Abstract
In recent years, circulating progenitors of endothelial cells and smooth muscle cells were identified in the peripheral blood. In our study, we evaluated the utilization of both cell types isolated and differentiated from peripheral porcine blood in terms for their use for tissue engineering purposes. By means of density gradient centrifugation, the monocyte fraction from porcine blood was separated, split, and cultivated with specific culture media with either endothelial cell growth medium-2 or smooth muscle cell growth medium-2 for the differentiation of endothelial cells or smooth muscle cells. Obtained cells were characterized at an early stage of cultivation before the first passage and a late stage (fourth passage) on the basis of the expression of the antigens CD31, CD34, CD45, nitric oxide synthase, and the contractile filaments smooth-muscle alpha-actin (sm-alpha-actin) and smoothelin. Functional characterization was done based on the secretion of nitric oxide, the formation of a coherent monolayer on polytetrafluoroethylene, and capillary sprouting. During cultivation in both endothelial cell growth medium-2 and smooth muscle cell growth medium-2, substantially two types of cells grew out: early outgrown CD45-positive cells, which disappeared during further cultivation, and in 85% (n = 17/20) of cultures cultivated with endothelial cell growth medium-2 colony-forming late outgrowth endothelial cells. During cultivation with smooth muscle cell growth medium-2 in 80% (n = 16/20) of isolations colony-forming late outgrowth smooth muscle cells entered the stage. Cultivation with either endothelial cell growth medium-2 or smooth muscle cell growth medium-2 had selective effect on the late outgrown cells to that effect that the number of CD31-positive cells increased from 34.8% ± 13% to 83.9% ± 8% in cultures cultivated with endothelial cell growth medium-2 and the number of sm-α-actin+ cells increased from 52.6% ± 18% to 88% ± 5% in cultures cultivated with smooth muscle cell growth medium-2, respectively. Functional analyses revealed significantly higher levels of nitric oxide secretion, endothelialization capacity, and capillary formation in not expanded cultures cultivated with endothelial cell growth medium-2 in comparison to later stages of cultivation and mature aortic cells. Blood seems to be a reliable and feasible source for the isolation of both endothelial and smooth muscle cells for application in tissue engineering approaches. Whereas, early co-cultures of early and late outgrowth cells provide functional advantages, the differentiation of cells can be directed selectively by the used culture medium for the expansion of highly proliferative late outgrowth endothelial cells and late outgrowth smooth muscle cells, respectively.
Keywords: Circulating progenitor cells, endothelial progenitor cells, early outgrowth endothelial cells, late outgrowth endothelial cells, late outgrowth smooth muscle cells, tissue engineering
Introduction
Since the description of circulating progenitor cells, which differentiate in vitro into endothelial cells (EC) and smooth muscle cells (SMC), both cell types can be isolated from peripheral blood probes. Thus, blood became a reasonable alternative source for the utilization of vascular cells in cardiovascular tissue engineering (TE).1,2 Origin and function of circulating progenitor cells in the peripheral blood are not clarified in detail despite intensive research in the last nearly 20 years. On one hand, inconsistent methodical protocols are used regarding isolation and cultivation of these cells in vitro. On the other hand, circulating progenitor cells, which were summed up under the term of endothelial progenitor cell in the past, apparently represent a heterogeneous composition of different cell types. At present, predominantly three cell types are described. These are termed according to their appearance during cultivation in vitro as early and late outgrowth endothelial cells (EOEC and LOEC, respectively).2–5 And as the third type—according to their endothelial counterparts—late appearing colony-forming SMC are termed as late outgrowth smooth muscle cells (LOSMC).1,6,7
However, despite remaining ambiguity regarding origin and function of circulating progenitor cells, in a number of studies attempts have been made to utilize these cells for therapeutic approaches, including graft seeding procedures in cardiovascular TE.8,9 For TE approaches, the use of circulating progenitor cells provides a number of actual and potential advantages. At first, these cells can be isolated from a sample of peripheral blood without the need of a surgical procedure for the isolation of cells from other sources. In addition, their use seems to be advantageous over that of mature vascular cells taken from blood vessels, which were predominantly used in the early days of TE regarding a decreased loss of functionality during expansion in vitro.10
Reduction of thrombogenicity by seeding of EC isolated from circulating progenitor cells was described.11 The seeding of distinct cell types on an appropriate scaffold material still remains the basic principle of TE. The most promising are approaches in which cells are seeded on a biological scaffold, which is gradually degraded when seeded cells build a “neotissue.” Nevertheless, although the establishment of coculture settings of EC with SMC has been found to be superior over the seeding of each cell type alone regarding their functionality, at present there is only one description about the use of both EC and SMC isolated and differentiated from peripheral blood probes in TE approaches.12–15
In this study, we evaluated a simple method for the isolation of circulating progenitor cells from peripheral porcine blood and their differentiation into EC and SMC by means of selective cultivation of the monocyte fraction with specific endothelial or smooth muscle culture media. Outgrowing EC and SMC were characterized in an early (before expansion) and late stage of cultivation (fourth passage) regarding their use in vascular TE in comparison with their respective mature counterparts isolated from porcine aorta.
