Abstract
Background:
Based on clinical practice guidelines, specific quality indicators are examined to assess the performance of a health care system for patients with end-stage renal disease (ESRD). We examined trends in the proportion of patients with ESRD referred late to nephrology, timing of dialysis initiation in those with chronic kidney disease, and proportion of patients with ESRD treated with pre-emptive kidney transplantation or peritoneal dialysis (PD).
Design:
This was a retrospective cohort study.
Setting:
The study was conducted in Alberta, Canada.
Patients:
Alberta residents aged 18 years or older with incident ESRD requiring renal replacement therapy between 2004 and 2013 were included.
Measurements:
Descriptive statistics, and log binomial and linear regression models were used for analysis.
Methods:
We determined the proportion of patients with ESRD who did not see a nephrologist within 90 days prior to starting dialysis (late referrals) and those who were receiving PD 90 days after dialysis initiation. Among those who had been seen by a nephrologist for at least 90 days, we also assessed the proportion who initiated dialysis with estimated glomerular filtration rate (eGFR) higher than or equal to 10.5 mL/min/1.73 m2, and underwent a pre-emptive transplant.
Results:
Our cohort included 5343 patients (mean age 61.8 years, 61.2% male). Over a 10-year period, there was a decrease in the proportion of late referrals (26.4% to 21.1%, P = .001). We also noted a decrease in the proportion of dialysis initiation with eGFR higher than or equal to 10.5 mL/min/1.73 m2 (21.2% to 14.7%, P < .001), with a significant increase in the proportion of patients initiating dialysis as an inpatient (38.8% to 45.2%, P = .001). There was a non-significant decrease in both the proportion of patients treated with a pre-emptive transplant and PD at 90 days over the 10-year period.
Limitations:
The use of administrative data restricted the availability of clinical data regarding underlying circumstances of each quality indicator, including patient symptoms, indications for dialysis initiation, and PD eligibility.
Conclusions:
We noted improvement in late referrals and early dialysis initiation over time. However, we also noted low and stable use of pre-emptive kidney transplantation and PD at 90 days, which warrants further exploration. These findings support the need for quality improvement initiatives designed to address these gaps in care and improve outcomes for patients with kidney failure.
Keywords: kidney failure, chronic, peritoneal dialysis, kidney transplantation, quality indicators, health care, renal replacement therapy
Abrégé
Mise en contexte:
Les indicateurs de la qualité, lorsqu’ils s’appuient sur des preuves solides, permettent d’évaluer efficacement la performance d’un système de soins de santé, y compris les soins dispensés aux patients atteints d’insuffisance rénale terminale (IRT). Nous avons examiné les tendances en matière d’indicateurs de la qualité, y compris la proportion de patients orientés tardivement en néphrologie, ainsi que la proportion de patients traités par dialyse péritonéale (DP) ou au moyen d’une greffe de rein comme modalité initiale de remplacement de la fonction rénale.
Type d’étude:
Il s’agit d’une étude de cohorte rétrospective.
Cadre:
L’étude a été menée en Alberta, au Canada.
Patients:
Il s’agit de patients albertains adultes nouvellement atteints d’IRT et nécessitant une thérapie continue de remplacement rénal.
Mesures:
Nous avons mesuré la proportion de patients qui n’avait pas vu un néphrologue dans les 90 jours précédant l’amorce de la dialyse (orientation tardive) ; la proportion de patients chez qui on avait amorcé la dialyse à la suite d’une mesure du DFGe ≥ 10.5 mL/min/1.73 m2 et la proportion de patients qui avaient subi une greffe comme modalité initiale de thérapie de remplacement de la fonction rénale et la proportion de ceux qui étaient traités par DP à 90 jours.
Méthodologie:
Nous avons utilisé des statistiques descriptives, un modèle logarithmique binomial ainsi que des modèles de régression linéaire pour évaluer les tendances des indicateurs de la qualité mentionnés ci-dessus.
Résultats:
Notre cohorte était formée de 5343 patients (61,2 % d’hommes) dont l’âge moyen se situait à 61.8 ans. Sur une période de 10 ans, nous avons observé que la proportion d’orientations tardives est passée de 26.4% à 21.1% (P = .001). Nous avons aussi noté une diminution de la proportion de patients chez qui on avait amorcé la dialyse avec une mesure du DFGe ≥ 10.5 mL/min/1.73 m2 (21.2% à 14.7%, P < .001). Toutefois, nous avons constaté une augmentation significative de la proportion de patients chez qui la dialyse avait été amorcée lors d’une hospitalisation (38.8% à 45.2%, P = .001). Enfin, nous avons noté une diminution non significative dans la proportion de patients traités par une greffe comme modalité initiale de remplacement de la fonction rénale ainsi que dans la proportion des patients traités par DP à 90 jours au cours de la même période.
Limites de l’étude:
L’utilisation de données administratives a limité la disponibilité des données cliniques en ce qui concerne les circonstances sous-jacentes à chaque indicateur de la qualité, incluant les symptômes du patient, les indications pour l’initiation de la dialyse et l’admissibilité à la dialyse péritonéale.
