Abstract
Introduction:
Tobacco cessation is the most important, cost-effective preventive maintenance that clinicians can offer study participants who use tobacco. There is lack of preparedness among primary care physicians in delivering cessation interventions. There are also limited studies which record the effectiveness of cessation interventions in the Indian context. This study is designed to evaluate the effectiveness of brief and intensive tobacco cessation interventions delivered by trained primary care providers in two states of India.
Methods and Analysis:
A quasi-experimental study design has been adopted for the study with around 20 primary care practices, selected from four districts of two states in India (Odisha and Rajasthan). Brief (3A) and Intensive tobacco (5A) cessation intervention services will be provided to two groups of tobacco users, respectively. Both groups will be followed up for 6 months to determine the effectiveness of the cessation interventions. The cost-effectiveness of the services will also be documented at the end of the study. The entire study will be completed in 24 months, of which the final 6 months will be reserved for study participant follow-up and quit rate evaluation. When comparing the two groups, differences between proportions will be assessed by chi-square test and differences between means with t-test. The conventional significance level of 0.05 will be used in all analyses in order to reject the null hypothesis of no difference between groups. We will use difference-in-differences methods to assess the impact of the interventions on physicians’ behavior to deliver tobacco cessation in their clinical practice.
Conclusion:
The study is in participant recruitment phase.
Keywords: Epidemiology, public health
Introduction
The epidemic of tobacco consumption is very complex in India which is the second largest producer and third largest consumer of tobacco in the world.1 Tobacco use is one of the leading causes of morbidity and mortality in India.2,3 The adverse health consequences of tobacco use not only compromise tobacco user’s quality of life but also entail significant direct and indirect economic costs on society.4 There are myriad forms of tobacco which are available in India and almost one in three adults (15 years and above) is a tobacco consumer.5,6 Stark inequalities exist in the socioeconomic, demographic and geographical distribution of tobacco use,7 making it imperative that tobacco cessation services is extended universally and is available for all. These cessation services are more likely to be strengthened in a cost-effective manner when linked with existing primary health care system8 especially in resource-constrained low and middle income country (LMIC) like India.
Tobacco cessation is the most important, cost-effective preventive maintenance that clinicians can offer study participants who use tobacco. It has been called the “gold standard” of prevention interventions by David Eddy, a leading authority on guidelines and cost-effectiveness analysis.9 Primary care physicians play a key role in identification, assessment and treatment of tobacco users. Evidence shows that physician’s message alone and counseling lead many patients to quit or significantly reduce their tobacco habit.10 Effective tobacco interventions are available but underutilized because nicotine is widely used and culturally accepted. Physicians do not inquire about tobacco usage, do not use available interventions, are under time constraints and may not believe the effort of tobacco cessation intervention is worth the benefit to the study participant.11 Recent data from Global Adult Tobacco Survey (GATS), India, show that less than half of smokers who visited health care providers were advised to stop smoking.12 Published data from India suggest that physicians lack skills in delivering brief intervention and counseling in tobacco cessation.13 One of the reasons identified for such lack of preparedness by physicians is the fact that there are no well-established evidence-based certified tobacco cessation training programs in the country. A recent evaluation of the National Tobacco Control Programme (NTCP) in India reported an urgent need for training in tobacco cessation among the health care providers.14 Findings from the Global Health Professionals Students Survey (GHPSS) showed a general lack of training on counseling techniques among dental, medical, nursing and pharmacy students.15 This training gap could be a major contributing factor to the low rates of tobacco cessation interventions delivered by physicians and highlights the urgent need for investments in both training physicians for delivering brief tobacco cessation interventions and creating a critical number of master trainers and resource networks for long-term sustainability. There are also limited data on the effectiveness of cessation interventions in Indian context.
We have designed a quasi-experimental study to evaluate the effectiveness of brief and intensive tobacco cessation interventions delivered by trained primary care providers in two states of India.
Study aim and objectives
The aim of this study is to evaluate the effectiveness of brief (3A)12 versus intensive (5A)16 tobacco cessation interventions in primary care practices of India.