Materials and methods
Cell isolation and cultivation
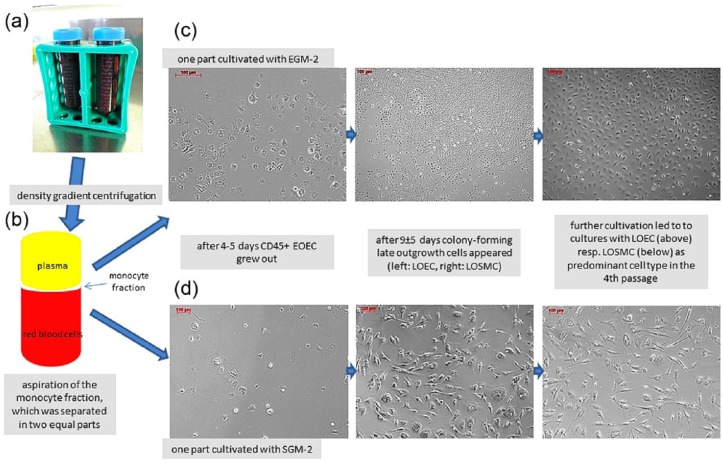
Blood (100 mL) was taken under sterile conditions via a catheter from pigs’ superior vena cava (German Hausschwein, 25–30 kg body weight, animals n = 20). Heparin (100 IU/mL, ratiopharm GmbH, Germany) was added to prevent premature clotting. The monocyte fraction was separated during density gradient centrifugation at 600g at 20°C for 12 min, washed twice in phosphate-buffered saline solution (PBS, Lonza, Switzerland), and partitioned into two equal aliquots. One aliquot was cultivated in endothelial cell growth medium-2 (EGM-2, Lonza) and the other one in smooth muscle cell growth medium-2 (SGM-2, Lonza). Each cell suspension was transferred separately into a 75-cm2 culture flask (Nunc, Denmark) coated with 1% gelatin (Sigma-Aldrich, Germany) (Figure 1). After reaching confluence, cells were detached with trypsin (TrypLE Select, Invitrogen, Germany), split 1:3, and further expanded until the fourth passage. Medium was changed every 3 days. For morphological and functional characterization, outgrown confluent not expanded cells and expanded cells from the fourth passage were seeded in chamber slides (Nunc, Denmark, 1 × 105 per cm2). Cell count showed 1.1 ± 1 × 108 cells in EC cultures and 1.7 ± 0.1 × 108 cells in SMC cultures after fourth passage.
Figure 1.
This figure shows a schematic overview of the experimental setting. (a) Blood was collected from pigs’ jugular vein. (b) By means of density gradient centrifugation, the monocyte fraction was separated, aspirated, and divided in two equal parts, which were cultivated with either EGM-2 or SGM-2. (c and d) In both culture settings after 4–5 days, small round-shaped EOEC appeared. During further cultivation in both settings, colony-forming clusters of late outgrowth cells appeared 9 ± 5 days after isolation: predominantly LOEC in cultures cultivated with EGM-2 and predominantly LOSMC in cultures cultivated with SGM-2 (c and d). Colony-forming late outgrown cells formed a coherent monolayer and overgrew the early outgrown cells.
For comparative studies, EC were isolated from porcine aorta (n = 10) by scrubbing them from the lumen with a scalpel. Cells were washed in PBS, resuspended in EGM-2, and transferred in a gelatin-coated culture flask. The remaining aortic segments were cut into pieces of 1 mm × 1 mm and placed in a gelatin-coated culture flask in SGM-2. Medium was changed every 3 days during SMC grew out. Reaching confluence cells were detached with trypsin, split 1:3, and further cultivated until the fourth passage. Morphological characterization was done under light microscope (Axio Observer, Zeiss Jena, Germany). Here, the cell count was 1 ± 0.1 × 108 for EC and 1.4 ± 0.2 × 108 for SMC after fourth passage.
Fluorescence staining
Immunofluorescence staining of cells seeded on chamber slides and fixed with paraformaldehyde was done as previously described13 (see Table 1 for the description of the used primary antibodies). Secondary antibodies linked to fluorescein isothiocyanate (FITC; Acris), tetramethylrhodamine (TRITC; Acris), and Cy3 (DAKO Cytomation, Denmark) were used. Specific staining was visualized by means of a fluorescence microscope (Axio Observer). Quantification of antigen expression was done by means of a flow cytometer (FACScalibur, BD Biosciences, USA) and processing with ImageJ (ImageJ, MD, USA).16
Table 1.
