Abstract
Neonatal respiratory distress syndrome due to surfactant deficiency is associated with high morbidity and mortality in preterm infants, and the use of less invasive surfactant administration (LISA) has been increasingly studied. This meta-analysis found that LISA via thin catheter significantly reduced the need for mechanical ventilation within the first 72 hours (relative risk [RR] = 0.677; P = .021), duration of mechanical ventilation (difference in means [MD] = −39.302 hours; P < .001), duration of supplemental oxygen (MD = −68.874 hours; P < .001), and duration of nasal continuous positive airway pressure (nCPAP; MD = −28.423 hours; P = .010). A trend toward a reduction in the incidence of bronchopulmonary dysplasia was observed (RR = 0.656; P = .141). No significant difference in overall mortality, incidence of pneumothorax, or successful first attempts was observed. LISA via thin catheter significantly reduces the need for mechanical ventilation within the first 72 hours as well as the duration of mechanical ventilation, supplemental oxygen, and nCPAP. LISA via thin catheter appears promising in improving preterm infant outcomes.
Keywords: surfactant, less invasive surfactant administration, minimally invasive, thin catheter, preterm, neonatal respiratory distress syndrome, bronchopulmonary dysplasia, meta-analysis
Introduction
Neonatal respiratory distress syndrome (RDS) due to surfactant deficiency is associated with high morbidity and mortality in preterm infants.1,2 Two thirds of preterm infants born prior to 33 weeks’ gestational age develop RDS after birth and require surfactant therapy.2 Surfactant administration traditionally involved endotracheal intubation and mechanical ventilation, which is associated with a risk of barotrauma and volutrauma.1,3,4 Activation of the complex inflammatory cascade typically ensues in these infants with mechanical ventilation, which increases their risk for developing bronchopulmonary dysplasia (BPD).1,3,4 In an effort to minimize mechanical lung damage, alternative approaches to surfactant delivery have been developed utilizing noninvasive ventilatory techniques, such as early nasal continuous positive airway pressure (CPAP).2,3,5 The COIN trial and SUPPORT trial demonstrated that early nasal CPAP (nCPAP) is a safe and efficacious alternative to intubation and prophylactic surfactant administration. In an effort to exploit the benefits of early surfactant administration while minimizing mechanical ventilation complications, Victorin et al introduced an approach involving intubation, surfactant administration during brief mechanical ventilation, and extubation, known as the INSURE technique.6 Furthermore, endotracheal intubation in neonates is associated with complications including neonatal desaturation, tracheal injury, and perforation.7,8
Attempts to achieve surfactant delivery while avoiding the need for intubation for even a brief period of time have been further studied using less invasive surfactant administration (LISA), also known as minimally invasive surfactant therapy (MIST).2,3,9 Various techniques including intratracheal surfactant installation with the use of a thin catheter, aerosolized or nebulized administration, pharyngeal administration, and laryngeal mask airway–guided administration have been evaluated.2,3 While all of these techniques administer surfactant while maintaining spontaneous breathing, the most commonly utilized technique involves the use of a thin catheter.2,3
At present, the existing literature regarding LISA via thin catheter techniques among preterm infants is limited.10-13 This current meta-analysis provides a comprehensive review of the use of LISA via thin catheter in preterm infants. Given the risk of barotrauma and volutrauma associated with endotracheal intubation as well as the high morbidity and mortality of BPD, LISA via thin catheter may have a prominent role in the future care of preterm infants with respiratory distress requiring surfactant administration.
Materials and Methods
Study Selection
A comprehensive literature search for all published randomized control trials (RCTs) evaluating LISA via thin catheter was conducted using PubMed, Cochrane Central Registry of Controlled Trials, and Google Scholar (from time of inception to 2016). Using the yielded results from these searches, additional searches were conducted. The last literature search was conducted on July 5, 2016. Searches were conducted using the keywords “less invasive,” “LISA,” “minimally invasive,” “MIST,” “surfactant administration,” “thin catheter,” and “preterm.” The following inclusion criteria were utilized: RCTs comparing the use of LISA via thin catheter with surfactant administration via the INSURE technique in preterm infants (<34 weeks gestational age) with signs of respiratory distress and requiring rescue surfactant administration within 2 hours of birth. In case of duplicate publications, only the most recent and updated report of the clinical trial was included. When necessary, the authors of the original trials were contacted for clarification of outcome data.