Conclusions:
Nous avons constaté une amélioration en ce qui concerne les orientations tardives et l’initiation d’une dialyse précoce au fil du temps. Cependant, nous avons remarqué, de façon constante dans le temps, un faible recours à la transplantation rénale comme modalité initiale et à la DP à 90 jours, ce qui devrait faire l’objet d’une étude plus poussée. Ces constatations constituent la première étape d’une proposition pour l’amélioration de la qualité conçue pour réduire les écarts observés au niveau des soins, et visant à améliorer les résultats pour les patients atteints d’insuffisance rénale.
What was known before
Dialysis registries in Canada and elsewhere have noted that the number of people on dialysis continues to increase, and that in many countries, the use of peritoneal dialysis continues to decline. More granular quality indicators reflecting overall care for patients with advanced kidney failure have not been available.
What this adds
We noted improvement in late referrals and early dialysis initiation for people initiating dialysis in Alberta over a 10 year period. However, we also noted low and stable use of pre-emptive kidney transplantation and PD at 90 days, supporting the need for quality improvement initiatives designed to address these gaps in care and improve outcomes for patients with kidney failure.
Introduction
Over the past 20 years, the prevalence of end-stage renal disease (ESRD) has increased, with a 123% increase in the number of patients treated with dialysis, and a doubling in the number of patients receiving a kidney transplant.1 ESRD, typically defined as estimated glomerular filtration rate (eGFR) less than 15 mL/min/1.73 m2, can be treated with renal replacement therapy (RRT) including pre-emptive kidney transplantation, hemodialysis (HD), or peritoneal dialysis (PD).
It is important that patients with ESRD receive quality care that also considers their personal circumstances to achieve optimal outcomes. Given the morbidity associated with ESRD,2-4 and its associated cost,5 measuring quality indicators routinely can provide a useful measure of performance for the health system, with the goals of designing strategies to address gaps and improve care and outcomes.
Based on guidelines,6 a variety of quality indicators in patients with advanced chronic kidney disease (CKD) have been identified, including the timing of referral to a nephrologist prior to dialysis initiation, the timing of dialysis initiation, and the proportion of patients treated with pre-emptive kidney transplantation or initiating dialysis on a home therapy (PD specifically). Regarding timing of referral to nephrology, if patients are referred to nephrology care late in the course of their CKD, this may result in a lack of adequate preparation and has been associated with adverse outcomes. If dialysis is initiated in people without uremic symptoms at a higher eGFR, it may result in unnecessary lifestyle hardships and a decrease in their quality of life.7 Furthermore, a recent clinical trial has shown no benefit and higher health care costs for those randomized to early initiation of dialysis in patients with progressive CKD.8
Pre-emptive transplantation is associated with improved survival following transplantation,9,10 higher quality of life, and lower costs compared with patients on HD.11 Given similar outcomes and significant health care savings in comparison with hemodialysis, the use of PD has been advocated for eligible candidates.
Using a cohort that included all adults with ESRD commencing RRT in a single Canadian province over a 10-year period, we examined trends in these quality indicators, including the proportion of patients referred late to a nephrologist, the timing of dialysis initiation, and the proportion of patients treated with pre-emptive transplantation or PD.
Materials and Methods
Study Cohort
We conducted a population-based analysis of Alberta adults (≥18 years) initiating chronic dialysis or receiving a kidney transplant between January 1, 2004, and December 31, 2013, identified from the Alberta Health administrative data and the Northern and Southern Alberta Renal Programs registry. Patients with acute renal failure who received dialysis for less than 90 days and recovered kidney function were excluded, but patients were included if they died within 90 days and the dialysis was intended to be chronic. Patients were considered to have chronic dialysis when (in the opinion of the health care team) the patient was unlikely to recover kidney function. When uncertain, chronic dialysis status was confirmed by review of electronic medical records.
Data Sources
Demographic and clinical data were obtained from Alberta Health administrative data files. The date of treatment initiation and treatment modality were obtained from the Northern and Southern Alberta Renal Program, and this registry was also used to identify the earliest incident chronic dialysis or transplant record for each patient.12 Laboratory data, specifically outpatient, inpatient, or emergency department serum creatinine measurements (obtained from the Alberta Kidney Disease Network/Interdisciplinary Chronic Disease Collaboration databases13) were used to estimate the last eGFR before dialysis initiation. The database includes laboratory data from hospitals and laboratories from across the province.
Quality Indicators
Consistent with current guidelines and good clinical practice, we studied 4 quality indicators relevant to patients with advanced kidney failure.
Late referral
The definition of late referral to a nephrologist varies in the literature from 1 to 6 months before starting RRT,14 although 90 days is most commonly used.15-18 Therefore, we defined late referral as patients with their first nephrology visit 90 days or less prior to starting dialysis. Given the possibility that some of the patients with no prior nephrology visit had acute kidney injury (or were not known to have CKD), in a secondary analysis, we limited the late referral cohort to patients who had at least 1 eGFR measurement below 30 mL/min/1.73 m2 between 90 days and 1 year before starting dialysis.