Parameters for the characterization of the isolated cells.
| Immunofluorescence stainings and flow cytometry |
| Primary antibodies were used against antigen |
| CD31 (Acris antibodies, Herford, Germany) |
| CD34 (USBiological, Biomol, Hamburg, Germany) |
| CD45 (Abcam, Cambridge, UK) |
| NO synthase (Acris antibodies) |
| Smooth muscle-α-actin (Imgenex, Biomol) |
| Smoothelin (Acris antibodies) |
| NO secretion |
| Each day over a period of 5 days |
| Endothelialization |
| Coverage of the surface when seeded on PTFE under static and flow conditions |
Measurement of nitric oxide
For quantification of nitric oxide (NO) secretion, cells were seeded on chamber slides (1 × 105 per cm2). NO concentration was determined by measurement of its degradation products nitrite and nitrate using a modified Griess’ reaction (Quantichrom NO assay Kit DINO 250, BioAssay Systems, California, USA) according to manufacturer’s instructions. In brief, samples were taken after an incubation period of 24 h and centrifuged at 600g at 4°C for 15 min. After deproteination with ZnSO4 and NaOH, nitrate was reduced into nitrite by addition of cadmium. Reagent solution was added and absorption measured in a microplate reader (Paradigm, Beckman/Coulter, USA) at 540 nm. Nitrite concentration was calculated from an established standard curve.
Endothelialization
Endothelialization was measured by seeding polytetrafluoroethylene (PTFE)-prostheses (Propaten, BBraun, Germany) with a density of 1 × 105 cells per cm2. Experiments were performed with cultures cultivated with EGM-2 before expansion and of the fourth passage as well as with aortic endothelial cells (aEC) before expansion and of the fourth passage (each n = 6) for comparative studies. Prostheses were cultivated in a bioreactor for 7 days either under static or pulsatile flow conditions in a bioreactor. The bioreactor consisted of a glass tube filled with EGM-2 with connectors for the perfusion of the PTFE segment, which were connected via silicon tubes with a reservoir and a roller pump (Masterflex, Germany). Perfusion was done with EGM-2 with a mean flow of 60 ± 2 mL/min and mean pressure of 50 ± 5 mmHg resulting in a shear stress of 8 dyne/cm2 (each n = 6). Specimens were taken and stained with calcein and ethidiumhomodimer-1 (Molecular probes, USA) for visualization of viable or dead cells. The percentage of the surface covered with EC was quantified using Axio Vision 4.8.2 (Carl Zeiss Microscopy GmbH, Göttingen, Germany).
Animal care
The study protocol was approved by the local authorities (Niedersaechsisches Landesamt fuer Verbraucherschutz und Lebensmittelsicherheit, reference number: 10/0214). All pigs received care according to the “Guide for the Care and Use of Laboratory Animals” (www.nap.edu/catalog/5140.html ed; Washington DC: National Academy Press, 1996). Animal housing and conduction of the experiments were done in the Institute for Laboratory Animal Science and Central Animal Facility of the Hannover Medical School. The animals were kept outdoors until 2 weeks before operation. The animals were given a general anesthesia with propofol, isoflurane, and fentanyl for the implementation of the harvesting of tissues and organs, which were used for other experiments of our laboratory. After finishing surgical procedures, all animals were euthanized in deep anesthesia with pentobarbital.
Statistical analysis
All statistical analyses were performed with GraphPad Prism, Version 6 (GraphPad Software Inc., USA). Mean and standard deviation (SD) were calculated for all continuous variables. The significance of differences was determined on the basis of Tukey’s test for analysis of variance (ANOVA). Differences were considered significant at p ≤ 0.05.
Results
Cell isolation and cultivation
Within the first 4–5 days of cultivation both in cultures with EGM-2 and SGM-2, small round cells appeared in the culture flask corresponding to the so-called early outgrowth endothelial cells (EOEC), which were positive for CD45 but negative for CD31, CD34, NOS, alpha-actin, and smoothelin (Figures 1(c) and (d) and 2). During further cultivation with both EGM-2 (in 85% of the isolations, n = 17/20) and SGM-2 (in 80% of the isolations, n = 16/20), clusters of colony-forming CD34-positive cells appeared (Figure 1) and grew to confluence. In cultures cultivated with EGM-2, colony-forming cells appeared within 5–12 (9 ± 5) days with an endothelial morphology according to the so-called LOEC (Figures 1(c) and 2), which reached confluence after 19 ± 7 days. In cultures cultivated with SGM-2, colony-forming cells appeared within 4–14 days (9 ± 5 days). Most cells had morphology similar to mature SMC corresponding to LOSMC (Figures 1(d) and 2). Here, confluence was reached after 16 ± 8 days. See also Table 2 for time course of expansion of the isolated cells.