Data Extraction
Articles retrieved from the searches were assessed for eligibility, and data relating to patients, LISA thin catheter and INSURE groups, clinical outcomes, and study methodology were extracted (Figure 1). The outcomes analyzed were the number of patients requiring mechanical ventilation within the first 72 hours, duration of mechanical ventilation, duration of oxygen supplementation, duration of nCPAP, overall mortality (prior to discharge), successful first attempts, and the incidence of various complications including pneumothorax and BPD (as defined by the trial).
Figure 1.

CONSORT diagram detailing the study selection process.
Statistical Analysis
For each study included in this meta-analysis, relative risk (RR) with a 95% confidence interval (CI) was calculated for the number of patients requiring mechanical ventilation, overall mortality, successful first attempts, and the incidence of pneumothorax and BPD. For continuous data, including duration of mechanical ventilation, duration of oxygen supplementation, and duration of nCPAP, difference in means (MD) and 95% CI were calculated. When a study reported a zero incidence in either the LISA or INSURE group, a continuity correction factor of 0.5 was applied and used to calculate the RR and variance. The decision to use either the fixed-effects model or random-effects model was made depending on the heterogeneity of the included studies. To assess for this heterogeneity, Cochrane’s Q statistic and I2 statistic was used and P < .05 or I2 > 50 was set as the cutoff for significance. Data were analyzed using a random-effects model when heterogeneity was considered significant, while a fixed-effects model was utilized in the absence of heterogeneity. The Cochrane Collaboration Risk of Bias tool was used to evaluate for risk of bias, and the Jadad score was used to assess the quality of the included studies.14 Each included study was assessed for randomization, blinding, and indication of patient dropouts and withdrawal, allowing for a Jadad score between 0 and 5.14 Publication bias regarding the RR of number of patients requiring mechanical ventilation was assessed visually with a funnel plot, and then quantified using Egger’s and Begg’s tests. Statistical significance was accepted at a level of P < .05 (2-tailed). Comprehensive Meta-Analysis software Version 3 (Biostat, Englewood, NJ) was used to conduct all meta-analysis of pooled study data.
Results
Demographic Characteristics of the Studies
A total of 3 RCTs meeting the inclusion criteria were identified, involving 328 infants (Table 1).11-13 A total of 166 infants received surfactant via thin catheter, and 162 infants received surfactant via INSURE.
Table 1.
Characteristics of All Published Randomized Control Trials Evaluating the Use of Less Invasive Surfactant Administration via Thin Catheter in Preterm Infants With Respiratory Distress Requiring Surfactant Administration (1966-2016).