Timing of dialysis initiation
Given the lack of survival or quality of life benefit demonstrated in a recent randomized clinical trial comparing early with deferred dialysis initiation8, we defined timing of dialysis initiation as the proportion of patients initiating dialysis (among those who saw a nephrologist more than 90 days prior to starting dialysis) with eGFR greater than or equal to 10.5 mL/min/1.73 m2.6,19 We used the most recent serum creatinine measurement (inpatient, outpatient, or emergency department) prior to initiating dialysis (and within 30 days before initiating dialysis) to estimate eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.20 Although an optimal definition of this quality indicator would include a measure of uremic symptoms, and guidelines recommend delaying dialysis initiation until the onset of uremic symptoms,21 this information was not available from our data sources. However, the metric we used is still a reflection of a center’s approach to dialysis initiation as patients are unlikely to have uremic symptoms at an eGFR above 10.5 mL/min/1.73 m2. As a secondary analysis, we limited the cohort to only patients who initiated dialysis as an outpatient, acknowledging that kidney function may not be in a steady state when dialysis is initiated as an inpatient.
Pre-emptive transplant
We defined pre-emptive transplant as receipt of a kidney transplant without previous chronic dialysis treatment at any time. As we did not have information on transplant eligibility, we assessed this quality indicator among those who had seen a nephrologist at least 90 days prior to the date of dialysis initiation or pre-emptive transplant, and among individuals likely to be considered eligible for a transplant.22,23 We defined this as individuals less than 60 years of age with no history of cancer or cardiovascular disease based on administrative data.
PD
Finally, the proportion of patients treated with PD was also assessed as a quality indicator, overall and across age groups separately. This was defined as the proportion of patients on PD at 90 days, among all patients initiating chronic dialysis (HD or PD) who were still receiving dialysis at that time. A 90-day period was used to ensure patients were stable on PD,24 and to also allow time for patients who initiated dialysis acutely on HD to be transferred to PD.
Definitions of Other Variables
Demographic and clinical variables including age, sex, First Nations status, and comorbid conditions were determined using Alberta Health administrative data files. Diabetes and hypertension were identified with a validated algorithm using physician claims, and hospital/ambulatory data.25,26 The International Classification of Diseases (ICD), Ninth Revision and ICD-10 coding algorithms were used for physician claims and hospital data, respectively.27 Other comorbidities including heart failure, stroke, myocardial infarction (MI), cancer, and peripheral vascular disease (PVD) were determined from the Deyo classification of Charlson comorbidities.27
The geographic location of residence (rural versus urban) was determined by linking the postal code for each individual to the postal code conversion file and the 2001, 2006, and 2011 Canadian census data. Consistent with the definition used by others, rural residence was defined as “a community size of less than 1000, or a population density of less than 400 patients per square km” with the criteria that “the community was located outside of a Census Metropolitan Area or Census Agglomeration.”28
Statistical Analysis
We described the data using means, standard deviations, percentages, and 95% confidence intervals (95% CIs), as appropriate. Results are presented by 2-year periods from January 1, 2004, to December 31, 2013. To determine trends in indicators over time, we regressed patients’ outcomes against their index dates, using log binomial or linear regression models, depending on whether the outcome was binary or continuous. For the outcome of patients receiving PD at 90 days, we included age group in the model to adjust for potential confounding by age. For all indicators, we also fit multivariable regression models, adjusting for possible confounders, to ensure that trends over time were not affected by patient differences. We obtained ethics approval from the Universities of Calgary and Alberta, and we are secondary users of the data collected, as defined by the Alberta Health Information Act. All statistical analysis was conducted using Stata 11.2.29
Results
Participant Characteristics
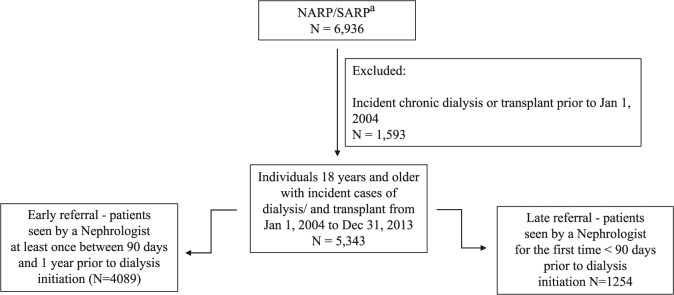
Our final cohort included 5343 patients who either initiated dialysis or received a pre-emptive transplant in Alberta between 2004 and 2013 (Figure 1). Most patients were aged 18 to 59 (41.1%; Table 1) and had a mean age of 61.8 (SD, 15.7) years. The proportion of males increased significantly from 56.9% to 63.1% (P for trend = .002) over the 10-year period, whereas the proportion of patients initiating dialysis from a rural setting decreased from 17.4% to 12.3% (P < .001, overall 14.4%), and the proportion of patients with diabetes and hypertension increased over time (P < .001 for both).
Figure 1.
Participant flow diagram.
aIdentified the earliest incident chronic dialysis or pre-emptive transplant record for each person in the NARP/SARP database who was 18 years or older at the time of the incident dialysis/transplant, and was present in the Alberta Health registry. NARP/SARP = Northern and Southern Alberta Renal Program.
Table 1.