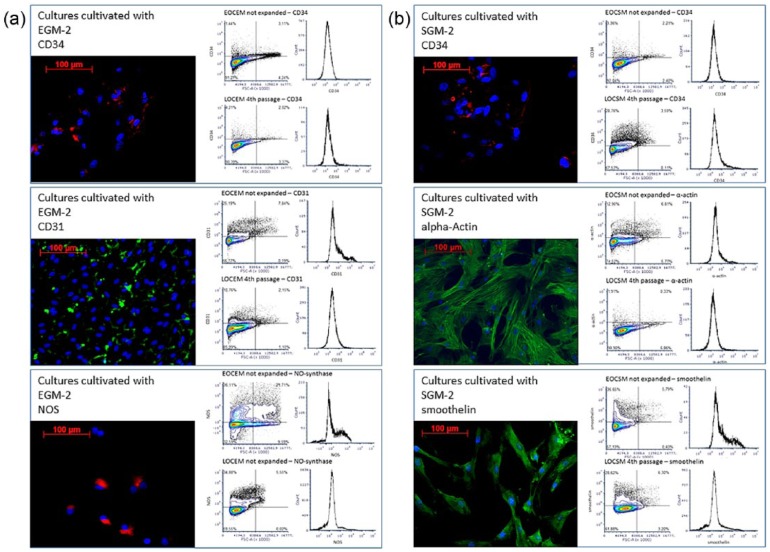
Figure 2.
This figure shows representative immunofluorescence staining and FACS analysis of outgrown cells cultivated with (a) EGM-2 and (b) SGM-2. In both settings, most cells expressed CD34 (red, upper row in both columns), whose portion decreased slightly during further cultivation. During cultivation with EGM-2, colony-forming cells positive for CD31 (green, middle row) and NOS (red, bottom both in the left column) became the predominant cell type. During cultivation with SGM-2, colony-forming LOSMC positive for sm-alpha-actin-positive (green, middle row) and about 57% positive for smoothelin (red, bottom row) were the predominant cell type. Compare Table 3 for antigen expression.
Table 2.
Time course of cell expansion.
| Cell type | Time to confluence in passage |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Outgrown cells cultivated with EGM-2 | 19 ± 7 | 9 ± 3 | 7 ± 2 | 5 ± 2 |
| Outgrown cells cultivated with SGM-216 | 16 ± 8 | 8 ± 5 | 7 ± 3 | 7 ± 3 |
| Aortic endothelial cells | 14 ± 4 | 4 ± 2 | 3 ± 1 | 3 ± 0 |
| Aortic smooth muscle cells | 24 ± 10 | 4 ± 1 | 4 ± 1 | 3 ± 0 |
Antigen expression in early and late stages of cultivation
In confluent but not expanded outgrown cell cultures cultivated with EGM-2, 7.7% ± 2% of the cells were CD45-positive, 89.2% ± 4% CD34-positive, 34.8% ± 13% CD31-positive, and 3.1% ± 3% expressed sm-alpha-actin (Figure 2(a) and (b)). In confluent not expanded outgrown cell cultures cultivated with SGM-2, 11.2% ± 5% of the cells were CD45-positive, 76.0% ± 3% CD34-positive, 11.2% ± 17% CD31-positive, and 52.6% ± 19% sm-alpha-actin-positive (Figure 2 and Table 3). In both settings, CD31-positive cells had an endothelial morphology, and sm-alpha-actin-positive cells were similar to SMC (Figures 1(c) and (d) and 2).
Table 3.
Antigen expression (expressed as percentage of positive cells by the total number of cells).
| Cells | Passage no. | CD31 | CD34 | CD45 | NOS | Alpha-actin | Smoothelin |
|---|---|---|---|---|---|---|---|
| Outgrown cells cultivated with | |||||||
| EGM-2 | ne | 34.8 ± 13 | 89.2 ± 4 | 7.7 ± 2 | 19.2 ± 3 | 3.1 ± 3 | 0.9 ± 1 |
| 4 | 83.9 ± 8 | 85.8 ± 8 | 0 ± 0 | 70.1 ± 11 | 1.1 ± 1 | 0.2 ± 0.3 | |
| ne | 11.2 ± 17 | 76.0 ± 3 | 11.2 ± 5 | 12.2 ± 11 | 52.6 ± 19 | 44.9 ± 9 | |
| 4 | 3.1 ± 0.1 | 61.7 ± 1 | 0 ± 0 | 2.5 ± 1 | 88.0 ± 5 | 56.6 ± 12 | |
NOS: NO synthase; ne: not expanded (confluent cultures before the first passage).
