Abstract
Background:
Induction of labour is the process of initiating the labour by artificial means from 24 weeks of gestation. The main aim of this study is to find out the maternal and foetal outcomes after induction of labour with misoprostol and oxytocin beyond 37 weeks of gestation.
Methods:
This was a hospital-based observational study carried out at Paropakar Maternity and Women’s Hospital, Nepal. Misoprostol of 25 µg was inserted in posterior fornix of vagina or oxytocin infusion was started from 2.5 units on whom induction was decided. Maternal and foetal/neonatal outcomes were observed. Collected data were analysed using SPSS and MS Excel.
Results:
General induction rate was found to be 7.2%. In this study, post-term pregnancy was found to be the most common reason for induction of labour. Analysis of onset of labour led to the finding that mean onset of labour was much rapid in oxytocin (6.6 h) than misoprostol (13.6 h). However, there is similarity in induction–delivery interval in both groups. Overall, the rate of normal delivery and caesarean section was found to be 64.9% and 33.2%, respectively. Similarly, normal delivery within 12 h was seen in 18.4% of the patients given with misoprostol and 43.5% in oxytocin group. Foetal distress was found as the most common reason for caesarean section. The overall occurrence of maternal complication was found to be similar in misoprostol and oxytocin groups, nausea/vomiting being the most common (36.7%) complication followed by fever (24.1%). Besides this, the most common neonatal complication found in overall cases was meconium stained liquor (49.2%).
Conclusion:
It was found that misoprostol was used most frequently for induction of labour compared to oxytocin. The onset of labour was found to be rapid in oxytocin than misoprostol. However, the occurrence of side effects was found to be similar in both misoprostol and oxytocin groups.
Keywords: Foetal outcome, induction of labour, maternal outcome, misoprostol, neonatal outcome, oxytocin
Introduction
Induction of labour (IOL) is the process to initiate labour by artificial means from 24 weeks of gestation.1 There can be risk of adverse events (caesarean section, prolonged labour, post-partum haemorrhage (PPH), traumatic birth, etc.) to both mother and infants if the pregnancy continues beyond term. IOL is practised widely to prevent such problems and helps to improve the health outcome.2 IOL occurs in over 20% of pregnancies and most commonly applies to cases where there are deviations from the normal physiological processes such as hypertension or diabetes or foetal problems such as foetal growth restriction or macrosomia.1,2
A variety of pharmacological and non-pharmacological methods are used for IOL. Pharmacological methods include oxytocin, prostaglandin (PG) analogues and smooth muscle stimulants such as herbs or castor oil, whereas non-pharmacological methods include mechanical methods such as digital stretching of the cervix and sweeping of the membranes, hygroscopic cervical dilators, balloon catheters, artificial rupture of the membranes and nipple stimulation.3
For an induction to be successful, the cervix needs to have undergone the changes that will ensure the uterine contractions are effective in the progressive dilation and effacement of the cervix. Assessing the ripeness of the cervix is done by means of a scoring system devised by Bishop in 1964.4 Induction is carried out by oxytocin in case cervix is favourable, that is, Bishop score of 6 or more, whereas in case the cervix is unfavourable, then usually a PG is placed in vagina or cervix to ripen the cervix to initiate the uterine contraction.5
PGs have been used for IOL since 1960s.6 The most effective agent found is intravaginal or intracervical prostaglandin E (PGE). PGs improve the rate of normal delivery and lower the rate of caesarean section.7 In comparison to other PGs, misoprostol is found to be cheap, widely available, stable at room temperature and has few side effects.8 Oxytocin is widely used for IOL, alone or in combination with other agents. Risks associated with the use of oxytocin infusion include foetal hypoxia and asphyxia, uterine rupture, fluid retention, PPH and amniotic fluid embolism.4,9–11
In this study, we have used onset of labour and induction-to-delivery interval to see effectiveness of the drugs. The outcomes of this study in terms of effectiveness and adverse effects of the drugs could generate helpful data to health-care personnel involved in pregnancy and childbirth. Thus, the main objective of this study was to describe the maternal and foetal outcomes for a consecutive cohort of women who underwent IOL with misoprostol and/or oxytocin in a hospital in Nepal.