Baseline Characteristics for Adult Incident ESRD Patients, by 2-Year Periods.
| Overalla (N = 5343) | 2-year perioda |
P for trend | |||||
|---|---|---|---|---|---|---|---|
| Jan 1, 2004 to Dec 31, 2005 (N = 1031) | Jan 1, 2006 to Dec 31, 2007 (N = 1036) | Jan 1, 2008 to Dec 31, 2009 (N = 1029) | Jan 1, 2010 to Dec 31, 2011 (N = 1075) | Jan 1, 2012 to Dec 31, 2013 (N = 1172) | |||
| Age, y, mean (SD) | 61.8 (15.7) | 61.8 (16.0) | 62.6 (16.2) | 62.2 (15.7) | 61.0 (15.3) | 61.5 (15.6) | .2 |
| Age category, y | |||||||
| 18-59 | 41.1 | 39.3 | 39.7 | 41.0 | 44.0 | 41.2 | .1 |
| 60-74 | 36.1 | 38.6 | 34.9 | 34.4 | 36.2 | 36.4 | .5 |
| 75+ | 22.8 | 22.1 | 25.4 | 24.6 | 19.8 | 22.4 | .2 |
| Male | 61.2 | 56.9 | 61.5 | 61.3 | 62.8 | 63.1 | .002 |
| First Nations | 6.8 | 7.9 | 7.1 | 6.0 | 6.6 | 6.6 | .1 |
| Rural residence | 14.4 | 17.4 | 15.8 | 13.6 | 13.2 | 12.3 | <.001 |
| Comorbid conditions | |||||||
| Diabetes | 54.4 | 51.9 | 50.1 | 55.3 | 54.3 | 59.5 | <.001 |
| Hypertension | 88.0 | 85.6 | 88.2 | 87.0 | 88.7 | 90.4 | <.001 |
| Heart failure | 37.6 | 38.0 | 40.3 | 35.7 | 37.6 | 36.4 | .3 |
| Stroke | 12.9 | 12.0 | 13.3 | 12.8 | 13.0 | 13.1 | .3 |
| Myocardial infarction | 21.0 | 22.9 | 23.3 | 21.2 | 19.3 | 18.9 | .004 |
| Cancer | 17.8 | 15.2 | 16.2 | 20.0 | 17.6 | 19.6 | .003 |
| Peripheral vascular disease | 16.8 | 19.7 | 18.7 | 15.1 | 15.4 | 15.4 | <.001 |
Note. ESRD = end-stage renal disease; SD = standard deviation.
Percent unless otherwise indicated.
Trends in Indicators of Quality Care
Late referral
Patients referred late to a nephrologist were less likely to have diabetes (37.6% vs 59.5%, P < .001) or hypertension (70.5% vs 93.4%, P < .001), and more likely to be First Nations (8.5% vs 6.3%, P = .009) or have cancer (21.1% vs 16.8%, P < .001), compared with patients referred early. The proportion of patients referred late to a nephrologist decreased from 26.4% in 2004/2005 to 21.1% in 2012/2013 (P for trend = .001; Table 2). Among patients who were seen by a nephrologist at least 90 days before dialysis initiation, 43% initiated dialysis as an inpatient, ranging from 38.8% to 45.2% over the 10-year period (P for trend = .001; Table 3). When we limited the late referral cohort to those with at least 1 eGFR measurement below 30 mL/min/1.73 m2 between 90 days and 1 year before starting dialysis, we found that the proportion of patients referred late to a nephrologist still declined significantly over the period, from 9.9% in 2004/2005 to 5.2% in 2012/2013 (P for trend = .001). The trends remained statistically significant when adjusted for potential confounders using multivariable regression models.
Table 2.
Proportion of Incident Dialysis Patients With a Late Referral (No Nephrologist Visit in the 90 Days Prior to Dialysis Initiation), by 2-Year Periods.
| 2-year period |
P for trend | |||||
|---|---|---|---|---|---|---|
| Jan 1, 2004 to Dec 31, 2005 (N = 988) | Jan 1, 2006 to Dec 31, 2007 (N = 999) | Jan 1, 2008 to Dec 31, 2009 (N = 999) | Jan 1, 2010 to Dec 31, 2011 (N = 1044) | Jan 1, 2012 to Dec 31, 2013 (N = 1130) | ||
| Late referral, % (95% CI) | 26.4 (23.7-29.3) | 25.1 (22.5-27.9) | 25.1 (22.5-27.9) | 23.0 (20.5-25.7) | 21.1 (18.7-23.6) | .001 |
Note. CI = confidence interval.
Table 3.
