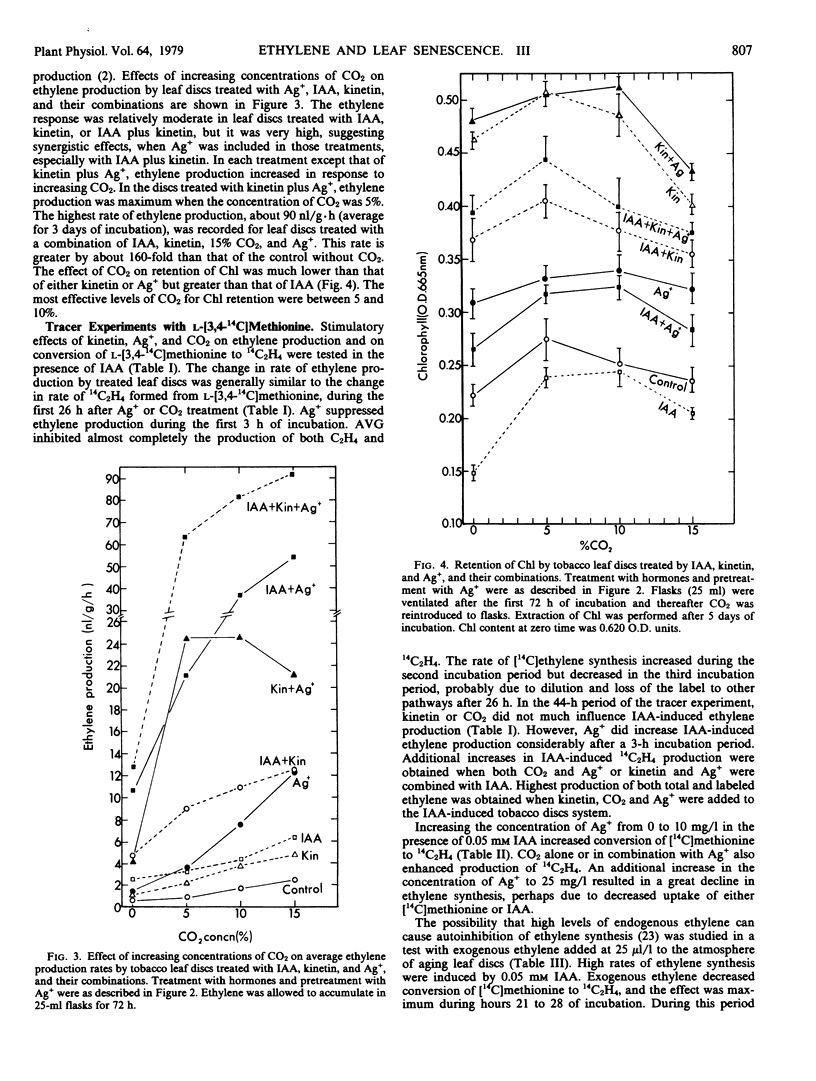
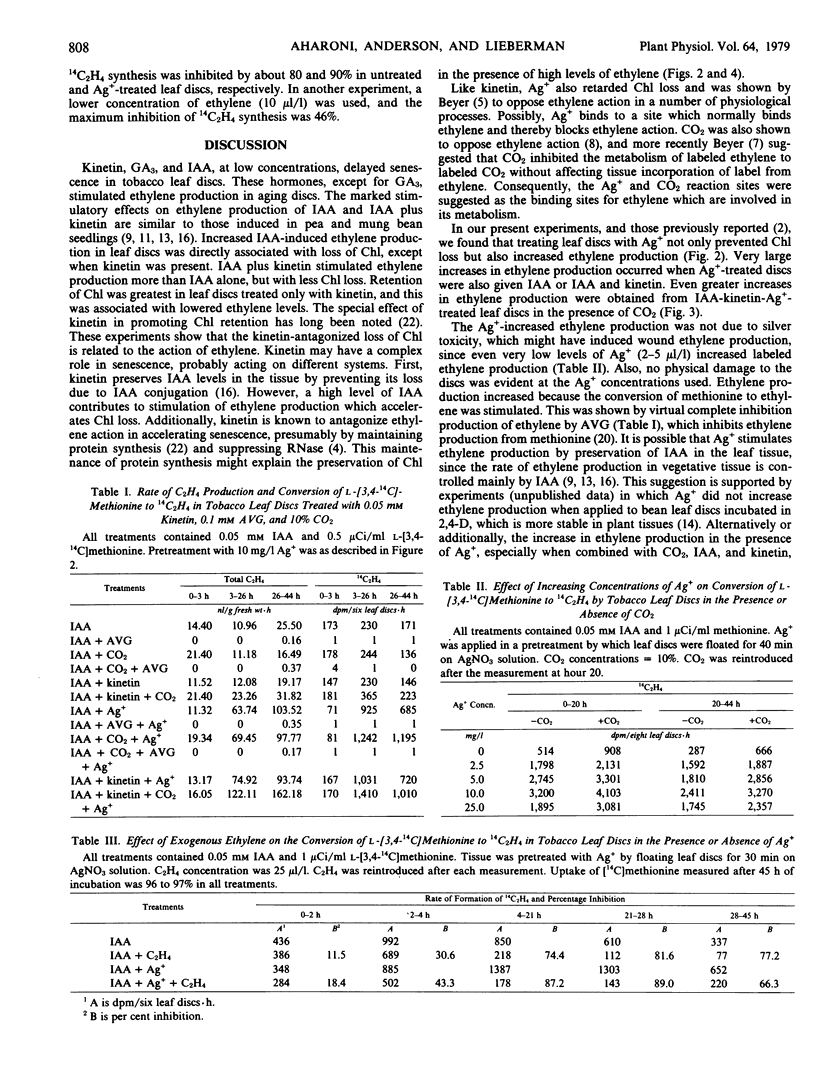
Abstract
Supraoptimal concentrations of indoleacetic acid (IAA) stimulated ethylene production, which in turn appeared to oppose the senescence-retarding effect of IAA in tobacco leaf discs. Kinetin acted synergistically with IAA in stimulating ethylene production, but it inhibited senescence. Silver ion and CO2, which are believed to block ethylene binding to its receptor sites, delayed senescence in terms of chlorophyll loss and stimulated ethylene production. Both effects of Ag+ were considerably greater than those of CO2. IAA, kinetin, CO2, and Ag+, combined, acted to increase ethylene production further. Although this combination increased ethylene production about 160-fold over that of the control, it inhibited senescence. Treatment with 25 μl/l of ethylene in the presence of IAA enhanced chlorophyll loss in leaf discs and inhibited by about 90% the conversion of l-[3,4-14C] methionine to 14C2H4 suggesting autoinhibition of ethylene production.
The results suggest that ethylene biosynthesis in leaves is controlled by hormones, especially auxin, and possibly the rate of ethylene production depends, via a feedback control system, on the rates of ethylene binding at its receptor sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A. Pitfalls in using sodium hypochlorite as a seed disinfectant in C incorporation studies. Plant Physiol. 1974 May;53(5):768–771. doi: 10.1104/pp.53.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Patterns of ehtylene production in senescing leaves. Plant Physiol. 1979 Nov;64(5):796–800. doi: 10.1104/pp.64.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad S. M., Mizrahi Y., Richmond A. E. Leaf water content and hormone effects on ribonuclease activity. Plant Physiol. 1973 Nov;52(5):510–512. doi: 10.1104/pp.52.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURG S. P., BURG E. A. ETHYLENE ACTION AND THE RIPENING OF FRUITS. Science. 