The key objectives of this study are as follows:
To test the real-world effectiveness of physician-delivered 3A and 5A interventions;
To determine the abstinence rates in 3A and 5A intervention;
To compare the cost in implementing the 3A and 5A interventions.
In our study, we define 3A and 5A interventions as follows:
Brief intervention (3A)
Short counseling/interaction session (approximately 2–3 min), that is, 3As—Ask, Advice & Assess;
Information, Education & Communication (IEC) materials will be shown to the tobacco user.
Intensive intervention (5A)
Elaborate counseling session (approximately 5–8 min), that is, 5As—Ask, Advice, Assess, Assist & Arrange;
IEC materials will be used extensively during the counseling;
Study participant education materials/leaflets will be given to the tobacco users;
Information on pharmacotherapy to quit tobacco;
Follow-up will be arranged.
Theory of change
Due to the lack of skills among primary care physicians on cessation services, we designed a training model using both online and class teaching methods (Figure 1).
Figure 1.
Theory of change.
This was based on evidence-based tobacco cessation practices in a comprehensive manner and its importance in routine clinical practice drawn from models of behaviour change. It provided logical and evidence based skills to implement cessation services in the healthcare facilities of India. The tobacco users who were counseled by the physicians to quit tobacco will be followed-up to record the abstinence rate. Appropriate evaluation methods will be used at each step.
Study design
A quasi-experimental study design has been adopted for the study with approximately 20 primary care practices, selected from four districts of two states in India (Odisha and Rajasthan). The study is a part of a larger project in which 500 physicians working in more than 250 government health practices have been trained on tobacco cessation interventions across 14 districts of the two states. It is the most appropriate design for the study in the presence of practical and ethical barriers to conducting randomized controlled trials (RCTs).17 Also, it is the design of choice to establish causal associations between an intervention and an outcome (in the absence of RCT—the “gold standard” of causal research design). It is one of the most common study designs to determine the efficacy of an intervention when there is time constraint in planning a randomized trial and randomization may not be a viable option.18 Study participants from selected primary care practices will be assessed on the date of study inclusion (D0) and after 1 (D0+1), 3 (D0+3) and 6 months (D0+6). Moreover, exit interviews of study participants and self-reported practices of physicians will also be conducted at the beginning and the end of the study. Finally, the intervention’s cost-effectiveness will be assessed.
Study duration
The entire study will be completed in 24 months, of which the final 6 months will be reserved for study participant follow-up and quit rate evaluation.
Study setting
Public Health Facilities (Primary Health Centre (PHC), Urban Health Centre (UHC), Community Health Centre (CHC) and District Headquarter Hospital (DHH)/Sub District Hospital (SDH)) located in selected districts.
Selection of health facilities
Health facilities were selected by systematic random sampling. The health facilities were chosen using systematic random sampling. All the health facilities providing primary care and satisfying the selection criteria in the district were listed. A total of 200 facilities were added to the list. The first health facility was selected at random and then every fifth health facility was selected for inclusion in the sample. The selection of health facilities was based on the following criteria:
The average outpatient load of non-communicable disease cases, 6 months prior to the study.
The facilities which had a trained physician and counselor were assigned as 5A center. The facilities with only a trained physician will be a 3A center.
The facilities were divided into ten 3A and ten 5A centers. Both types of centers were present in each of the selected district.
Recruitment of study participants and sampling
The study participants will be tobacco users visiting the selected health practices during the study duration.
Selection of study participants
Tobacco users who visited the health facility during the recruitment period and agreed to participate in the study were included until the desired sample size was reached.
Following inclusion criteria were employed to select the study participants:
Study participants should be current tobacco user;
Study participants should be older than 18 years;
Study participants should not suffer from any major chronic illness (patients who may not be able to adhere to the follow-up plan due to the underlying illness);
Study participants should be a resident of the area under the jurisdiction of the health facility.