Characteristics of Dialysis Initiation Among Patients Seen by a Nephrologist at Least 90 Days Before Dialysis Initiation, by 2-Year Periods.
| 2-year period |
P for trend | |||||
|---|---|---|---|---|---|---|
| Jan 1, 2004 to Dec 31, 2005 (N = 727) | Jan 1, 2006 to Dec 31, 2007 (N = 748) | Jan 1, 2008 to Dec 31, 2009 (N = 748) | Jan 1, 2010 to Dec 31, 2011 (N = 804) | Jan 1, 2012 to Dec 31, 2013 (N = 892) | ||
| Proportion who started dialysis as an inpatient, % (95% CI) | 38.8 (35.2-42.4) | 42.0 (38.4-45.6) | 40.6 (37.1-44.3) | 47.6 (44.1-51.2) | 45.2 (41.9-48.5) | .001 |
| Proportion whose last eGFR was >10.5 mL/min/1.73 m2,a % (95% CI) | 25.6 (22.4-29.0) | 21.2 (18.3-24.3) | 20.1 (17.3-23.2) | 17.3 (14.7-20.1) | 14.7 (12.4-17.2) | <.001 |
| Last eGFR before dialysis (mL/min/1.73 m2)a, mean (95% CI) | 9.3 (8.8-9.9) | 8.6 (8.3-8.9) | 8.4 (8.1-8.8) | 8.2 (7.9-8.4) | 7.7 (7.5-8.0) | <.001 |
| Proportion whose last eGFR was <6 mL/min/1.73 m2,a % (95% CI) | 21.5 (18.5-24.8) | 22.8 (19.8-26.1) | 23.5 (20.5-26.8) | 26.6 (23.5-29.8) | 29.9 (26.9-33.1) | <.001 |
Note. A total of 108 patients were excluded because their most recent serum creatinine measurement was done more than 30 days prior to RRT, or they had no prior serum creatinine measurement. CI = confidence interval; eGFR = estimated glomerular filtration rate.
Among incident dialysis patients were those who were followed by a nephrologist for more than 90 days and had a serum creatinine measurement taken 30 days or less before dialysis initiation.
Timing of dialysis initiation
Among patients who were seen by a nephrologist at least 90 days before dialysis initiation, we observed a reduction in the proportion of patients initiating dialysis with eGFR greater than 10.5 mL/min/1.73 m2 (25.6% to 14.7%, P for trend < .001) and an increase in the proportion of patients starting dialysis with their last eGFR less than 6 mL/min/1.73 m2 (21.5% to 29.9%, P for trend < .001). Similarly, there was a significant decrease in the mean eGFR at dialysis initiation, from 9.3 mL/min/1.73 m2 to 7.7 mL/min/1.73 m2 (P for trend < .001). We noted that approximately 40% of patients started dialysis as inpatients, a proportion that has increased significantly over time. When we restricted the analysis to those initiating dialysis as outpatients, we still observed a significant reduction in the proportion initiating dialysis with eGFR greater than 10.5 mL/min/1.73 m2 over the 10 years (24.2% to 11.8%, P for trend < .001) and a significant increase in the proportion with eGFR less than 6 mL/min/1.73 m2 (17.2% to 27.7%, P for trend < .001). There was also a significant decrease in the mean eGFR at dialysis initiation, from 9.2 mL/min/1.73 m2 to 7.5 mL/min/1.73 m2 (P for trend < .001). All trends remained statistically significant when adjusted for potential confounders using multivariable regression models.
Pre-emptive kidney transplantation
Over the 10-year period, 921 patients initiating RRT were eligible to receive a kidney transplant based on our criteria, and 97 (10.5%) of these patients underwent a pre-emptive transplant (Table 4). The proportion of pre-emptive transplants among potentially eligible patients was stable over the 10-year period (12.9% to 9.9%; P for trend = .11). There was no significant trend in eGFR prior to pre-emptive transplantation (mean eGFR 12.6 mL/min/1.73 m2 overall).
Table 4.
Incident RRT Patients Who Received a Pre-emptive Transplant Among Those Potentially Eligible,a by 2-Year Periods.
| Overall | 2-year period |
P for trend | |||||
|---|---|---|---|---|---|---|---|
| Jan 1, 2004 to Dec 31, 2005 | Jan 1, 2006 to Dec 31, 2007 | Jan 1, 2008 to Dec 31, 2009 | Jan 1, 2010 to Dec 31, 2011 | Jan 1, 2012 to Dec 31, 2013 | |||
| No. of potentially eligible patients | 921 | 186 | 171 | 173 | 188 | 203 | — |
| No. of pre-emptive transplants | 97 | 24 | 22 | 17 | 14 | 20 | — |
| Proportion of pre-emptive transplants among eligible patients, % (95% CI) | 10.5 (8.6-12.7) | 12.9 (8.4-18.6) | 12.9 (8.2-18.8) | 9.8 (5.8-15.2) | 7.4 (4.1-7.2) | 9.9 (6.1-14.8) | .11 |
| Last eGFR before pre-emptive transplant in eligible patients (mL/min/1.73 m2), mean (95% CI) | 12.6 (11.6-13.6) | 12.7 (9.9-15.6) | 11.6 (10.0-13.2) | 15.3 (12.9-17.7) | 13.0 (10.6-15.3) | 11.2 (9.7-12.6) | .37 |
Note. RRT = renal replacement therapy; CI = confidence interval.
Younger than 60 years of age with no history of cardiovascular disease or cancer, who had seen a nephrologist at least 90 days before dialysis initiation or pre-emptive transplant.