During further cultivation with EGM-2, confluence was reached in the fourth passage after 40 ± 9 (Figure 2 and Table 2). At this point, most of these cells expressed CD31 (83.9% ± 8%) and CD34 (85.8% ± 8%) (Figure 2 and Table 3). When cultivated with SGM-2, confluence in the fourth passage was reached after 37 ± 10 days (Table 3). Here, most cells expressed CD34 (61.7% ± 1%) and alpha-actin (88.0% ± 5%) (Figure 2 and Table 3). In both culture settings, CD45-positive cells were not detected in the fourth passage (Table 3).
Capillary sprouting
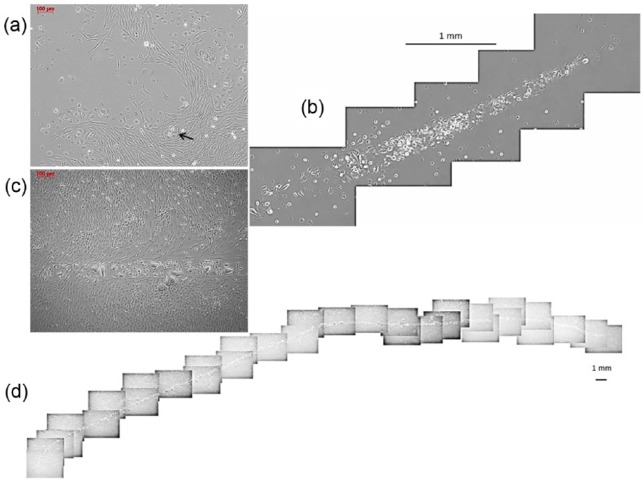
Extensive sprouting of capillaries was found only in outgrown cell cultures cultivated with SGM-2 (n = 5). Sprouting obviously started from clusters of EOEC surrounded by LOEC (Figure 3(a), arrow). Different from the others, these cultures could be cultivated for more than 4 weeks without becoming over-confluent. During this time, capillaries became more and more complex with a length up to 4.5 cm (Figure 3(d)) and an increasing involvement of LOSMC into the formation of capillary walls (Figure 3(b) and (c)). In capillary-forming cultures, the percentage of CD31-positive cells was twice as high as (21.7% ± 14% vs 11.2% ± 17%) in the other cultures cultivated with SGM-2.
Figure 3.
(a) First appearance of capillary sprouting was seen when colony-forming LOEC proliferated and surrounded EOEC (arrow). Formation of long tubular capillaries was found only in the presence of LOSMC in cultures cultivated with SGM-2. During cultivation, (b) these structures became more and more complex and (c) LOSMC participated in the tube formation. These cultures could be cultivated for about 4 weeks without signs of overgrowth. (d) In this time, capillaries grew up to a length of up to 4.5 cm.
Isolation and cultivation of native aortic EC and SMC
Isolation of aortic EC and SMC was successful in 100%. EC reached confluence in the first passage after 14 ± 4 and in the fourth passage after 24 ± 6 days, SMC after 24 ± 10 and 35 ± 10 days (Table 1).
Endothelialization
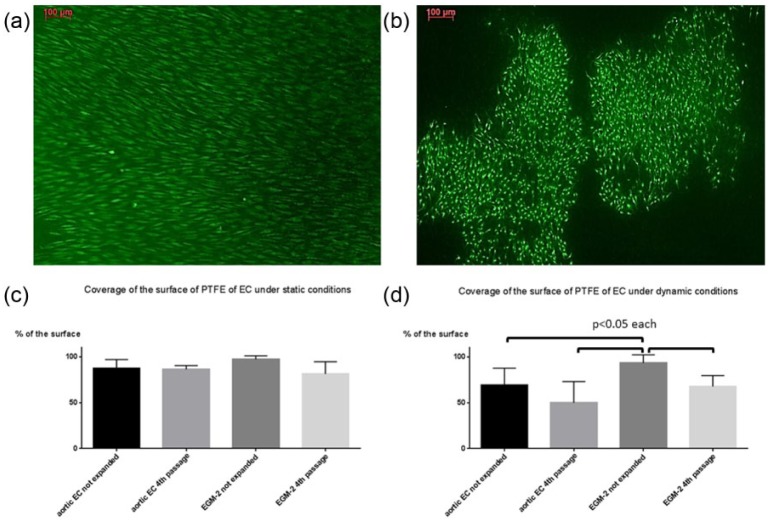
Not expanded cultures of outgrown cells cultivated with EGM-2 formed a nearly complete monolayer after seeding on PTFE both under static and dynamic conditions (Figure 4(a)). After perfusion, these cells covered 93.5% ± 9% of the surface, which was significantly higher in comparison to expanded LOEC from the fourth passage and aortic EC (Figure 4(b) and (d)). Under static conditions, not expanded cells cultivated with EGM-2 covered 97.2% ± 4% of the surface without significant difference to expanded cells and aortic cells (Figure 4(c)).
Figure 4.