| Kanmaz et al (2013)13 | Mohammadizadeh et al (2015)11 | Bao et al (2015)12 | |
|---|---|---|---|
| Total number of infants (# LISA, # ETT) | 200 (100, 100) | 38 (19, 19) | 90 (47, 43) |
| Inclusion criteria | <32 weeks, with signs of RDS (FiO2 ≥0.4 to maintain SpO2 85-92%) | ≤34 weeks; 1000-1,800 g, with signs of RDS (FiO2 >30% to maintain SpO2 87-92%) | 28 weeks to 32 weeks GA, with RDS (based on clinical diagnosis confirmed by radiological imaging) |
| Country | Turkey | Iran | China |
| Mean age of infants in weeks (LISA, ETT) | 28 ± 2, 28.3 ± 2 | 30 ± 2, 31 ± 2 | 29.1 ± 1.5, 29.3 ± 1.6 |
| Mean weight of infants in grams (LISA, ETT) | 1093 ± 270, 1121 ± 270 | 1289 ± 219, 1428 ± 272 | 1034 ± 221, 1087 ± 198 |
| APGAR scores (mean) | |||
| 1-minute | NR | NR | 6.4, 6.3 |
| 5-minute | 7, 7 | NR | 8.7, 8.8 |
| 10-minute | NR | NR | NR |
| Gender, % (LISA, ETT) | |||
| Male | 60%, 50% | 52.6%, 57.9% | 59.6%, 60.4% |
| Female | 40%, 50% | 47.4%, 42.1% | 40.4%, 39.6% |
| Cesarean births, % (LISA, ETT) | 75%, 83% | 100%, 89.5% | 74.5%, 76.7% |
| Birth, % (LISA, ETT) | |||
| Single | NR | 47.4%, 31.6% | 44.4%, 44.2% |
| Multiples | NR | 52.6%, 68.4% | 65.6%, 65.8% |
| Antenatal corticosteroids, % (LISA, ETT) | 73%, 81% | 84.2%, 89.5% | 89.4%, 93.0% |
| LISA thin catheter | 5-F flexible, sterile nasogastric tube | 4-F end hole feeding tube | 16 gauge, 130-mm vascular catheter (16G Angiocath, BD, Sandy, UT) |
| Control group | Orally intubated with double-lumen endotracheal tube, surfactant administered, promptly extubated, recommence nCPAP | Orally intubated with 2.5-F or 3-F endotracheal tube, surfactant administered, CPAP for at least one minute or until SpO2 >87%, promptly extubated, recommenced on nCPAP | Orally intubated, brief mechanical ventilation, surfactant administered, extubated as soon as clinically possible, switched to nCPAP (entire process took about 3 minutes) |
| Definition of BPD | National Institutes of Child Health and Development diagnostic criteria | NR | National Institutes of Child Health and Development diagnostic criteria |
| Threshold for intubation and mechanical ventilation | 1. Sustained CPAP pressure beyond 7 cm H2O along with a FiO2 of 0.6 | 1. FiO2 ≥70% for >2 hours or >40% for more than 12 hours to maintain SpO2 ≥87% | 1. FiO2 >0.6 |
| 2. pH <7.2 | 2. pH <7.2 or PaCO2 >65 mm Hg | 2. pH <7.2 | |
Abbreviations: APGAR, Appearance, Pulse, Grimace, Activity, Respiration score; BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; ETT, endotracheal intubation; FiO2, fraction of inspired oxygen; GA, gestational age; LISA, less invasive surfactant administration; nCPAP, nasal continuous positive airway pressure; NR, not reported; PaO2, arterial partial pressure of oxygen; RDS, respiratory distress syndrome; SpO2, oxygen saturation.
Risk of Bias Among Included Studies
The risk of bias assessment among the included studies was assessed and all included studies had low to moderate risk of bias (Supplementary Table S1, available online at http://gph.sagepub.com/supplemental). Most bias was attributed to lack of blinding of the participants and researchers (performance bias) as well as during the outcome assessment (detection bias).
Effects of LISA via Thin Catheter on the Need for Mechanical Ventilation
Data on the number of infants requiring mechanical ventilation within the first 72 hours in both the LISA thin catheter and INSURE groups were reported in all trials. Fewer infants in the thin catheter group required mechanical ventilation within the first 72 hours, compared to the control group who received surfactant via INSURE (40/166 [24.1%] vs 58/162 [35.8%]). There was no significant heterogeneity between trials (P = .980, I2 < 0.001), and a fixed-effects model was assumed. Meta-analysis revealed a 32.3% reduction in the need for mechanical ventilation within the first 72 hours with the use of thin catheter compared to INSURE (RR = 0.677; 95% CI = 0.486-0.942; P = .021; Figure 2).
Figure 2.
Forest plot evaluating the relative risk of mechanical ventilation requirement (within the first 72 hours of life) associated with less invasive surfactant administration via thin catheter in preterm infants with respiratory distress requiring surfactant administration.