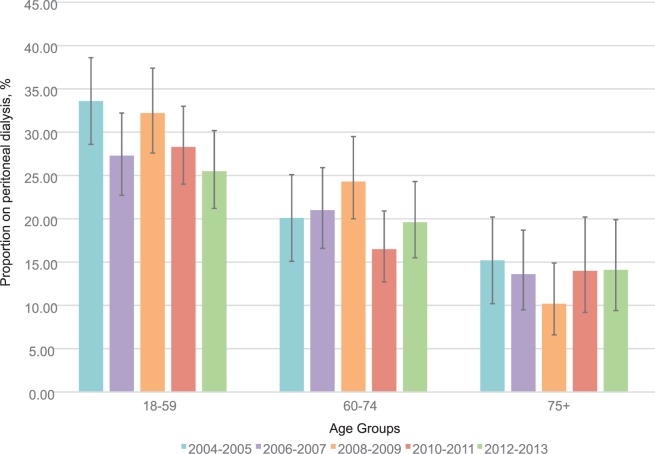
Use of PD at 90 days after dialysis initiation
Within each age group, the proportion of patients receiving PD 90 days after dialysis initiation was relatively stable (Figure 2). For those aged 18 to 59, there was a trend toward a reduction in use of PD at 90 days over time (P = .08). This age group also had the highest proportion of PD use at 90 days compared with older age groups. After adjusting for age, the overall P value for trend was not statistically significant (P = .14).
Figure 2.
Proportion of incident dialysis patients receiving PD at 90 days after dialysis initiation, by age group and 2-year periods.
Note. Error bars show 95% confidence intervals. PD = peritoneal dialysis.
Discussion
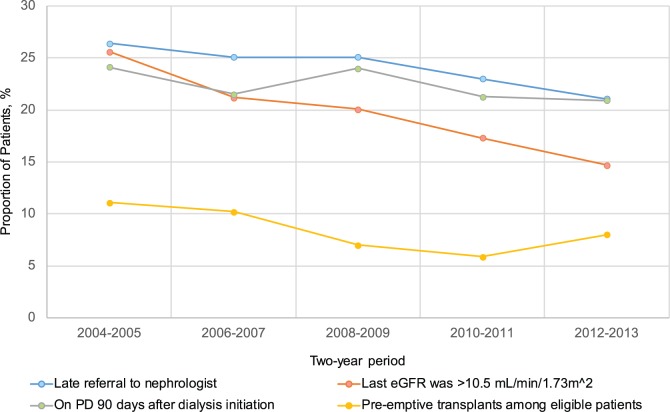
In our cohort of 5343 patients in a Canadian province with access to universal health care, we noted changes in some of the indicators that we measured to reflect quality care in advanced kidney failure over a 10-year period. Specifically, the proportion of patients who had seen a nephrologist for less than 90 days prior to dialysis initiation (late referral) was low (particularly when the cohort was restricted to those with a prior eGFR <30 mL/min/1.73 m2) and decreased over time. Furthermore, there was a decrease in the proportion of patients initiating dialysis with eGFR greater than 10.5 mL/min/1.73 m2 over the 10-year period, and an increase in the proportion starting dialysis with eGFR less than 6 mL/min/1.73 m2. The proportion of individuals receiving pre-emptive transplants among those potentially eligible and the proportion receiving PD at 90 days after dialysis initiation was stable. These results, which are summarized in Figure 3, were confirmed when using alternate definitions for these quality indicators.
Figure 3.
Proportion of patients who were referred late to a nephrologist, had a last eGFR greater than 10.5 mL/min/1.73 m2 before chronic dialysis initiation, were on PD 90 days after chronic dialysis initiation, and had a pre-emptive transplant among eligible patients.
Note. eGFR = estimated glomerular filtration rate; PD = peritoneal dialysis.
There are a variety of potential factors that may have influenced these trends over the 10-year period including changes in patient characteristics, practice patterns, health care policies, or new clinical evidence. The observed decrease in dialysis patients with a late referral may have been influenced by improved awareness of referral guidelines,6 or increased use of automated laboratory reporting of eGFR. Automated reporting of eGFR was implemented October 15, 2004, in Alberta, including guidance for referral to nephrology for patients with eGFR lower than 30 mL/min/1.73 m2. Previous studies30-32 have also found reductions in late referrals to a nephrologist.
Trends in use of PD may be influenced by physician remuneration. In Alberta, prior to April 1, 2002, there was no physician remuneration for home dialysis, but between 2002 and 2010, remuneration for home dialysis increased to the point where in 2010, remuneration became equal for home and in-center dialysis. As use of PD did not rise over this time, this change did not appear to have an impact of use of home dialysis.
