medium supplemented with 10% fetal bovine serum (FBS), 100 mg/mL ascorbic acid, 100 U penicillin, and 100 mg/mL streptomycin, seeded on SPCHT scaffolds (described below), and cultured for 14 days.
Scanning electron microscopy
Morphological analysis of scaffolds, with or without cells seeded, was performed using a JSM-5410 (Jeol USA Inc., Peabody, MA, USA) scanning electron microscope as previously reported.5 All specimens were coated with a conductive layer of sputtered gold. An accelerating voltage of 20 kV was used to obtain micrographs.
Histology and immunohistochemistry
SPCHT scaffolds from different days of culture were fixed for 24 h with 4% formaldehyde at room temperature and embedded in paraffin. Five-micrometer-thick sections were obtained and standard hematoxylin and eosin staining was carried out. The number of cells was counted throughout the 14 days of culture on the entire surface of each section. Type I and II collagen expression was evaluated using immunohistochemistry with specific mouse anti-human antibodies (C2456 Sigma [1:50 dilution] and CP18 Calbiochem [1:50 dilution], respectively). The sections were deparaffined and rehydrated through a graded ethanol series, rinsed in distilled water, and treated with 0.3% H2O2 and 10% normal horse serum to block endogenous peroxidase and nonspecific binding, respectively. Antigen retrieval for type I collagen was performed by boiling in a pressure cooker for 3 min in Envision™ FLEX Target Retrieval Solution low pH (Dako, Barcelona, Spain). For type II collagen, Envision FLEX Target Retrieval Solution high pH (Dako) was used. The Dako Envision amplification system (Cytomation Envision + System labeled polymer-HRP anti-mouse) was then used, followed by development with 3,3′-diaminobenzidine (Dako) as the chromogen according to the manufacturer’s instructions, which resulted in brown staining in the immunoreactive structures. Finally, the sections were counterstained with Mayer’s hematoxylin (Sigma-Aldrich).
Statistical analysis
Data are presented as mean ± standard error (SE) of n = 3. Statistical analyses were performed using two-way analysis of variance (ANOVA) followed by Bonferroni’s tests using GraphPad Prism software (GraphPad, San Diego, CA, USA). Significance was accepted for p values <0.05.
Results
Generation of combined αP-PRP/SPCHT scaffolds
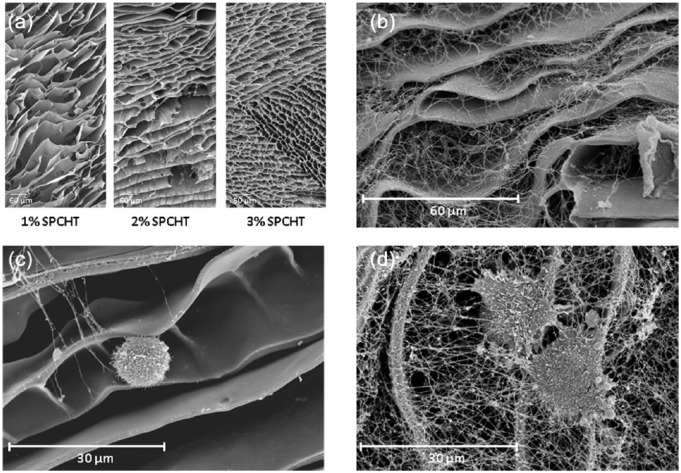
Scaffolds were constructed using 1%, 2%, or 3% chitosan solution and evaluated using scanning electron microscopy (SEM). The results revealed a characteristic honeycomb structure with chitosan forming thin walls just a few microns thick. As the chitosan concentration in the solution used in the freeze extraction decreased, the porosity of the scaffold increased. A lower structural deformity and a better conservation of the interconnectivity and pore distribution was observed when 3% chitosan was used, so 3% SPCHT scaffolds were used thereafter (Figure 1(a)). In these scaffolds, the pores presented with an elongated morphology with a short length (the distance between chitosan layers) in the order of tens of microns and large dimensions of >100 µm.
Figure 1.