1965 May 28;148(3674):1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Beyer E. M. A potent inhibitor of ethylene action in plants. Plant Physiol. 1976 Sep;58(3):268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. M. Effect of silver ion, carbon dioxide, and oxygen on ethylene action and metabolism. Plant Physiol. 1979 Jan;63(1):169–173. doi: 10.1104/pp.63.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic Acid. Plant Physiol. 1968 Jul;43(7):1069–1074. doi: 10.1104/pp.43.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Molecular requirements for the biological activity of ethylene. Plant Physiol. 1967 Jan;42(1):144–152. doi: 10.1104/pp.42.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRELIN E. S. Mitosis in adult cartilage. Science. 1957 Apr 5;125(3249):650–650. doi: 10.1126/science.125.3249.650. [DOI] [PubMed] [Google Scholar]
- Chalutz E., Lieberman M. Inhibition of Ethylene Production in Penicillium digitatum. Plant Physiol. 1978 Jan;61(1):111–114. doi: 10.1104/pp.61.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y., Lieberman M. Effects of Kinetin, IAA, and Gibberellin on Ethylene Production, and Their Interactions in Growth of Seedlings. Plant Physiol. 1968 Dec;43(12):2029–2036. doi: 10.1104/pp.43.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Hanson A. D. Relationship between Ethylene Evolution and Senescence in Morning-Glory Flower Tissue. Plant Physiol. 1976 Apr;57(4):523–527. doi: 10.1104/pp.57.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Knegt E. Influence of Ethylene on Indole-3-acetic Acid Concentration in Etiolated Pea Epicotyl Tissue. Plant Physiol. 1977 Oct;60(4):475–477. doi: 10.1104/pp.60.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurchie E. J., McGlasson W. B., Eaks I. L. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972 May 26;237(5352):235–236. doi: 10.1038/237235a0. [DOI] [PubMed] [Google Scholar]
- Owens L. D., Lieberman M., Kunishi A. Inhibition of ethylene production by rhizobitoxine. Plant Physiol. 1971 Jul;48(1):1–4. doi: 10.1104/pp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