Methods
This was a hospital-based observational study which was carried out at Paropakar Maternity and Women’s Hospital, Thapathali, Kathmandu, Nepal, during the period from July 2014 to September 2014. The sample population for the study was those patients in whom IOL was decided after admission in the hospital for delivery.
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria in this study were alive singleton pregnancy, cephalic presentation and gestational age of 37 completed weeks and above.
Exclusion criteria
The exclusion criteria in this study were grand multiparity (>5 deliveries), women with previous lower segment caesarean section (LsCs), antepartum haemorrhage and prelabour rupture of membrane (PROM).
Study variables
The study variables are as follows: onset of labour, induction-to-delivery interval, gestational age, parity, induction method, mode of delivery, maternal and neonatal outcome.
Data collection tools
Structured questionnaire and patient’s record file was used as a tool for collection of information. Bishop’s scoring and Apgar scoring system was used to check cervix status and neonatal outcome, respectively.4
Data collection technique/methods
Before administration of drugs, women were asked to empty the bladder. Bishop’s scoring was done. In case of IOL with misoprostol, 25 µg was inserted in the posterior fornix of the vagina. Doses of 25 µg were repeated every 6 h according to the requirement of the patient with maximum up to two doses.
In case of administration of Syntocinon (oxytocin), the infusion started from 2.5 units given with 500 mL of dextrose or normal saline at 10 drops per minute. The rate was increased by 10 drops per minute in every 30 min. This was done until a good contraction pattern (three contractions in 10 min each lasting >40 s) was established maximum up to 60 drops per minute. If a good contraction pattern was not established, then the concentration of Syntocinon was increased up to 5 units, and the infusion rate was adjusted to 30 drops per minute. The rate was again increased by 10 drops up to 60 drops per minute. Uterine contractions (for 10 min) and foetal heart rate (for 1 min) were monitored hourly by staff nurses. Foetal Heart Sound(FHS) was monitored every 30 min in case of infusion of Syntocinon.
All eligible women were observed for the occurrence of any side effects (vomiting, diarrhoea, pyrexia, tachycardia, tachysystole, hyperstimulation and uterine rupture). After delivery, neonatal condition was observed. Finally, overall maternal and neonatal outcomes were recorded. Collected data were compiled, managed, analysed and presented using Student version of Statistical Package for Social Sciences (SPSS) software in 21.0 versions and MS Excel. As this was a non-randomized observational study in which the method of IOL for each woman was determined on clinical grounds, no formal comparisons were made between the treatment groups.
Ethical consideration
Ethical approval was granted by the Institutional Review Committee of the hospital to conduct the study. Confidentiality was maintained, and respondents were not forced to answer the questions.
Results
During the study period, there were a total of 3211 cases of delivery. Out of total admitted cases, 231 underwent for IOL. Of these, 205 met the eligibility criteria. General induction rate was found to be 7.2%. No any maternal/neonatal mortality was seen in IOL group.
The majority of the sample population fell under the age group of 20–24 years (91, 44.4%) followed by 25–29 years (77, 37.6%), below 20 years (24, 11.7%) and 30–35 years (13, 6.3%). There was the highest prevalence of nulliparous women (127, 61.9%), whereas 78 (38.1%) of them were multiparous. Looking at gravida, the highest sample population induced was primigravida 127 (61.9%) followed by 60 (29.3%), 15 (7.3%) and 3 (1.5%) second, third and fourth gravida, respectively. The gestational age of the patient varied from 37 weeks to 43 weeks, out of which the highest proportion (94, 45.9%) of women were found in 41 weeks of gestational age (Table 1).
Table 1.