Overall, our results are similar to those noted in reports from other countries. With the same definition of a late referral as our study, the proportion of patients with a late referral was 26% of patients in Australia,17 35% in the United States,15 and 48% in Spain.16 Our results regarding timing of dialysis initiation are consistent with previous reports from Ontario, which indicated mean eGFR at dialysis initiation decreased from 9.9 mL/min/1.73 m2 in 2010 to 8.3 mL/min/1.73 m2 in 2015.33 The reduction in the proportion of patients initiating dialysis with eGFR higher than 10.5 mL/min/1.73 m2 was likely influenced by evidence from the IDEAL trial published in 2010, which found no benefit for earlier dialysis initiation.8 These results suggest that initiation of dialysis has been delayed over time, as recommended by recent clinical practice guidelines.21 As we do not have data on patients’ symptoms at dialysis initiation, we are unable to definitively comment on the appropriateness of timing but note that there has been a 7% increase in the proportion of patients who initiated dialysis as an inpatient over the 10-year period. We noted that 31% of inpatient dialysis initiations had eGFR less than 6 mL/min/1.73 m2 versus 21% of those who initiated as outpatients, but are unable to determine the reason for this increase in inpatient dialysis starts with our data. In our multivariable analysis, we found that diabetes, hypertension, cancer, PVD, MI, age more than 60 years, as well as eGFR less than 6 mL/min/1.73 m2 were all independently associated with increased risk of an inpatient start, but the trend to increasing inpatient starts remained significant after adjusting for these covariates.
Transplantation, pre-emptive transplantation specifically, is the optimal form of RRT given the increased patient survival and quality of life9; however, we observed a non-significant decline in the proportion of pre-emptive transplants in Alberta among eligible patients over the 10-year time period. There are several possible explanations for this trend, which may be related to delays in referral, delays in the transplant work-up, or possibly a decrease in the availability of living donors. Furthermore, United States Renal Data System data show that among incident cases of ESRD initiating RRT (HD, PD, or transplantation), 2.6% were pre-emptive transplants and the number of pre-emptive transplants increased by 59.2% in a 13-year period.34
We found that the proportion of patients using PD at 90 days has been stable over time, with a non-significant decrease over time in patients aged 18 to 59 years old. National data from Canada report a constant proportion of patients initiating dialysis on PD from 2004 to 20131 and Ontario reported relatively constant rates of incident PD use from 18.6% in 2010 to 20.7% in 2015.33 The use of PD also varies by country—in Korea, the percentage of patients on PD has decreased from 15% to 10% over a 23-year period.35 In the United States, though there was a decline from 10.9% to 7.1% over an 8-year period36 until 2011, there recently has been an increase in the use of PD. Between 2011 and 2013, there was a 15% relative increase in the number of patients on home dialysis among US dialysis providers after a change to remuneration for PD.37 The reasons for the lack of an increase in PD over time in Alberta are likely to be multi-faceted and related to patient and health system factors.
Our study has limitations that should be considered when interpreting the results. First, our study was based primarily on administrative data that did not include detailed clinical data such as patient symptoms and the indications for dialysis initiation, information about eligibility for PD, or information on vascular access at dialysis initiation. This limits a full understanding of the underlying reasons for these quality indicators and future studies should seek to understand factors (and identify modifiable factors where possible) that are responsible for the changes in these quality indicators over time. In addition, we were not able to identify whether patients were eligible for a kidney transplant, so we developed an inclusion criteria in accordance with the available literature.22,23 Finally, our study was based on a single Canadian province, and the generalizability to other jurisdictions is uncertain—we recommend that other health systems conduct similar analyses to understand the current care and guide quality improvement activities.
Conclusion
In conclusion, we noted changes in some indicators reflective of quality kidney care, including a reduction in the proportion of patients who had seen a nephrologist for less than 90 days prior to initiating dialysis (late referral), and in the proportion of patients initiating dialysis with eGFR greater than 10.5 mL/min/1.73 m2. Despite recommendations to increase use of pre-emptive transplantation and use of PD, the use of these therapies did not increase over time. These data serve as the first step in a quality improvement process. Further studies are required to determine the barriers to improving these quality indicators and the optimal strategies for improving patient care and outcomes.
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Health Research Ethics boards in Calgary and Edmonton, Alberta, and a waiver of consent was accepted.
Consent for Publication: All of the authors have read and provide consent to the publication of this work.
Availability of Data and Materials: The authors conducted this research after ethics approval, and in the context of a contract with Alberta Health enabling this research. Given the constraints of this contract, the health administrative data used to conduct this research can not be shared.
Authors’ Note: This study is based in part on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the view of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta, Alberta Health nor Alberta Health Services express any opinion in relation to this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Interdisciplinary Chronic Disease Collaboration (ICDC) is funded through an Alberta Innovates–Health Solution (AI-HS) Collaborative Research and Innovation Opportunities team grant. Dr Manns is supported by the Svare Chair in Health Economics and an Alberta Innovates Health Scholar award, Dr Tonelli is supported by the David Freeze Chair in Health Services Research, Helen Tam-Tham is supported by an AI-HS graduate studentship, and Dr Hemmelgarn is supported by the Roy and Vi Baay Chair in Kidney Research.