Ultrastructure of chondrocytes seeded on SPCHT and αP-PRP/SPCHT scaffolds. Representative scanning electron microscopy images of 1%–3% SPCHT scaffolds: (a) αP-PRP/3% SPCHT scaffolds, (b) chondrocytes cultured for 14 days on 3% SPCHT scaffolds, (b) chondrocytes cultured for 14 days on αP-PRP/3% SPCHT scaffolds, and (d) the data shown are representative of three separate experiments.
To generate αP-PRP/SPCHT scaffolds, three different protocols were tested. In the first method, 50 µL of PRP was injected in the SPCHT scaffold. It was then activated 5 min later by the injection of 0.45 mM CaCl2. Second, 0.45 mM CaCl2 was added to the SPCHT scaffold and then 50 µL of PRP was injected. Third, 0.45 mM CaCl2 was mixed with 50 µL of PRP and then the mixture was injected in the SPCHT scaffolds. The first method was selected because it yielded a more homogeneous distribution (on the surface and inside the pores) of the fibrin network (Figure 1(b) than the other protocols, as evaluated using SEM (data not shown).
αP-PRP increases cell nesting on SPCHT scaffolds
Cultured chondrocytes were isolated and 20 × 103 cells were seeded on the surface of 3% SPCHT scaffolds, with or without αP-PRP combination, for up to 14 days. Cells grown on SPCHT exhibited a round morphology and a smaller size (Figure 1(c)) compared with those grown on αP-PRP/SPCHT, which exhibited a flattened morphology with a larger number of longer processes and a better attachment to the scaffold because they were covered by a fibrin network that was also adhered to the SPCHT scaffold (Figure 1(d) and Figure 2(a)).
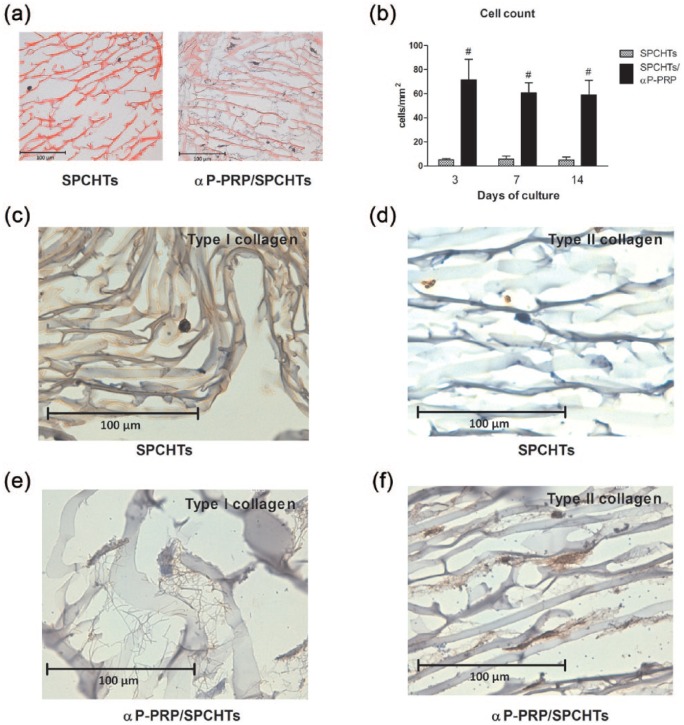
Figure 2.
αP-PRP increased the nesting and differentiation of chondrocytes in vitro. Chondrocytes were cultured on SPCHT or αP-PRP/SPCHT scaffolds for up to 14 days (a, at 3 days of culture, stained with hematoxylin and eosin). (b) The number of cells throughout the 14 days of culture (mean ± SE, n = 3). #p < 0.05 versus the SPCHT group by Bonferroni test. Cell differentiation was determined using immunohistochemistry against type I (c and e) or type II (d and f) collagen in SPCHT and αP-PRP/SPCHT scaffolds. Immunohistochemistry images are representative of three separate experiments.