Demographic details of the study subjects.
| Category | Group | No. of population, n (%) |
|---|---|---|
| Age (years) | Below 20 | 24 (11.7) |
| 20–24 | 91 (44.4) | |
| 25–29 | 77 (37.6) | |
| 30–35 | 13 (6.3) | |
| Parity | Nulliparous | 127 (61.9) |
| Multiparous | 78 (38.1) | |
| Gravidity | G1 | 127 (61.9) |
| G2 | 60 (29.3) | |
| G3 | 15 (7.3) | |
| G4 | 3 (1.5) | |
| Gestational week | 37 | 6 (2.9) |
| 38 | 8 (3.9) | |
| 39 | 22 (10.7) | |
| 40 | 59 (28.8) | |
| 41 | 94 (45.9) | |
| 42 | 13 (6.3) | |
| 43 | 3 (1.5) |
It was found that post-dated pregnancy was the major indication for IOL, that is, 144 (70.2%) followed by gestational hypertension 15 (7.3%). The indications for induction are shown in Table 2.
Table 2.
Indication of induction.
| Indication of induction | No. of patients, n (%) |
|---|---|
| Post-dated pregnancy | 144 (70.2) |
| Gestational hypertension | 15 (7.3) |
| Chronic hypertension | 1 (0.5) |
| Hypothyroidism | 2 (0.9) |
| Oligohydramnios | 3 (1.5) |
| Intrauterine growth restriction (IUGR) | 1 (0.5) |
| Previous history of unexplained intrauterine death | 2 (0.9) |
| Low Amniotic Fluid Index (AFI) | 1 (0.5) |
| Long spacing | 2 (0.9) |
| Rh-negative mother | 3 (1.5) |
| Cholestasis of pregnancy | 3 (1.5) |
| Polyhydramnios | 2 (0.9) |
| History of sub-fertility | 2 (0.9) |
| Previous history of stillbirth | 1 (0.5) |
| Other maternal indication | 10 (4.9) |
| Foetal indication | 10 (4.9) |
| Other reasons | 3 (1.5) |
| Total patients | 205 |
Out of 205 cases, 138 (67.3%) women were induced with misoprostol, 50 (24.4%) were induced with oxytocin. In total, 17 (8.3%) were induced with oxytocin only after failure of misoprostol which is placed in ‘other’ group (Figure 1). The modes of delivery after induction are depicted in Table 3. After induction, out of 205 cases, the rate of normal delivery was found to be 133 (64.9%), caesarean section 68 (33.2%) and vacuum delivery 4 (1.9%). The majority of the women provided with misoprostol (98, 71.1%) and oxytocin (33, 66%) underwent normal delivery, whereas caesarean section was maximum (15, 88.2%) in other group.
Figure 1.
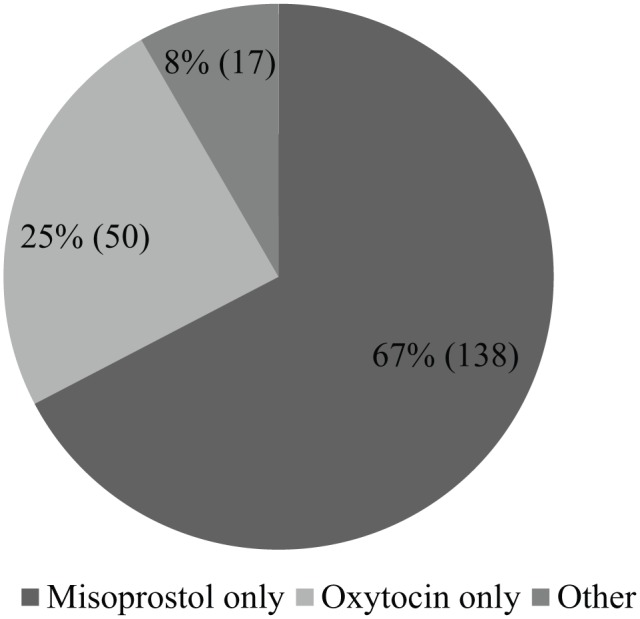
Method of induction.
Table 3.