Cells which were cultivated with EGM-2 and taken from (a) not expanded cultures and (b) from the fourth passage, seeded on PTFE after 7 days of cultivation in a bioreactor (staining with calcein AM and ethidiumhomodimer-1, magnification 100×). Not expanded cells cultivated with EGM-2 formed a nearly coherent monolayer (a) in contrast to cells taken from the fourth passage (b). The percentage of the surface covered with LOEC and EC is shown in the both graphs (c under static conditions and d under dynamic conditions). (c) Under static conditions no significant differences were found between the different cell types. After perfusion in a bioreactor under dynamic conditions, coverage of the surface was significantly highest in setting with not expanded cells cultivated with EGM-2 in comparison to all other groups (p < 0.05), while no significant differences were found between the other settings.
NO secretion
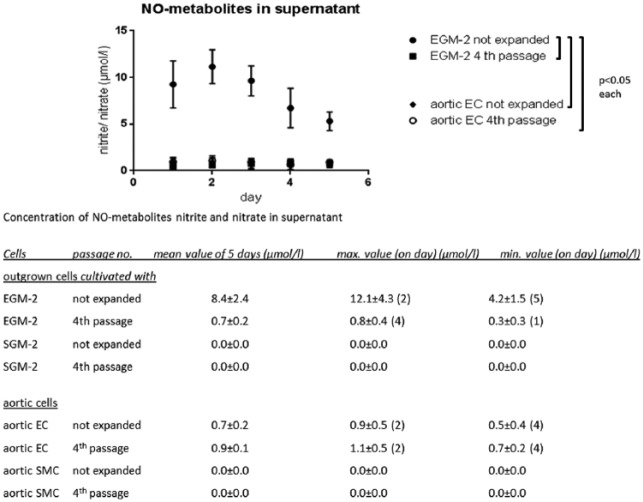
NO secretion was significantly highest in not expanded cultures cultivated with EGM-2 (in mean 8.4 ± 2.4 µmol/L) in comparison to all other settings (p < 0.05). No significant differences were found between expanded LOEC (fourth passage) (0.7 ± 0.2 µmol/L) and aortic EC before (0.7 ± 0.2 µmol/L) and after expansion (0.9 ± 0.1 µmol/L). Almost no NO metabolites were detected in cultures cultivated with SGM-2 and aortic SMC (Figure 5).
Figure 5.
Significant higher levels of NO metabolites were measured in not expanded cell cultures cultivated with EGM-2 (EOEC and LOEC) in comparison to expanded cultures cultivated with EGM-2 in the fourth passage and aortic EC. The concentration of expanded cultures cultivated with EGM-2 was comparable to those of not expanded and expanded aortic EC. Almost no NO formation was found in cultures cultivated with SGM-2 and aortic SMC.
Discussion
Both cell types with endothelial and smooth muscle expression can be isolated by means of the minimal invasive procedure of a venipuncture. At least three types of progenitor cells have been identified over the last years, which were successfully isolated and differentiated in this study from the same blood samples by means of cultivation with selective culture media. According to their appearance in culture, morphology, and growth capacity, circulating progenitor cells are differentiated in EOEC, LOEC, and LOSMC, respectively.
In both settings, early and late outgrowth cells were observed. Irrespective of whether the monocyte fraction was cultivated with EGM-2 or SGM-2, CD45-positive EOEC appeared within the first days. Since these cells express monocytic antigens such as CD14 and CD45, they are considered to originate from the monocytic cell lineage and therefore also referred to as angiogenic monocytes.17–19 EOEC are considered to induce and regulate vascular remodeling processes such as (re-)endothelialization and angiogenesis by the secretion of vasoactive substances, such as cytokines, vascular endothelial growth factor, hepatocyte growth factor, granulocyte colony–stimulating factor, stromal cell-derived factor-1, tissue factor, NO, and prostaglandin I2.4,20–22 Colony-forming late outgrowth cells entered the stage a few days later (in mean at day 9 ± 5) in both settings forming a co-population with EOEC. Today, late outgrowing colony-forming CD31-positive cells are considered to be precursors of EC and to originate from CD34-positive cells, as was confirmed by the antigen expression of isolated cells in our study.17,18,23,24
While EOEC have a secretory activity, LOEC were found to be highly sensitive to cytokines.17,25–27 This makes both cell types probably synergistic during vasculogenesis in vivo.18 Here, in this study, functional characterization revealed some beneficial synergistic effects of these co-populations regarding to their use in TE approaches. A significant higher capacity to form a monolayer on a synthetic surface (endothelialization) and a significant higher secretion of NO was found, although less than 20% of the cells expressed NOS. Naturally, a high potential for endothelialization is of great interest for TE approaches to achieve antithrombogenicity of bioartificial devices. NO is one key factor maintaining and regulating endothelial functionality such as the inhibition of platelet aggregation, vascular tone, and the inhibition of neointimal hyperplasia.28,29 Today, in vascular TE, a rethinking is emerging away from the time- and cost-intensive in vitro engineering of a complete neo-vessel toward a reduction of the manufacturing process to a fully biocompatible matrix supporting and undergoing a remodeling process resulting in the formation of a neo-artery in vivo.15 Due to the high secretory activity of EOEC in combination with synergistic effects by the LOEC in terms of remodeling in vivo, seeding of co-populations of EOEC and LOEC seems to be advantageous in such settings.