Cell number was estimated by the double-blinded counting of hematoxylin and eosin–stained slides of SPCHT scaffolds with and without combination to αP-PRP. The results revealed a significant increase in cell number in αP-PRP/SPCHT compared with SPCHT scaffolds (Figure 2(b); p < 0.05 by two-way ANOVA). No significant differences were observed over time in culture within the same experimental group because cell number remained constant until day 14.
αP-PRP induces chondrocyte differentiation on αP-PRP/SPCHT scaffolds
Immunohistochemistry was performed for type I and II collagen in 3% SPCHT scaffolds with and without combination to αP-PRP to investigate the effectiveness of αP-PRP in the induction of chondrocyte differentiation. After 14 days of culture, chondrocytes grown in SPCHT expressed high levels of type I collagen, whereas type II collagen was not detectable (Figure 2(c) and (d), respectively). In contrast, cells grown on αP-PRP/SPCHT expressed high levels of type II collagen, but type I collagen was almost undetectable (Figure 2(e) and (f)).
Discussion
Restoring the structure and function of articular cartilage is considered a clinical problem in the patients in which different pathological factors affect the management of the lesion. In these cases, tissue engineering–based strategies including the use of cells, factors, and scaffolds are gaining relevance. Chitosan is among the long list of biomaterials studied for cartilage regeneration. Although the chemical, physical, and biomechanical properties of this polymer are useful for tissue engineering, different strategies have been used to improve its usefulness in the regeneration of different tissues, including its combination with PRP. These mixed scaffolds have different advantages. First, the porous structure of chitosan stabilizes the fibrin network formed before PRP activation, which establishes an adequate 3D environment for cell growth and differentiation.6 Second, the combined scaffold releases several growth and differentiation factors in a controlled and sustained manner, including TGF-β and platelet-derived growth factor-AB (PDGF-AB).11 Finally, the biochemical factors released after PRP activation influence the entire joint environment, which exerts beneficial effects on synoviocytes, meniscal cells, and local mesenchymal stem cells (MSCs).8
The synergy between chitosan and PRP has proven useful in different in vitro and in vivo models of bone and wound healing regeneration.12,13 In this study, the benefits of mixed chitosan-PRP scaffolds in cartilage regeneration were investigated. With regard to scaffold preparation, scaffolds with three different porosities were tested. Electron microscopic analysis of the resulting constructs revealed less structural deformity and better conservation of the interconnectivity and pore distribution when 3% chitosan was used in the freeze extraction process, which is consistent with previous studies;6 therefore, 3% chitosan was chosen for our research. With regard to PRP preparation, a single centrifugation method was used because previous studies have demonstrated that different centrifugation procedures have no effect on the levels of platelet activation.14 We observed a significant increase in the number of cells both at the surface and within the pores of the chitosan scaffolds in the experimental groups that combined SPCHT with αP-PRP. This is also consistent with previous observations of the use of mesenchymal or adipose stem cells for bone regeneration.6,15 The effects of αP-PRP were also evident at the cellular differentiation level. On one hand, the morphology of the cells analyzed by SEM was compatible with differentiating chondrocytes. On the other hand, cells grown in SPCHT combined with αP-PRP expressed almost undetectable levels of type I collagen, as detected by immunohistochemistry, but expressed high levels of type II collagen, which is characteristic of hyaline cartilage. However, cells grown in the absence of αP-PRP expressed higher levels of type I collagen and non-detectable levels of type II.
Finally, it is important to acknowledge that all these effects were evident at an early time of culture (3 days for cell number and 14 days for cell differentiation). This finding is important because it suggests that the use of αP-PRP with SPCHT scaffolds could improve cell nesting in cases where the use of cellularized scaffold needs a previous in vitro culture phase before implant in vivo. In summary, the current results demonstrate that αP-PRP improves the nesting and differentiation of human chondrocytes cultured on chitosan scaffolds. Therefore, this combination could represent a new strategy to regenerate articular cartilage in cases where conventional treatments fail.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants MAT2016-76039-C4-2-R (M.S.-T. and C.C.) and MAT 2013-46467-C4-1-R (M.A.G.-G. and J.L.G.R.) from the Ministry of Economy and Competitiveness of the Spanish Government and by the program VLC-Bioclínic from the University of Valencia and INCLIVA (Spain). CIBER-BBN and CIBERER are funded by the VI National R&D&I Plan 2008-2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions, and financed by the Instituto de Salud Carlos III with the assistance of the European Regional Development Fund. M.A.G.-G. acknowledges a grant from the BES-2011-044740.