Modes of delivery.
| Mode of delivery | Misoprostol only | Oxytocin only | Other | Total |
|---|---|---|---|---|
| Normal | 98 (71.1%) | 33 (66%) | 2 (11.8%) | 133 (64.9%) |
| Caesarean | 39 (28.3%) | 14 (28%) | 15 (88.2%) | 68 (33.2%) |
| Vacuum | 1 (0.7%) | 3 (6%) | – | 4 (1.9%) |
| Total | 138 | 50 | 17 | 205 |
Of the total 68 caesarean section cases, foetal distress was found to be the most common reason for caesarean 42 (61.8%, of total caesarean section cases) followed by failure of induction 16 (23.5%; Table 4).
Table 4.
Indication for caesarean section.
| Indication for caesarean | Misoprostol only | Oxytocin only | Other | Total |
|---|---|---|---|---|
| Foetal distress | 30 (76.9%) | 9 (64.3%) | 3 (20%) | 42 (61.8%) |
| Failed IOL | 2 (5.1%) | 4 (28.6%) | 10 (66.7%) | 16 (23.5%) |
| Not progress of labour | 3 (7.7%) | – | 1(6.7%) | 4 (5.9%) |
| Emesis for chorioamnionitis | 1 (2.6%) | – | – | 1 (1.5%) |
| Other indications | 3 (7.7%) | 1 (7.1%) | 1 (6.7%) | 5 (7.4%) |
| Total no. of patients | 39 | 14 | 15 | 68 |
IOL: induction of labour.
It was found that second dose of misoprostol was required in 58 (42.1%) cases (Table 5). Among those, the requirement of additional misoprostol dose was much higher (n = 82, 59%) in nulliparous women (n = 56, 40.6%) than in multiparous women.
Table 5.
Details of requirement of additional misoprostol dose.
| Requirement of additional misoprostol dose | Total cases |
|---|---|
| Yes | 58 (42.1%) |
| No | 80 (57.9%) |
| Total | 138 |
Maternal and foetal outcomes
Maternal and foetal outcome were seen in 188 cases which were induced with only misoprostol and only oxytocin. However, maternal and foetal complications were seen in all three groups to find the overall complication of IOL.
Onset of labour and induction–delivery interval
Onset of labour and induction–delivery interval was seen among 188 cases in which only misoprostol and only oxytocin were given. Besides this, the results are based on data, excluding the samples in which failure of induction was seen, that is, an effective sample size of 182 (12 in misoprostol group and 46 in oxytocin group).
The mean (standard error (SE)) onset of action for oxytocin was 6.6 h (1.2 h), whereas it was 13.6 h (0.9 h) for misoprostol (Table 6). Similarly, the mean (SE) induction–delivery interval was found to be 17.9 h (1.3 h) in misoprostol-given group, whereas it was 16.9 h (2.3 h) in oxytocin-given group (Table 7). Similarly, looking at delivery according to hour intervals, it was seen that more number of patients delivered in <12 h in oxytocin group than in misoprostol group. In total, 60 women in misoprostol group and 14 women of oxytocin group delivered within 12–24 h, whereas other 51 women in misoprostol and 12 women in oxytocin-given group were delivered within 48 h of induction (Table 8).
Table 6.
Mean onset time of action.
| Induction method | Sample size (n) | Mean (SE) (h) |
|---|---|---|
| Misoprostol | 136 | 13.6 (0.9) |
| Oxytocin | 46 | 6.6 (1.2) |
SE: standard error.
Table 7.
Induction-to-delivery interval.
| Induction method | Sample size (n) | Mean (SE) (h) |
|---|---|---|
| Misoprostol | 136 | 17.9 (1.3) |
| Oxytocin | 46 | 16.9 (2.3) |
SE: standard error.
Table 8.
Details of delivery according to hour interval of delivery.
| Induction interval (h) | Misoprostol | Oxytocin |
|---|---|---|
| <12 | 25 (18.4%) | 20 (43.5%) |
| 12–24 | 60 (43.1%) | 14 (30.4%) |
| 24–48 | 51 (37.5%) | 12 (26.1%) |
| Total patients | 136 | 46 |
Maternal complication
Maternal complication was seen in total 205 sample size. Among which, maternal morbidity was seen in 79 (38.5%) patients. Nausea/vomiting was the most common side effect seen in 29 (36.7%) patients followed by fever in 19 (24.1%) patients. The overall occurrence of maternal complications was 38 (27.5%) for misoprostol group, 23 (46%) for oxytocin group and 8 (47.1%) for other group. The incidence of diarrhoea was seen only in misoprostol-treated group. The occurrence and distribution of maternal complications is presented in Table 9.