Effects of LISA via Thin Catheter on the Duration of Mechanical Ventilation Required
The duration of mechanical ventilation required was reported in all trials. Meta-analysis showed a significant reduction in the duration of mechanical ventilation required with the use of thin catheter (MD = −39.302 hours; 95% CI = −60.513 to −18.090; P < .001) compared to INSURE.
Effects of LISA via Thin Catheter on the Need for Supplemental Oxygen
The duration of supplemental oxygen required was reported in all trials. Meta-analysis showed a significant reduction in the need for supplemental oxygen with the use of thin catheter (MD = −68.874 hours; 95% CI = −95.783 to −41.965; P < .001) compared to INSURE.
Effects of LISA via Thin Catheter on the Need for nCPAP
The duration of supplemental oxygen required was reported in all trials. Meta-analysis showed a significant reduction in the need for nCPAP with the use of thin catheter (MD = −28.423; 95% CI = −50.116 to −6.730; P = .010) compared to INSURE.
Effects of LISA via Thin Catheter on Bronchopulmonary Dysplasia
Data on the incidence of BPD was reported in all trials involving. Fewer infants in the thin catheter group developed BPD as compared to the INSURE group (18/166 [10.8%] vs 27/162 [16.7%]). Meta-analysis showed a significant 34.4% reduction in the risk of BPD with the use of thin catheter compared to INSURE (RR = 0.656; 95% CI = 0.375-1.149; P = .141), although results failed to reach statistical significance (Figure 3).
Figure 3.
Forest plot evaluating the relative risk of bronchopulmonary dysplasia associated with less invasive surfactant administration via thin catheter in preterm infants with respiratory distress requiring surfactant administration.
Effects of LISA via Thin Catheter on Mortality
Data on mortality were reported in all trials. Meta-analysis showed no significant difference in the risk of mortality between the thin catheter and INSURE groups (RR = 1.137; 95% CI = 0.603-2.143; P = .691; Figure 4).
Figure 4.
Forest plot evaluating the relative risk of mortality associated with less invasive surfactant administration via thin catheter in preterm infants with respiratory distress requiring surfactant administration.
Effects of LISA via Thin Catheter on Incidence of Pneumothorax
The incidence of pneumothorax was reported in 2 trials involving 290 infants (147 infants with thin catheter and 143 infants with INSURE). Fewer infants in the thin catheter group developed a pneumothorax compared to the INSURE group (11/147 [7.5%] vs 13/143 [9.1%]). Meta-analysis showed a 17.6% reduction in the risk of pneumothorax (RR = 0.824; 95% CI = 0.378-1.793; P = .625); however, the difference failed to reach statistical significance.
Effects of LISA via Thin Catheter on Successful First Attempts
The incidence of successful first attempt at catheterization or intubation was reported in 2 trials involving 128 infants (66 infants with thin catheter and 62 infants with INSURE). There was a greater proportion of successful first attempts with the use of thin catheter compared to INSURE (57/66 [86.4%] vs 46/62 [74.2%]). Meta-analysis showed no significant difference in first attempt success between the thin catheter and INSURE groups (RR = 1.079; 95% CI = 0.929-1.252; P = .321).
Quality of the Evidence Assessment
Quality of the evidence was assessed using the Jadad scale (Supplementary Table S2, available online at http://gph.sagepub.com/supplemental). Two of the studies had a Jadad score of ≥3, while one of the studies had a Jadad score of 2.