Exclusion criteria included the following:
Patients below 18 years of age;
Patients who do not have the mental capacity to provide informed consent and complete study protocol;
Migrant patients will not be included.
To prevent cross contamination with the study, none of the selected practices had any established smoking cessation programs. Each facility is staffed by at least one physician trained on tobacco cessation interventions as part of the project. Detailed summary of the study is provided in Figure 2.
Figure 2.
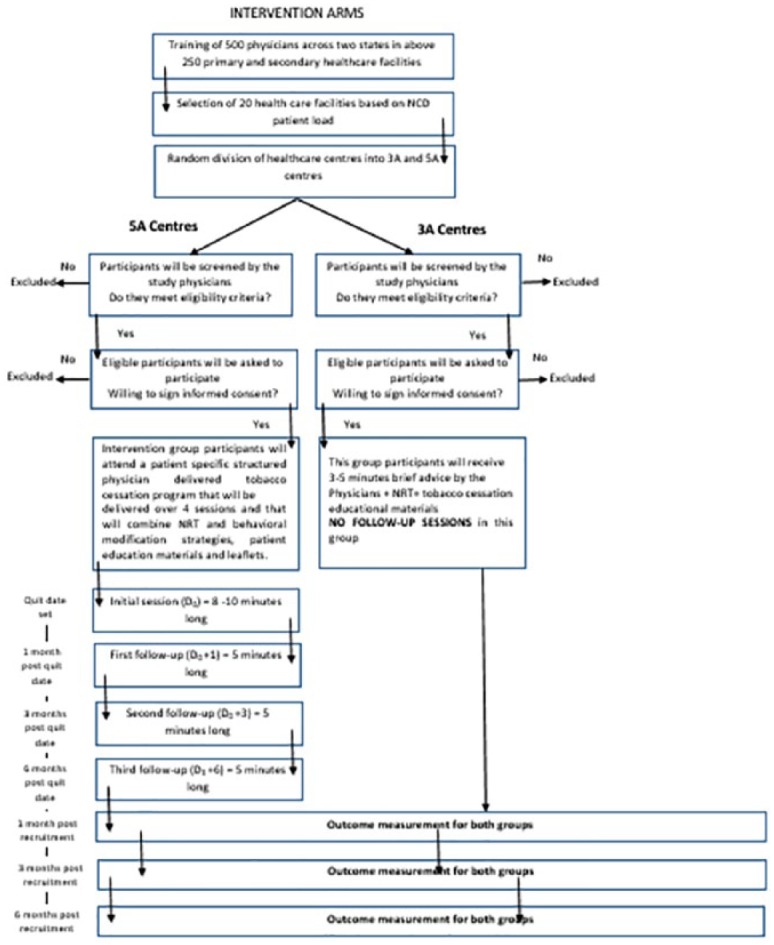
Summary of the study.
Measures and evaluation
Measures
To determine the quit rates, an initial assessment questionnaire on the tobacco status of each registered study participant (3A and 5A centers), sociodemographic data, knowledge, attitude and type of tobacco use will be recorded by the physician during the first visit. A final questionnaire to evaluate tobacco status will be completed at D0 + 6 months (D0 in the study refers to the first visit of the study participant):
A study participant follow-up facilitator will be identified from the selected facility who will assist the trained physician.
The identified facilitator will be oriented about the project and his or her role. He or she will be provided with a register with a standard reporting format to track the study participants. They will maintain the database for the study participants receiving cessation advice at their respective facility. One facilitator will be identified at each selected facility.
Further the enrolled study participants will be randomly selected for follow-up until the desired sample size is reached.
Follow-up will be done at 1, 3 and 6 months. Figure 3 depicts the follow-up intervals of the study.
The follow-up will aim to record the abstinence rate among the study participants.
Self-reported abstinence will be recorded for all study participants during D0+1 and D0+3 months. Abstinence validation in the last follow-up visit D0+6 for smokers will be done by the use of smokerlyzers and self-reported abstinence will be recorded for smokeless study participants.
Figure 3.