References
- 1. Muzzarelli RA, Greco F, Busilacchi A, et al. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym 2012; 89(3): 723–739. [DOI] [PubMed] [Google Scholar]
- 2. Xie A, Nie L, Shen G, et al. The application of autologous platelet-rich plasma gel in cartilage regeneration. Mol Med Rep 2014; 10(3): 1642–1648. [DOI] [PubMed] [Google Scholar]
- 3. Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, et al. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int 2015; 2015: 821279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim J, Lin B, Kim S, et al. TGF-β1 conjugated chitosan collagen hydrogels induce chondrogenic differentiation of human synovium-derived stem cells. J Biol Eng 2015; 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. García Cruz DM, Salmerón-Sánchez M, Gómez-Ribelles JL. Stirred flow bioreactor modulates chondrocyte growth and extracellular matrix biosynthesis in chitosan scaffolds. Biomed Mater Res A 2012; 100(9): 2330–2341. [DOI] [PubMed] [Google Scholar]
- 6. Shimojo AA, Perez AG, Galdames SE, et al. Performance of PRP associated with porous chitosan as a composite scaffold for regenerative medicine. Scientific World Journal 2015; 2015: 396131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen J, Gao Q, Zhang Y, et al. Autologous platelet-rich plasma promotes proliferation and chondrogenic differentiation of adipose-derived stem cells. Mol Med Rep 2015; 11(2): 1298–1303. [DOI] [PubMed] [Google Scholar]
- 8. Krüger JP, Ketzmar AK, Endres M, et al. Human platelet-rich plasma induces chondrogenic differentiation of subchondral progenitor cells in polyglycolic acid-hyaluronan scaffolds. J Biomed Mater Res B Appl Biomater 2014; 102(4): 681–692. [DOI] [PubMed] [Google Scholar]
- 9. Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 2014; 7(4): 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sancho-Tello M, Forriol F, Gastaldi P, et al. Time evolution of in vivo articular cartilage repair induced by bone marrow stimulation and scaffold implantation in rabbits. Int J Artif Organs 2015; 38(4): 210–223. [DOI] [PubMed] [Google Scholar]
- 11. Shimojo AA, Perez AG, Galdames SE, et al. Stabilization of porous chitosan improves the performance of its association with platelet-rich plasma as a composite scaffold. Mater Sci Eng C Mater Biol Appl 2016; 60: 538–546. [DOI] [PubMed] [Google Scholar]
- 12. Oktay EO, Demiralp B, Demiralp B, et al. Effects of platelet-rich plasma and chitosan combination on bone regeneration in experimental rabbit cranial defects. J Oral Implantol 2010; 36(3): 175–184. [DOI] [PubMed] [Google Scholar]
- 13. Mohammadi R, Mehrtash M, Mehrtash M, et al. Effect of platelet rich plasma combined with chitosan biodegradable film on full-thickness wound healing in rat model. Bull Emerg Trauma 2016; 4(1): 29–37. [PMC free article] [PubMed] [Google Scholar]
- 14. Kutlu B, Tiğlı Aydın RS, Akman AC, et al. Platelet-rich plasma-loaded chitosan scaffolds: preparation and growth factor release kinetics. J Biomed Mater Res B Appl Biomater 2013; 101(1): 28–35. [DOI] [PubMed] [Google Scholar]
- 15. Bi L, Cheng W, Fan H, et al. Reconstruction of goat tibial defects using an injectable tricalcium phosphate/chitosan in combination with autologous platelet-rich plasma. Biomaterials 2010; 31(12): 3201–3211. [DOI] [PubMed] [Google Scholar]