Publication Bias
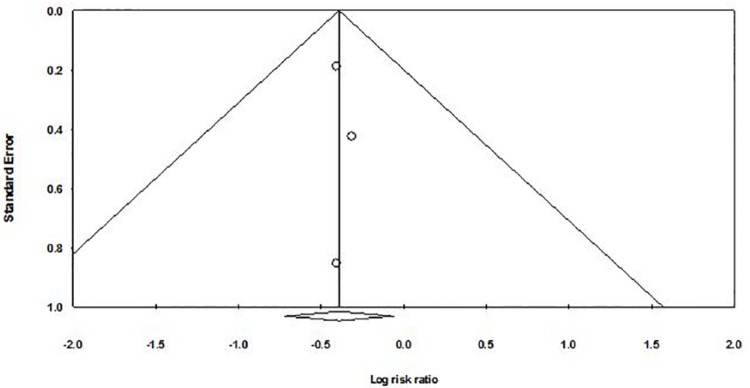
A funnel plot was used to qualitatively assess for publication bias, and Egger’s and Begg’s tests were done to calculate publication bias. There was no obvious evidence of asymmetry on the funnel plot (Figure 5). Furthermore, there was no evidence of publication bias for the primary end point of this study (the need for mechanical ventilation within the first 72 hours with LISA via thin catheter) by either Egger’s (P = .664) or Begg’s test (P = .602).
Figure 5.
Funnel plot assessing publication bias (analyzing the effect of less invasive surfactant administration via thin catheter on the need for mechanical ventilation within the first 72 hours in preterm infants with respiratory distress requiring surfactant administration).
Discussion
BPD remains the most common complication in preterm infants affecting over a third of infants born <1250 g.4 Despite surfactant use and technological advances allowing premature infants to survive longer, the incidence of BPD remains high.3 In recent years, the use of noninvasive respiratory support in BPD infants has significantly improved outcomes for premature patients.15 Stroustrup et al conducted a retrospective study involving 9 542 032 patients from the Nationwide Inpatient State database and reported a significant reduction in the incidence of BPD by 3.3% annually between 1993 and 2006 (P = .0009), which coincided with a 3.5-fold increase (from 6.4% in 1993 to 22.6% in 2006%; P < .0001) in the use of noninvasive respiratory support, such as CPAP, in patients with BPD.15
The use of less invasive approaches without the need for intubation has been increasingly studied. The use of a thin catheter has been the most investigated technique, with others including aerosolized or nebulized surfactant, administration via laryngeal mask, and nasopharyngeal administration.2,3,5 Aerosolized surfactant administration was first studied in a feasibility study by Jorch et al, who demonstrated improvements in ventilation and oxygenation among the 20 infants with RDS treated with aerosolized surfactant.16 Dijk et al studied 9 infants and reported efficacy rates of <10% with surfactant nebulization and noted that most aerosolized surfactant was expired and did not reach the lungs.17 More recent RCTs have reported minimal benefits at best.18,19 Berggren et al conducted an RCT involving 32 infants (16 treated with nebulized surfactant and 16 who did not receive surfactant) and reported no significant difference in the requirement of mechanical ventilation or the incidence of BPD (P > .05).20 Minocchieri et al conducted an RCT involving 9 infants and reported a 43.7% reduction in the need for mechanical ventilation in the first 72 hours (RR = 0.563; 95% CI = 0.341-0.929; P = .032) with the use of nebulized surfactant and CPAP compared to CPAP alone.21
Surfactant administration using a laryngeal mask, first attempted by Trevisanuto et al in 2005 has shown to be of limited benefit.22 Attridge et al conducted the lone RCT involving 26 infants (13 infants with laryngeal mask and CPAP and 13 infants with CPAP alone) and reported a reduction in the mean fraction of inspired oxygen requirement for the first 12 hours following intervention with the use of laryngeal mask (mean FiO2 0.27 vs 0.40, P = .04) but no difference in the need for mechanical ventilation (8% vs 23%, P = .59).23 Efficacy rates of <10% have been attributed to the large particle size of the surfactant and the small airways of preterm neonates.16-18,20,21
Nasopharyngeal surfactant administration was first studied by the Ten Centre Study Group involving 328 infants (159 infants with nasopharyngeal surfactant, 149 infants without surfactant) and reported an average of 20 hours less ventilation among infants receiving the surfactant and a significant reduction in mortality (19% vs 30%, P < .01).24 Kattwinkel et al conducted a feasibility study involving 23 neonates treated with nasopharyngeal surfactant and reported that 13 of the 15 infants delivered vaginally and 3 of the 8 neonates born via Cesarean section were weaned to room air relatively quickly without requiring further surfactant or endotracheal intubation.25 The amount of surfactant that was actually delivered to the trachea remains uncertain.25