Table 9.
Overall distribution of maternal complications.
| Misoprostol only | Oxytocin only | Other | Total | |
|---|---|---|---|---|
| Maternal complications, n (%) | ||||
| Nausea/vomiting | 18 (47.4) | 8 (34.8) | 3 (37.5) | 29 (36.7) |
| Diarrhoea | 2 (5.3) | – | – | 2 (2.5) |
| Headache | – | 2 (8.7) | – | 2 (2.5) |
| Fever | 11 (28.9) | 7 (30.4) | 1 (12.5) | 19 (24.1) |
| Shortness of breath (SOB) | 2 (5.3) | 2 (8.7) | 2 (25) | 6 (7.6) |
| Post-partum haemorrhage (PPH) | 5 (13.2) | 4 (17.4) | 1 (12.5) | 10 (12.7) |
| Overall occurrence, n (%) | 38 (27.5) | 23 (46) | 8 (47.1) | 79 (38.5) |
Neonatal outcome/complication
For neonatal outcome, Apgar score was used. Neonates mean (SE) Apgar score at 2 min was 5.7 (0.9) and 5.3 (0.2) for misoprostol- and oxytocin-treated cases, respectively, whereas it was 7.5 (0.1) and 7.3 (0.1), respectively, at 5 min (Table 10).
Table 10.
Details of Apgar score.
| Induction method | Mean Apgar score (SE) |
|---|---|
| Apgar score at 2 min | |
| Misoprostol | 5.7 (0.9) |
| Oxytocin | 5.3 (0.2) |
| Apgar score at 5 min | |
| Misoprostol | 7.5 (0.1) |
| Oxytocin | 7.3 (0.1) |
SE: standard error.
The occurrence and distribution of neonatal/foetal complication is presented in Table 11. Similarly, of the total effective population of 205, 130 (63.4%) had some foetal complications. Meconium stained liquor (MSL) was the most frequently encountered foetal complication in 64 (49.2%) patients followed by requirement of suction for resuscitation in 28 (21.6%) patients, baby unit admission in 27 (20.8%) patients, irregular foetal heart rate (FHR) in 9 (6.9%) patients and foetal bradycardia in 2 (1.5%) patients. Furthermore, Apgar score of <7 at 5 min was found in 11 (18.0%) and 5 (21.7%) misoprostol- and oxytocin-treated patients, respectively.
Table 11.
Overall occurrence and distribution of neonatal complications.
| Misoprostol only | Oxytocin only | Other | Total | |
|---|---|---|---|---|
| Foetal/neonatal complications, n (%) | ||||
| Irregular FHR | 7 (8.1) | 2 (5.7) | – | 9 (6.9) |
| Foetal bradycardia | 1 (1.2) | – | 1 (11.1) | 2 (1.5) |
| MSL | 42 (48.8) | 16 (45.7) | 6 (66.7) | 64 (49.2) |
| Suction/oxygen resuscitation | 18 (20.9) | 9 (25.7) | 1 (11.1) | 28 (21.5) |
| Baby unit admission | 18 (20.9) | 8 (22.9) | 1 (11.1) | 27 (20.8) |
| Overall occurrence, n (%) | 86 (62.3) | 35 (70) | 9 (52.9) | 130 (63.4) |
FHR: foetal heart rate; MSL: meconium stained liquor.