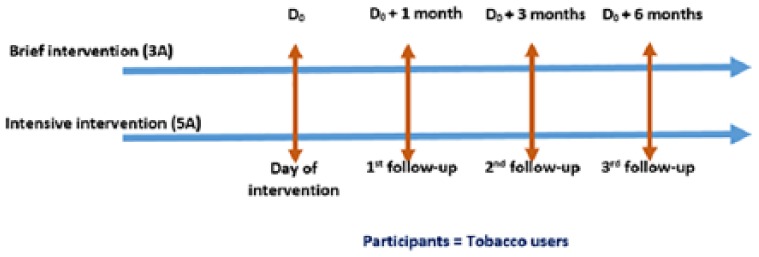
Follow-up of participants.
Outcome evaluation
Primary and secondary outcomes are assessed on the individual participant level 6 months after the initial consultation with the physician. The primary outcome is the rate of tobacco abstinence after 6 months. The rate of abstinence in the study is defined as the number of study participants who quit tobacco at D0 + 6 months relative to the total number of study participants at D0, where D0 is the day of intervention inception. Abstinence is defined here as quitting tobacco for at least 1 month.
Abstinence validation for smokers will be done by the use of smokerlyzers and self-reported abstinence will be recorded for smokeless study participants.
Secondary outcome measures
Point prevalence of study participants (no tobacco used during the preceding 7 days) at the end of the intervention period and at 3- and 6-month follow-up.
Self-reported number of quit attempts and temporary or complete relapse.
Study participant’s willingness to quit tobacco within the 6 months after the intervention.
Rate of withdrawal from the intervention among the study participants after 6 months.
Process evaluation
The process evaluation is performed at the facility level and based on semi-structured interviews that will be conducted with physicians assigned to both the intervention groups at the beginning and end of the study (0 and 6 months). During the interviews, focus will be placed on routine cessation practices, confidence in delivering cessation services, knowledge on cessation, perceived barriers and facilitators to the implementation of the intervention. Further questions will concern experiences with implementing the intervention under routine conditions. In addition, physicians will be asked about their experiences in motivating study participants. Exit interviews with study participant will also be conducted at the beginning and end of the study. The interviews will assess the type of tobacco use, quit attempts, practice and awareness on cessation services as well as advantages of such services. The cost of implementation of the intervention will also be calculated at the end of study. This will include the costs of delivering the interventions, that is, remuneration of the physicians and counselors (in case of 5A), course instructors, course materials and room rent.
Study procedure
Study participants visiting the selected facility during the study and who meet the inclusion criteria will be included in the study. Study participants who wish to participate in the study will be asked to provide their contact details on a separate form along with demographic details. Study participant follow-up facilitators will keep a list of study participants who participate in the study. Physicians will specify study participant’s smoking status and the severity on a documentation sheet. At the end of the survey phase, the lists and documentation sheets will be sent to the study center at the Public Health Foundation of India. A D0+1 and D0+3 month follow-up will be carried out via telephone by a research assistant using the information provided by study participants on the contact information sheet. The final follow-up (D0+6) will be done through house-to-house visit or by calling the study participant to the respective health practices.
Statistical analysis
Descriptive statistics on the study participants will be presented as proportions for categorical variables and as mean values with corresponding standard deviation (SD) for continuous variables. When comparing the two groups, differences between proportions will be assessed by chi-square test and differences between means with t-test. The conventional significance level of 0.05 will be used in all analyses in order to reject the null hypothesis of no difference between groups.
We will use difference-in-differences (DID) methods to assess the impacts of the interventions on physicians’ behavior to make study participants aware about tobacco cessation. DID estimators exploit available information on observables at two or more points of time across longitudinal data (or cross sectional data, as appropriate) by defining “intervention” and “control” groups on the basis of any interventions.19 In our study, both interventions will be used as mutual controls. For ease of analysis, 5A will be used as intervention group and 3A as control group. In the simplest form, DID can be written as
| (1) |
where T1 = outcome among intervention groups in the end line, C1 = outcome in control group in the baseline, T0 = outcome in among the intervention group in the baseline and C0 = outcome in the control group in the baseline.