Surfactant administration with the use of a thin catheter, first described by Verder et al in 1992, has been the most studied less invasive technique.26 During this procedure, a laryngoscope is used to visualize the glottis, and a thin catheter is inserted into the trachea. The laryngoscope is removed, and the surfactant is injected into the trachea through the thin catheter.3 Kribs et al conducted the first observational study involving 42 infants, evaluating surfactant administration using a thin, flexible intratracheal catheter and reported a 12% mortality compared to historical trends of 35% and concluded that thin catheter was a feasible approach.27 Further RCTs using this method of a thin flexible catheter (diameter of 2.5-5 F) have demonstrated reductions in the need for mechanical ventilation.13,28 Dargaville et al attempted a modified approach with a 16-gauge semirigid vascular catheter, and reported similar results, with reductions in the need for mechanical ventilation within the first 72 hours among infants 25 to 28 weeks gestational age (32% vs 68% historical controls, P = .0011) and infants 29 to 34 weeks gestational age (0% vs 15% historical controls, P = .057).29
Early studies involving LISA via thin catheter have demonstrated equivalent efficacy to the INSURE technique. Aguar et al conducted a cohort study involving 44 preterm infants with RDS receiving surfactant via a gastric tube placed in the trachea and reported no significant difference in the need for mechanical ventilation (25% vs 33%, P = .44), duration of required mechanical ventilation (115 hours vs 150 hours, P > .05) or oxygen supplementation (102 hours vs 117 hours, P > .05), BPD (4.5% vs 6.5%, P > .05), neonatal intensive care length of stay (20 day vs 22 days, P > .05), or mortality (4.5% vs 3.2%, P > .05), compared to historical trends using the INSURE technique.30 In contrast, Krajewski et al conducted a cohort study involving 26 preterm infants with RDS receiving surfactant via thin catheter and reported significant reductions in the need for mechanical ventilation (19.2% vs 65%, P < .05) and BPD (15.4% vs 40%, P < .05) compared to historical results with the INSURE technique.31
The current meta-analysis found that LISA via thin catheter is associated with a significant reduction in the need for mechanical ventilation within the first 72 hours and the duration of mechanical ventilation, supplemental oxygen, and nCPAP required, with no significant difference in overall mortality. A trend toward a reduction in the incidence of BPD was observed, but failed to reach statistical significance likely due to inadequate sample size. Although there is no difference in overall mortality, LISA via thin catheter appears safe to use, with no significant difference in the risk of complications compared to the INSURE technique. Fear of failure with the use of thin catheter has also been raised; however, LISA via thin catheter is associated with similar rates of successful first attempts.10-12
Despite the lack of side effects associated with the use of thin catheter for surfactant administration, this technique is not without its own disadvantages. Despite being considered minimally invasive and not requiring intubation, this procedure still involves the use of a laryngoscope to visualize the vocal cords in a relatively awake infant, which can be difficult and traumatic, especially among untrained individuals.3 Mastering these skills to successfully administer surfactant to the infant while maintaining a child’s comfort and avoiding the need for sedatives and analgesics remains a significant clinical challenge.3
The precise amount of surfactant that should optimally be deposited and determining the amount of surfactant reaching the lungs with the use of LISA via thin catheter remains uncertain. Although no study has investigated this in a human model to date, Niemarkt et al investigated the effects of LISA via thin catheter on oxygenation and pulmonary surfactant distribution in preterm lambs.32 Lambs receiving surfactant via thin catheter had similar PaO2 values compared to the intubated lambs, but significantly higher PaO2 values than the lambs receiving only CPAP.32 The amount of surfactant found in the lungs of the lambs receiving surfactant via thin catheter was only 17.4% compared to the amount of surfactant found in the lungs of the lambs that were intubated (P < .05).32 Lambs receiving surfactant via thin catheter experienced improved oxygenation, similar to conventional surfactant techniques, despite lower surfactant deposition (P < .05) and lung compliance (P = .23).32