Discussion
There is a potential risk for the health of mother and infant if pregnancy continues beyond term and because of which IOL is desired.9,12 In a study conducted in Norway, it was found that IOL and post-term pregnancy are the prognostic factors for poor outcome.10 Even though routine IOL at 41 weeks of gestation is suggested to reduce perinatal mortality, induction is associated with other obstetric complications.13
It was seen that misoprostol was quite frequently used in this study. Misoprostol is safe, cost-effective and easy to administer and store because of which it has become a drug of choice in poor nations, and 25 µg intravaginal misoprostol has been included in the World Health Organization (WHO) complementary list as drug for IOL.14 The gestational age of the patient varied from 37 weeks to 43 weeks in our study which is similar to other studies.15,16
IOL is indicated for various reasons regarding maternal and foetal conditions. Post-term pregnancy was the most frequently encountered reason for induction in this study which is similar to the findings of other studies.9,10,17–19 Other indications found in our study were gestational hypertension, chronic hypertension, hypothyroidism, oligohydramnios, intrauterine growth restriction (IUGR), history of unexplained intrauterine foetal death, low Amniotic Fluid Index (AFI), Rh-negative mother, cholestasis of pregnancy, polyhydramnios, history of sub-fertility, previous history of stillbirth, maternal indications and other foetal indications. In a study by Goldberg and Wing,18 other indications were included such as decreased foetal movement at term and pregnancy-induced hypertension.
Kelly and Tan11 and Escudero and Contreras20 reported that oxytocin is an effective method of labour induction. In these studies, the time duration from initiation of induction to delivery was shorter in groups induced with oxytocin, and majority delivered within 24 h after intravenous oxytocin induction. In our study, the mean onset of action for oxytocin was found to be rapid than misoprostol. Furthermore, this study shows that there is not much difference in induction–delivery interval between two drugs. The induction–delivery interval in misoprostol group was similar to another study,18 whereas this differs from other studies where shorter induction–delivery interval was seen in misoprostol than oxytocin.21,22
The overall success rate of normal delivery and caesarean section was found to be 64.9% and 33.2%, respectively. Normal delivery in patients administered only by misoprostol was little higher (71.1%) than oxytocin (66%) group. According to different studies, the incidence of normal delivery was similar to this study.16,17 Most of the other studies,22–26 have found that caesarean section rate was significantly less in misoprostol than other methods for induction. A study reported that though more incidences of caesarean section were encountered with oxytocin, it appeared to be safe.11 However, another study reported that the incidence of caesarean section was similar in both oxytocin and misoprostol groups, no differences were observed between groups in perinatal and post-partum adverse outcomes and misoprostol use was considered safe.20 This incidence of caesarean was similar in both misoprostol (28.3%) and oxytocin groups (28%) in our study.
Heffner et al.15 reported that IOL, age of mother and gestational age over 40 weeks were some factors that increased the risk for caesarean delivery. As we studied different reasons for caesarean section, it was seen that the most common reason for caesarean was found to be foetal distress which is similar to a study.17 In another study, failed induction was found to be the second highest indication for caesarean like in this study.15
IOL is not free from unwanted effects. This study indicates that both misoprostol and oxytocin were associated with several complications. Overall, maternal morbidity resulting from misoprostol was found to be nausea/vomiting, diarrhoea, headache, fever, shortness of breath (SOB) and PPH with nausea/vomiting being the most common followed by fever. Several studies27,28 have reported uterine hyperstimulation and tachysystole with misoprostol, but in this study, no such cases were found. According to different studies, there is less risk of hyperstimulation with lower dose of misoprostol, but it also decreases the effectiveness for labour induction.27–29 The side effects found in this study is similar to another study conducted in Nepal16 except for fever, which was seen in only one case.
Regarding neonatal outcomes, the overall occurrence of MSL (49.2%) was found to be higher. Other complications seen were requirement of suction for resuscitation, baby unit admission, irregularity in FHR, foetal bradycardia and Apgar score of <7. Noted complications were similar to other studies.9,13 In this study, very less difference was seen in Apgar score between misoprostol and oxytocin group. The rate of neonates with Apgar score of < 7 at 2 and 5 min was higher than a study conducted by Heimstad et al.13
According to Chitrakar,23 a 25 µg intravaginal misoprostol reduces passes of meconium in foetus and is safe. A study by Hofmeyr and Gülmezoglu3 also suggests that even though administration of misoprostol increases the passes of meconium in the foetus, neonatal adverse effect is less even at higher doses. Many other studies have reported that there is an increase of risk for stillbirth and perinatal mortality after 41 weeks of gestational age.13,30–32 One study also reported maternal and neonatal death15 which was not seen in this study.