Considering the two periods, baseline (pre-intervention) and end line (post-intervention) and two population groups (treatment and control groups), a regression specification for arriving at a basic DID estimators is written as
| (2) |
where yit is the outcome of interest for individual physician i in time period t and dT is a dummy for the group with treatment. dt is a dummy variable for the post-intervention time period (t1) as against the pre-intervention time period (t0). The DID estimate is given by which is nothing but the coefficient for the interaction between the treatment and the post-treatment period, that is, dummy . The dummy equals unity for the treatment group in the post-intervention period and captures the effect of the treatment over time. is the error term. Since equation (1) does not control for any socioeconomic characteristic (confounders), β3 represents a “simple difference” in the mean outcome of the respective groups.
Furthermore, since there is a possibility that physician may get changed at the facility levels owing to transfer/retirement or more number of physicians may join the practices located in the intervention primary setting units (PSUs) between the baseline and end line, we control for personal attributes of physicians. Accordingly, we modify equation (2) as follows
| (3) |
where the additional term Xit in equation (3) represents a vector of personal attributes of the physicians. These attributes are, gender, age, location, education and years of experience.
Finally, since our outcome indicators are categorical, instead using a “linear probability model” we used logistic regression model as mentioned in equation (4)
| (4) |
Using equation (3), we report odds ratios of the DID estimators for different outcome indicators. Equation (3) can be used to provide estimates of the following:
Estimates for the control group in the baseline period (t0)—represented by the constant term ;
Simple difference in outcome for the control group in the end line—as represented by ;
Simple difference in outcome for the treatment group (treat) in the baseline period (t0)—represented by the coefficient ;
Difference-in-differences estimates for outcome indicators—represented by .
Sample size
Meta-analyses show that 5% tobacco users quit after brief advice from a physician20 and that 15% quit after more intensive intervention.21 A 10% advantage of the new method over brief advice for point prevalence quitting was considered clinically significant22—a fairly large advantage for motivational consulting would be necessary to justify training large numbers of clinicians to change their consulting style. The sample target size was 600 study participants, with 300 in each arm of the study. Allowing for up to 33% loss to follow-up, this would provide 80% power to detect a 10% difference in smoking cessation outcomes (15% vs 5%) at a two-tailed significance level of 5%. Equal number of study participants will also be followed up in the control arm (n = 300). The average non-communicable diseases (NCD) patients seen per day at the health facilities of Odisha and Rajasthan were 5, 15, 20 and 55 in PHC, UHC, CHC and DHH, respectively. To achieve the desired sample size, 5%, 16%, 21% and 58% patients were taken from PHC, UHC, CHC and DHH, respectively.
Privacy and confidentiality
Information sheets and consent forms will be used to obtain written consent for each stage of the study. It will be clearly stated that the respondent is free to withdraw from the study at any time for any reason without prejudice to future care, and with no obligation to give the reason for withdrawal. Consent to respondent will be obtained in a two-stage process. Physician will initially establish verbal consent to check on eligibility to take part and then screen respondent based on the pre-decided inclusion/exclusion criteria. Study participants who then are positive on screening will be invited into the brief/intensive intervention and will be required to give their written consent. The study participants will be informed about the intervention in detail (time, number of follow-ups, type of services). They will be allowed as much time as wished to consider the information and the opportunity to ask questions in order to decide whether they want to participate in the study. Written informed consent will then be obtained by means of participant dated signature and dated signature of the person who presented and obtained the informed consent. The study staff will ensure that the respondent’s anonymity is maintained. A separate room will always be available for the intervention to be administered. However, if the respondents disclose information that may result in them or anyone else being put at risk of harm the relevant authorities may have to be informed.
Discontinuation/withdrawal of participants from study
Each participant has the right to withdraw study at any time. In addition, the investigator may discontinue a participant from the study at any time if the investigator considers it necessary for any reason including:
Ineligibility (either arising during the study or retrospective having been overlooked at screening); Significant protocol deviation;Significant non-compliance with treatment regimen or study requirements; Consent withdrawn.