Although the results of this meta-analysis are significant, there are limitations to this study due to the variation and heterogeneity of the RCTs. The enrollment criteria used in each study differed with regard to gestational age and birth weights, which are known risk factors of BPD. Most existing studies have included infants <34 weeks or <32 weeks gestational age, and no study included infants <28 weeks who are at highest risk of developing BPD. Additional randomized placebo-control trials studying the safety and efficacy of LISA via thin catheter in this unique population are required. The type and diameter of catheter varied within each study as well as between studies, which may also affect the efficacy and success rates. The specific protocol utilized in each RCT also varied. The definition of BPD varied slightly between studies. BPD was defined according to the individual studies. While some studies were nonspecific in defining BPD, Kanmaz et al11 and Bao et al12 specifically referenced literature and defined BPD according to the National Institute of Child Health and Development diagnostic criteria. Furthermore, the threshold for CPAP failure varied between studies. The studies ranged from a FiO2 requirement of >0.611,12 to >0.7 for >2 hours or >0.4 for >12 hours.13 The optimal type and dose of surfactant required is usually unclear and requires further investigation. With a trend toward noninvasive ventilator techniques, studies comparing the use of LISA via thin catheter with nasal CPAP are required.
Despite these limitations, this study found that LISA via thin catheter is a valuable technique in preterm infants. Given the often grim prognosis of these infants, the significant reduction in the need for mechanical ventilation, duration of mechanical ventilation, supplemental oxygen, and nCPAP, and the apparent lack of significant adverse side effects and relative ease of administration, implies that LISA via thin catheter may have a role in the future care of preterm infants requiring surfactant administration. LISA via thin catheter appears promising in improving preterm infant outcomes, but additional RCTs are required.
Author Contributions
CL: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
RC: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SS: Contributed to conception and design; contributed to acquisition, analysis, and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Isayama T, Chai-Adisaksopha C, McDonald SD. Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 2015;169:731-739. [DOI] [PubMed] [Google Scholar]
- 3. More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: a meta-narrative review. JAMA Pediatr. 2014;168:901-908. [DOI] [PubMed] [Google Scholar]
- 4. Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305-1311. [DOI] [PubMed] [Google Scholar]
- 5. Lista G, La VA, Castoldi F. LISA: surfactant administration in spontaneous breathing. Which evidence from the literature? Acta Biomed. 2015;86(suppl 1):24-26. [PubMed] [Google Scholar]
- 6. Victorin LH, Deverajan LV, Curstedt T, Robertson B. Surfactant replacement in spontaneously breathing babies with hyaline membrane disease—a pilot study. Biol Neonate. 1990;58:121-126. [DOI] [PubMed] [Google Scholar]
- 7. Lagoo JY, Jose J, Kilpadi KA. Tracheal perforation in a neonate: a devastating complication following traumatic endotracheal intubation. Indian J Anaesth. 2013;57:623-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Endotracheal intubation attempts during neonatal resuscitation: success rates, duration, and adverse effects. Pediatrics. 2006;117:e16-e21. [DOI] [PubMed] [Google Scholar]