For the engineering of complex tissue structures in vitro, the formation of a capillary network for nutrient and oxygen supply of seeded and ingrown cells has emerged as a key problem. Here, we obtained the formation of complex capillary structures in cultures with LOSMC containing EOEC and a high fraction of LOEC, which was twice as high as in the cultures without the formation of capillaries. In contrast, no capillary sprouting was found in expanded cell cultures, indicating the participation of EOEC, especially since clusters of EOEC were identified as alleged starting points of capillary sprouting. However, further experiments are needed to identify the exact composition of participating cells and putative additives for a reproduction in different experimental settings with expanded and different cell types. Whereas tube formation in cultures of EOEC and LOEC was already described by others, it was not described in such complex extent as we found here in the presence of LOSMC.23
While these properties of co-populations can prove useful for cardiovascular TE, and we have already converted the seeding protocol of bioartificial vascular grafts to the use of not expanded EOEC/LOEC co-populations, the only small number of circulating progenitor cells in the peripheral blood is a considerable disadvantage.30 The number of cells which are capable to differentiate into EC in vitro is estimated to only 0.05–0.2 cells per mL blood.31 Thus, in many applications, an expansion of the cells will be necessary in vitro in order to obtain a sufficiently large number of cells. The cultivation of the monocyte fraction separated by means of density gradient centrifugation is a common technique, although it is not selective, and in all cultures in our study both CD31+ and sm-alpha-actin+ cells grew out. However, corresponding to the finding that the culture medium is a contributing factor for the differentiation of outgrowing cells, during cultivation with specific commercial culture media highly proliferative LOEC and LOSMC, respectively, increasingly became the dominant cell type to the extent that in the fourth passage more than 80% of the cells are either CD31+ or sm-alpha-actin+.3 The proliferation rate determined as time to confluence until the fourth passage was insignificantly lower in comparison to aortic cells. In contrast, in expanded cultures of the fourth passage, when EOEC disappeared, endothelialization and NO secretion became similar to that level of aortic and of expanded LOEC described in other studies.26,32 Although EOEC were present in early stage of cultivation with SGM-2, no NO secretion was found in those cultures, indicating that EOEC do not secrete NO.
Little was studied so far about origin and function of LOSMC. Our results indicate that these cells originate from CD34+ cells with a similar origin to LOEC. During cultivation of LOSMC, a maintained high-degree expression of smoothelin was found as marker for a high contractility capacity, which was found to be decreased to a hardly detectable amount during cultivation of mature SMC.33 Although the maintained high smoothelin expression in the LOSMC cultures indicate a high stability of contractile phenotypical expression during cultivation in vitro, this property was found to be disadvantageous in a previous study, when outgrown SMC seeded in a coculture setting with outgrown EC both isolated from blood hardly expressed extracellular matrix proteins diminishing a maturation process in vitro.13 Nevertheless, induction of a secreting phenotype should be possible and is the target of further studies.
Conclusion
Separation of the monocyte fraction and their cultivation with specific media is a feasible isolation technique of both EC and SMC from blood. Thereby isolated cells represent a putative more powerful tool for TE in comparison to mature vascular cells. Nevertheless, for a better understanding and particularly reliability of the use of blood-derived cells, the exact composition of different outgrowing cell types and mechanism responsible for the observed advantages in early stages of cultivation has to be explored in future experiments.
Acknowledgments
M.W. contributed equally to this work.
Footnotes
Declaration of conflicting interests: The authors state that there are commercial associations that might create a conflict of interest including financial interests in connection with this manuscript.
Funding: This study was funded by the Deutsche Forschungsgemeinschaft (DFG).