The reason for withdrawal will be recorded. Data from withdrawn participants will still be included in the final data analysis.
Discussion
This study is the first quasi-experimental study using a hybrid tobacco cessation training model in India conducted to test the effect of a physician-delivered smoking cessation program on tobacco cessation rates. This could prove to be an effective low cost cessation model, ideal for government health care settings in India. Even a modest success rate could have a large effect on the availability and uptake of cessation services in health care. This will make a valuable contribution to lowering tobacco prevalence in the country.
Moreover, the results will provide information regarding the feasibility of implementing 5A and 3A services under routine conditions. This information is vital as NTCP of India has laid special emphasis on capacity building of physicians and other health care providers in tobacco cessation.23 From policy perspective, the study will provide the critical evidence to inform policy both at the state and the country levels. We reason that showcasing a well-developed cessation model will be the best advocacy tool and NTCP will itself be in a position to implement such models in all health care practices of India. All these activities will eventually strengthen the “National Tobacco Control Programme.”
Key findings from the formative research on establishing a tobacco control network in India validates the fact that there is an interest among different health care professionals in learning about cessation and integrating it in their clinical practices. This study will further help in informing and updating the existing resources in cessation. This will help in the building a structured tobacco dependence treatment services in the country based on both national and global evidence.
Generalizability of the study results may be limited because physicians and study participants willing to participate in a study on smoking cessation could be more interested and more engaged in smoking cessation activities compared to the general population. In addition, tobacco consumption will be measured via questionnaire, and consequently, study participants may over- or underreport the amount of tobacco consumed. However, measuring tobacco consumption via self-report measures is generally accurate and is most appropriate because the study design does not allow for biochemical validation. A possible source of bias is that will be recruited after primary care practices have been randomly allocated to the 3A or 5A group. In this case, the allocation schedule is known, which may lead to biased participant recruitment within medical practices.24
Conclusion
It will be interesting to see the results of the study which to a great extent will reflect the real-world effectiveness of the interventions used in the Indian health settings. More studies should be conducted on larger scales to coming up with daunting evidence on tobacco cessation models which can work in Indian settings.
Acknowledgments
Facility selection has been completed and participant recruitment started in March 2016 and is expected to be completed in April 2106. This is the first version of the study protocol and no amendments have been made to the study design. R.P., M.R.M. and S.P. conceptualized and designed the study. R.P. wrote the grant proposal and discussion section and undertook the critical revisions of the paper for substantial intellectual content. M.R.M. directed the development of data analysis plan. S.M., M.R.M. and K.G. worked on the development of methodology and analytic plan of the study. S.P. contributed to the background and aims and objective section of this paper. All the authors approved the final version to be published.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Public Health Foundation of India (PHFI; approval number: TRC-IEC-258/15).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by IGLC (Independent Grants for Learning & Change; grant number 13197253) by Pfizer.