Thus, we see that the use of misoprostol and oxytocin during IOL is associated with maternal and foetal adverse effects, and we believe that it is the clinician’s judgement that determines the safety while minimizing the risks. So, any differences observed between the treatment regimens may also have been influenced by the decision process taken to determine which women underwent each regime. Similarly, this study was a single-centred study. Inclusion of multicentre data could have made the analysis much more representative.
Conclusion
It was found that misoprostol was the most frequently used drug for IOL as compared to oxytocin. The induction–delivery interval and onset of labour were observed, which showed that there is not much difference in induction-to-delivery interval within those drugs, whereas the onset of labour was found to be rapid in oxytocin than misoprostol. However, the occurrence of side effects was found to be similar in both misoprostol and oxytocin groups.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval was granted by the Institutional Review Committee of Paropakar Maternity and Women’s Hospital to conduct the study. Hospital Institutional Review committee ethical approval number: 54-11ka-1736.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Informed verbal consent was taken from the patients.
References
- 1. World Health Organization. WHO recommendations for induction of labour, http://apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf
- 2. Wing DA. Induction of labor. In: Queenan JT, Hobbins JC, Spong CY. (eds) Protocols for high-risk pregnancies. Hoboken, NJ: Wiley-Blackwell, 2010, pp. 140–147. [Google Scholar]
- 3. Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev 2003; (1): CD000941. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Preparations WECoSfP – quality assurance of pharmaceuticals: meeting a major public health challenge. 2014, http://apps.who.int/medicinedocs/documents/s21390en/s21390en.pdf
- 5. Gülmezoglu AM, Crowther CA, Middleton P, et al. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2012; (6): CD004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly AJ, Malik S, Smith L, et al. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database Syst Rev 2009; (4): CD003101. [DOI] [PubMed] [Google Scholar]
- 7. Royal College of Obstetricians and Gynaecologists. Induction of labour: evidence-based clinical guideline number 9. 2001, http://www.perinatal.sld.cu/docs/guiasclinicas/inductionoflabour.pdf
- 8. Tang O, Gemzell-Danielsson K, Ho P. Misoprostol: pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynecol Obstet 2007; 99: S160–S167. [DOI] [PubMed] [Google Scholar]
- 9. Daniel-Spiegel E, Weiner Z, Ben-Shlomo I, et al. For how long should oxytocin be continued during induction of labour? BJOG 2004; 111(4): 331–334. [DOI] [PubMed] [Google Scholar]
- 10. Heimstad R, Romundstad PR, Eik-Nes SH, et al. Outcomes of pregnancy beyond 37 weeks of gestation. Obstet Gynecol 2006; 108(3 Pt 1): 500–508. [DOI] [PubMed] [Google Scholar]
- 11. Kelly AJ, Tan B. Intravenous oxytocin alone for cervical ripening and induction of labour. Birth 2001; 28(4): 280–281. [DOI] [PubMed] [Google Scholar]
- 12. Gülmezoglu AM, Crowther CA, Middleton P. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database Syst Rev 2006; (4): CD004945. [DOI] [PubMed] [Google Scholar]
- 13. Heimstad R, Skogvoll E, Mattsson L-Å, et al. Induction of labor or serial antenatal fetal monitoring in postterm pregnancy: a randomized controlled trial. Obstet Gynecol 2007; 109(3): 609–617. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. The selection and use of essential medicines: report of the WHO Expert Committee, 2005 (including the 14th model list of essential medicines). 2006, http://apps.who.int/iris/bitstream/10665/43292/1/WHO_TRS_933_eng.pdf [PubMed]