References
- 1. Simper D, Stalboerger PG, Panetta CJ, et al. Smooth muscle progenitor cells in human blood. Circulation 2002; 106(10): 1199–1204. [DOI] [PubMed] [Google Scholar]
- 2. Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275(5302): 964–967. [DOI] [PubMed] [Google Scholar]
- 3. Guan XM, Cheng M, Li H, et al. Biological properties of bone marrow-derived early and late endothelial progenitor cells in different culture media. Mol Med Rep 2013; 8(6): 1722–1728. [DOI] [PubMed] [Google Scholar]
- 4. Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003; 107(8): 1164–1169. [DOI] [PubMed] [Google Scholar]
- 5. Rehman J, Li J, Parvathaneni L, et al. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol 2004; 43(12): 2314–2318. [DOI] [PubMed] [Google Scholar]
- 6. Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 2003; 93(11): 1023–1025. [DOI] [PubMed] [Google Scholar]
- 7. Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92(2): 362–367. [PubMed] [Google Scholar]
- 8. Capiod JC, Tournois C, Vitry F, et al. Characterization and comparison of bone marrow and peripheral blood mononuclear cells used for cellular therapy in critical leg ischaemia: towards a new cellular product. Vox Sang 2009; 96(3): 256–265. [DOI] [PubMed] [Google Scholar]
- 9. Kawamoto A, Katayama M, Handa N, et al. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells 2009; 27(11): 2857–2864. [DOI] [PubMed] [Google Scholar]
- 10. Prasad Chennazhy K, Krishnan LK. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 2005; 26(28): 5658–5667. [DOI] [PubMed] [Google Scholar]
- 11. Zhou M, Liu Z, Liu C, et al. Tissue engineering of small-diameter vascular grafts by endothelial progenitor cells seeding heparin-coated decellularized scaffolds. J Biomed Mater Res B Appl Biomater 2012; 100(1): 111–120. [DOI] [PubMed] [Google Scholar]
- 12. Leung BM, Sefton MV. A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Ann Biomed Eng 2007; 35(12): 2039–2049. [DOI] [PubMed] [Google Scholar]
- 13. Aper T, Teebken OE, Kruger A, et al. Development of implantable autologous small-calibre vascular grafts from peripheral blood samples. Zentralbl Chir 2013; 138(2): 173–179. [DOI] [PubMed] [Google Scholar]
- 14. Yu H, Dai W, Yang Z, et al. Smooth muscle cells improve endothelial cell retention on polytetrafluoroethylene grafts in vivo. J Vasc Surg 2003; 38(3): 557–563. [DOI] [PubMed] [Google Scholar]
- 15. Cleary MA, Geiger E, Grady C, et al. Vascular tissue engineering: the next generation. Trends Mol Med 2012; 18(7): 394–404. [DOI] [PubMed] [Google Scholar]
- 16. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9(7): 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 2004; 24(2): 288–293. [DOI] [PubMed] [Google Scholar]
- 18. Pearson JD. Endothelial progenitor cells—an evolving story. Microvasc Res 2010; 79(3): 162–168. [DOI] [PubMed] [Google Scholar]
- 19. Medina RJ, O’Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics 2010; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med 2012; 2012: 918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Ingram DA, Murphy MP, et al. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol 2009; 295(5): 1675–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kusuyama TO, Nishiya D, Enomoto S, et al. The effects of HMG-CoA reductase inhibitor on vascular progenitor cells. J Pharmacol Sci 2006; 101(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 23. Neumuller J, Neumuller-Guber SE, Lipovac M, et al. Immunological and ultrastructural characterization of endothelial cell cultures differentiated from human cord blood derived endothelial progenitor cells. Histochem Cell Biol 2006; 126(6): 649–664. [DOI] [PubMed] [Google Scholar]
- 24. Timmermans F, Plum J, Yoder MC, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med 2009; 13(1): 87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aburakawa Y, Kawabe J, Okada M, et al. Prostacyclin stimulated integrin-dependent angiogenic effects of endothelial progenitor cells and mediated potent circulation recovery in ischemic hind limb model. Circ J 2013; 77(4): 1053–1062. [DOI] [PubMed] [Google Scholar]
- 26. Brown MA, Wallace CS, Angelos M, et al. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A 2009; 15(11): 3575–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bompais H, Chagraoui J, Canron X, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood 2004; 103(7): 2577–2584. [DOI] [PubMed] [Google Scholar]
- 28. Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987; 2(8567): 1057–1058. [DOI] [PubMed] [Google Scholar]
- 29. Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 2009; 89(2): 481–534. [DOI] [PubMed] [Google Scholar]
- 30. Aper T, Wilhelmi M, Gebhardt C, et al. Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater 2016; 29: 21–32. [DOI] [PubMed] [Google Scholar]
- 31. Prater DN, Case J, Ingram DA, et al. Working hypothesis to redefine endothelial progenitor cells. Leukemia 2007; 21(6): 1141–1149. [DOI] [PubMed] [Google Scholar]
- 32. Wu JR, Hsu JH, Dai ZK, et al. Activation of endothelial NO synthase by a xanthine derivative ameliorates hypoxia-induced apoptosis in endothelial progenitor cells. J Pharm Pharmacol 2016; 68(6): 810–818. [DOI] [PubMed] [Google Scholar]
- 33. Van Eys GJ, Niessen PM, Rensen SS. Smoothelin in vascular smooth muscle cells. Trends Cardiovasc Med 2007; 17(1): 26–30. [DOI] [PubMed] [Google Scholar]