Informed consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Mishra G, Pimple S, Shastri S. An overview of the tobacco problem in India. Indian J Med Paediatr Oncol 2012; 33: 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh RB, Singh S, Chattopadhya P, et al. Tobacco consumption in relation to causes of death in an urban population of north India. Int J Chron Obstruct Pulmon Dis 2007; 2: 177–185. [PMC free article] [PubMed] [Google Scholar]
- 3. Pednekar M, Sinha D, Singh G, et al. Tobacco use and cessation counseling in India—data from the Global Health Professions Students Survey, 2005–09. Indian J Cancer 2012; 49: 425–430. [DOI] [PubMed] [Google Scholar]
- 4. El Hajj M, Kheir N, Al Mulla A, et al. Assessing the effectiveness of a pharmacist-delivered smoking cessation program in the State of Qatar: study protocol for a randomized controlled trial. Trials 2015; 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhawna G. Burden of smoked and smokeless tobacco consumption in India—results from the Global adult Tobacco Survey India (GATS-India) 2009–2010. Asian Pac J Cancer Prev 2013; 14: 3323–3329. [DOI] [PubMed] [Google Scholar]
- 6. Grills NJ, Singh R, Singh R, et al. Tobacco usage in Uttarakhand: a dangerous combination of high prevalence, widespread ignorance, and resistance to quitting. BioMed Res Int 2015; 2015: 132120 (10 pp.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thakur J, Prinja S, Bhatnagar N, et al. Widespread inequalities in smoking & smokeless tobacco consumption across wealth quintiles in States of India: need for targeted interventions. Indian J Med Res 2015; 141: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Senkubuge F, Modisenyane M, Bishaw T. Strengthening health systems by health sector reforms. Glob Health Action 2014; 7: 23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eddy DM. David Eddy ranks the tests. Harv Health Lett 1992; 17(Suppl.): 10–11. [Google Scholar]
- 10. Thankappan KR, Mini GK, Daivadanam M, et al. Smoking cessation among diabetes patients: results of a pilot randomized controlled trial in Kerala, India. BMC Public Health 2013; 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greden JF, Pomerleau O. Caffeine-related disorders and nicotine-related disorders. In: Kaplan HI, Sadock BJ, Cancro R. (eds) Comprehensive textbook of psychiatry. 6th ed Baltimore: Williams & Wilkins, pp. 799–810 [Google Scholar]
- 12.http://www.ncsct.co.uk/usr/pub/interventions-in-secondary-care-june-10-oncology-study participants-factsheet.pdf (accessed 14 April 2016).
- 13. Panda R, Persai D, Venkatesan S. Missed opportunities for brief intervention in tobacco control in primary care: study participants’ perspectives from primary health care settings in India. BMC Health Serv Res 2015; 15: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panda R. Examining physicians’ preparedness for tobacco cessation services in India: findings from primary care public health facilities in two Indian states. Australas Med J 2013; 6: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saade G, Warren CW, Jones NR, et al. Tobacco use and cessation counseling among health professional students: Lebanon Global Health Professions Student Survey. J Med Liban 2009; 57(4): 243–247. [PubMed] [Google Scholar]
- 16. Carson KV, Verbiest ME, Crone MR, et al. Training health professionals in smoking cessation. Cochrane Database Syst Rev 2012; 16(5): CD000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grimshaw J, Campbell M, Eccles M, et al. Experimental and quasi-experimental designs for evaluating guideline implementation strategies. Fam Pract 2000; 17: S11–S16. [DOI] [PubMed] [Google Scholar]
- 18. Harris AD, McGregor JC, Perencevich EN, et al. The use and interpretation of quasi-experimental studies in medical informatics. J Am Med Inform Assoc 2006; 13(1): 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abadie A. Difference-in-difference estimators In: Steven ND, Lawrence EB. (eds), The new Palgrave dictionary of economics. 2nd ed Palgrave Macmillan: London, 2008. [Google Scholar]
- 20. Kottke TE, Battista RN, DeFriese GH, et al. Attributes of successful smoking cessation interventions in medical practice: a meta-analysis of 39 controlled trials. JAMA 1988; 259: 2883–2889. [DOI] [PubMed] [Google Scholar]
- 21. Wu L, He Y, Jiang B, et al. The effect of a very brief smoking-reduction intervention in smokers who have no intention to quit: study protocol for a randomized controlled trial. BMC Public Health 2015; 15: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butler CC, Rollnick S, Cohen D, et al. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. Br J Gen Pract 1999; 49(445): 611–616. [Google Scholar]
- 23. Ministry of Health and Family Welfare. Global Adult Tobacco Survey, India 2009–10. Ministry of Health and Family Welfare, Government of India, 2010, http://mohfw.nic.in/WriteReadData/l892s/1455618937GATS%20India.pdf [Google Scholar]
- 24. Hahn S, Puffer S, Torgerson DJ, et al. Methodological bias in cluster randomised trials. BMC Med Res Methodol 2005; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]