References
- 1. The Canadian Institute for Health Information. Canadian Organ Replacement Register Annual Report: Treatment of End-Stage Organ Failure in Canada, 2004 to 2013. Ottawa, Ontario: Canadian Institution for Health Information; 2015. [Google Scholar]
- 2. Collins AJ, Foley RN, Gilbertson DT, Chen S-C. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(suppl 1):S5-S11. [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489-495. [DOI] [PubMed] [Google Scholar]
- 4. Mix TCH, St. Peter WL, Ebben J, et al. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis. 2003;42(5):972-981. [DOI] [PubMed] [Google Scholar]
- 5. Manns BJ, Mendelssohn DC, Taub KJ. The economics of end-stage renal disease care in Canada: incentives and impact on delivery of care. Int J Health Care Finance Econ. 2007;7(2):149-169. [DOI] [PubMed] [Google Scholar]
- 6. National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Am J Kidney Dis. 2013;3(1):1-163. [Google Scholar]
- 7. Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5(10):1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Eng J Med. 2010;363(7):609-619. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura T, Ushigome H, Nakao T, et al. Advantages and disadvantages of pre-emptive kidney transplantation: results from a single transplantation center. Transplant Proc. 2015;47(3):626-629. [DOI] [PubMed] [Google Scholar]
- 10. Asderakis A, Augustine T, Dyer P, et al. Pre-emptive kidney transplantation: the attractive alternative. Nephrol Dial Transplant. 1998;13(7):1799-1803. [DOI] [PubMed] [Google Scholar]
- 11. Rambod M, Shabani M, Shokrpour N, Rafii F, Mohammadalliha J. Quality of life of hemodialysis and renal transplantation patients. Health Care Manag (Frederick). 2011;30(1):23-28. [DOI] [PubMed] [Google Scholar]
- 12. Manns BJ, Mortis GP, Taub KJ, McLaughlin K, Donaldson C, Ghali WA. The Southern Alberta Renal Program database: a prototype for patient management and research initiatives. Clin Invest Med. 2001;24:164-170. [PubMed] [Google Scholar]
- 13. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10(1): 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baer G, Lameire N, Van Biesen W. Late referral of patients with end-stage renal disease: an in-depth review and suggestions for further actions. NDT Plus. 2010;3(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr, Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int. 2001;60(4):1547-1554. [DOI] [PubMed] [Google Scholar]
- 16. Lorenzo V, Martín M, Rufino M, Hernández D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: an observational cohort study. Am J Kidney Dis. 2004;43(6):999-1007. [DOI] [PubMed] [Google Scholar]
- 17. Cass A, Cunningham J, Arnold P, Snelling P, Wang Z, Hoy W. Delayed referral to a nephrologist: outcomes among patients who survive at least one year on dialysis. Med J Aust. 2002;177:135-138. [DOI] [PubMed] [Google Scholar]
- 18. Roubicek C, Brunet P, Huiart L, et al. Timing of nephrology referral: influence on mortality and morbidity. Am J Kidney Dis. 2000;36(1):35-41. [DOI] [PubMed] [Google Scholar]
- 19. Sood MM, Manns B, Dart A, et al. Variation in the level of eGFR at dialysis initiation across dialysis facilities and geographic regions. Clin J Am Soc Nephrol. 2014;9(10):1747-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470. [DOI] [PubMed] [Google Scholar]
- 21. Nesrallah GE, Mustafa RA, Clark WF, et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186(2):112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173(10):S1-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. British Columbia Transplant. Clinical guidelines for kidney transplantation. http://www.transplant.bc.ca/sites/default/files/documents/files/Clinical%20Guidelines%20for%20Kidney%20Transplantation.pdf. Published 2015. Accessed May 23, 2016.
- 24. Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. [DOI] [PubMed] [Google Scholar]
- 26. Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54(6):1423-1428. [DOI] [PubMed] [Google Scholar]
- 27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 28. Catalogue SC. Postal Code Conversion File Plus (PCCF+) reference guide. 2014. http://data.library.utoronto.ca/datapub/codebooks/cstdli/pccf_health/pccf6a1/82-F0086-XDB-2014v6a-eng.pdf
- 29. StataCorp. Stata statistical software: release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 30. Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA. 2010;303(12):1151-1158. [DOI] [PubMed] [Google Scholar]
- 31. Foote C, Clayton PA, Johnson DW, Jardine M, Snelling P, Cass A. Impact of estimated GFR reporting on late referral rates and practice patterns for end-stage kidney disease patients: a multilevel logistic regression analysis using the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Am J Kidney Dis. 2014;64(3):359-366. [DOI] [PubMed] [Google Scholar]
- 32. Jun M, Hemmelgarn BR. Automated estimated GFR reporting and late referral: are we expecting automatic benefits? Am J Kidney Dis. 2014;64(3):319-321. [DOI] [PubMed] [Google Scholar]
- 33. Ontario Renal Network. Ontario 2016 CKD System Atlas: Trends in Kidney Disease and Care. Toronto, Canada: Ontario Renal Network; 2016. [Google Scholar]
- 34. United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;1;1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jin D. Major changes and improvements of dialysis therapy in Korea: review of end-stage renal disease registry. Korean J Intern Med. 2015;30(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mehrotra R, Kermah D, Fried L, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18(10):2781-2788. [DOI] [PubMed] [Google Scholar]
- 37. Golper TA. The possible impact of the US prospective payment system (“bundle”) on the growth of peritoneal dialysis. Perit Dial Int. 2013;33(6):596-599. [DOI] [PMC free article] [PubMed] [Google Scholar]