- 15. Heffner LJ, Elkin E, Fretts RC. Impact of labor induction, gestational age, and maternal age on cesarean delivery rates. Obstet Gynecol 2003; 102(2): 287–293. [DOI] [PubMed] [Google Scholar]
- 16. Dongol A, Shakya S, Chawla C. Safety and efficacy of misoprostol for induction of labour. J Nepal Health Res Counc 2010; 8(1): 27–30. [PubMed] [Google Scholar]
- 17. Prager M, Eneroth-Grimfors E, Edlund M, et al. A randomised controlled trial of intravaginal dinoprostone, intravaginal misoprostol and transcervical balloon catheter for labour induction. BJOG 2008; 115(11): 1443–1450. [DOI] [PubMed] [Google Scholar]
- 18. Goldberg AB, Wing DA. Induction of labor: the misoprostol controversy. J Midwifery Womens Health 2003; 48(4): 244–248. [DOI] [PubMed] [Google Scholar]
- 19. Yawn BP, Wollan P, McKeon K, et al. Temporal changes in rates and reasons for medical induction of term labor, 1980–1996. Am J Obstet Gynecol 2001; 184(4): 611–619. [DOI] [PubMed] [Google Scholar]
- 20. Escudero F, Contreras H. A comparative trial of labor induction with misoprostol versus oxytocin. Int J Gynecol Obstet 1997; 57(2): 139–143. [DOI] [PubMed] [Google Scholar]
- 21. Kramer RL, Gilson GJ, Morrison DS, et al. A randomized trial of misoprostol and oxytocin for induction of labor: safety and efficacy. Obstet Gynecol 1997; 89(3): 387–391. [DOI] [PubMed] [Google Scholar]
- 22. Hofmeyr G, Gülmezoglu A, Alfirevic Z. Misoprostol for induction of labour: a systematic review. BJOG 1999; 106(8): 798–803. [DOI] [PubMed] [Google Scholar]
- 23. Chitrakar NS. Comparison of misoprostol versus dinoprostone for pre-induction cervical ripening at-term. J Nepal Health Res Counc 2012; 10: 10–15. [PubMed] [Google Scholar]
- 24. Sanchez-Ramos L, Kaunitz AM, Wears RL, et al. Misoprostol for cervical ripening and labor induction: a meta-analysis. Obstet Gynecol 1997; 89(4): 633–642. [DOI] [PubMed] [Google Scholar]
- 25. Indiana Perinatal Network. Levels of hospital perinatal care in Indiana (retrieved from the Indiana Section of ACOG and AAP, Indiana Chapter). 2008, https://c.ymcdn.com/sites/www.indianaperinatal.org/resource/resmgr/policy_makers/locfinal1.6.12.pdf
- 26. Hofmeyr G, Gülmezoglu A. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy. Cochrane Database Syst Rev 2000; (2): CD000941. [DOI] [PubMed] [Google Scholar]
- 27. Dällenbach P, Boulvain M, Viardot C, et al. Oral misoprostol or vaginal dinoprostone for labor induction: a randomized controlled trial. Am J Obstet Gynecol 2003; 188(1): 162–167. [DOI] [PubMed] [Google Scholar]
- 28. Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol with vaginally administered misoprostol for cervical ripening and labor induction. Am J Obstet Gynecol 1999; 180(5): 1155–1160. [DOI] [PubMed] [Google Scholar]
- 29. Shakya R, Shrestha J, Thapa P. Safety and efficacy of misoprostol and dinoprostone as cervical ripening agents. JNMA J Nepal Med Assoc 2010; 49(177): 33–37. [PubMed] [Google Scholar]
- 30. Ingemarsson I, Källén K. Stillbirths and rate of neonatal deaths in 76,761 postterm pregnancies in Sweden, 1982–1991: a register study. Acta Obstet Gynecol Scand 1997; 76(7): 658–662. [DOI] [PubMed] [Google Scholar]
- 31. Hilder L, Costeloe K, Thilaganathan B, et al. Prolonged pregnancy: evaluating gestation-specific risks of fetal and infant mortality. BJOG 1998; 105(2): 169–173. [DOI] [PubMed] [Google Scholar]
- 32. Divon MY, Haglund B, Nisell H, et al. Fetal and neonatal mortality in the postterm pregnancy: the impact of gestational age and fetal growth restriction. Am J Obstet Gynecol 1998; 178(4): 726–731. [DOI] [PubMed] [Google Scholar]