- 9. Aguar M, Nunez A, Cubells E, Cernada M, Dargaville PA, Vento M. Administration of surfactant using less invasive techniques as a part of a non-aggressive paradigm towards preterm infants. Early Hum Dev. 2014;90(suppl 2):S57-S59. [DOI] [PubMed] [Google Scholar]
- 10. Kribs A, Roll C, Gopel W, et al. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2015;169:723-730. [DOI] [PubMed] [Google Scholar]
- 11. Mohammadizadeh M, Ardestani AG, Sadeghnia AR. Early administration of surfactant via a thin intratracheal catheter in preterm infants with respiratory distress syndrome: feasibility and outcome. J Res Pharm Pract. 2015;4:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao Y, Zhang G, Wu M, Ma L, Zhu J. A pilot study of less invasive surfactant administration in very preterm infants in a Chinese tertiary center. BMC Pediatr. 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013;131:e502-e509. [DOI] [PubMed] [Google Scholar]
- 14. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 15. Stroustrup A, Trasande L. Epidemiological characteristics and resource use in neonates with bronchopulmonary dysplasia: 1993-2006. Pediatrics. 2010;126:291-297. [DOI] [PubMed] [Google Scholar]
- 16. Jorch G, Hartl H, Roth B, et al. Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr Pulmonol. 1997;24:222-224. [DOI] [PubMed] [Google Scholar]
- 17. Dijk PH, Heikamp A, Piers DA, Weller E, Bambang OS. Surfactant nebulisation: safety, efficiency and influence on surface lowering properties and biochemical composition. Intensive Care Med. 1997;23:456-462. [DOI] [PubMed] [Google Scholar]
- 18. Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arroe MP, Albertsen P, Bode S, Greisen G, Jonsbo F. Inhalation of aerosolized surfactant (Exosurf) to neonates treated with nasal continuous positive airway pressure. Prenat Neonatal Med. 1998;3:346-352. [Google Scholar]
- 20. Berggren E, Liljedahl M, Winbladh B, et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr. 2000;89:460-464. [DOI] [PubMed] [Google Scholar]
- 21. Minocchieri S, Knoch S, Schoel WM, Ochs M, Nelle M. Nebulizing poractant alfa versus conventional instillation: ultrastructural appearance and preservation of surface activity. Pediatr Pulmonol. 2014;49:348-356. [DOI] [PubMed] [Google Scholar]
- 22. Trevisanuto D, Grazzina N, Ferrarese P, Micaglio M, Verghese C, Zanardo V. Laryngeal mask airway used as a delivery conduit for the administration of surfactant to preterm infants with respiratory distress syndrome. Biol Neonate. 2005;87:217-220. [DOI] [PubMed] [Google Scholar]
- 23. Attridge JT, Stewart C, Stukenborg GJ, Kattwinkel J. Administration of rescue surfactant by laryngeal mask airway: lessons from a pilot trial. Am J Perinatol. 2013;30:201-206. [DOI] [PubMed] [Google Scholar]
- 24. Ten centre trial of artificial surfactant (artificial lung expanding compound) in very premature babies. Ten Centre Study Group. Br Med J (Clin Res Ed). 1987;294:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kattwinkel J, Robinson M, Bloom BT, Delmore P, Ferguson JE. Technique for intrapartum administration of surfactant without requirement for an endotracheal tube. J Perinatol. 2004;24:360-365. [DOI] [PubMed] [Google Scholar]
- 26. Verder H, Agertoft L, Albertsen P, et al. Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study [in Danish]. Ugeskr Laeger. 1992;154:2136-2139. [PubMed] [Google Scholar]
- 27. Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks). Paediatr Anaesth. 2007;17:364-369. [DOI] [PubMed] [Google Scholar]
- 28. Gopel W, Kribs A, Ziegler A, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378:1627-1634. [DOI] [PubMed] [Google Scholar]
- 29. Dargaville PA, Aiyappan A, De Paoli AG, et al. Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed. 2013;98:F122-F126. [DOI] [PubMed] [Google Scholar]
- 30. Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr. 2014;103:e229-e233. [DOI] [PubMed] [Google Scholar]
- 31. Krajewski P, Chudzik A, Strzalko-Gloskowska B, et al. Surfactant administration without intubation in preterm infants with respiratory distress syndrome—our experiences. J Matern Fetal Neonatal Med. 2015;28:1161-1164. [DOI] [PubMed] [Google Scholar]
- 32. Niemarkt HJ, Kuypers E, Jellema R, et al. Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res. 2014;76:166-170. [DOI] [PubMed] [Google Scholar]