Abstract
Auxins and cytokinins support cell division in tissue and cell cultures. In cytokinin-independent pear (Pyrus communis) cells, omission of 2,4-dichlorophenoxyacetic acid (2,4-D) from the medium for two successive transfers leads to rapid cell lysis, unless the osmolarity is raised to 0.4 molar with mannitol. Use of this system (nutrients plus mannitol minus 2,4-D) for the study of cell senescence was explored both in batch culture and in a system designed to permit medium renewal without withdrawal of live cells.
In both systems, an initial period (1-6 days) of limited increase in cell number is characterized by a continuous decrease in the respiratory activity and in protein and RNA synthesis to very low basal rates. In batch culture, cell death occurs after 13 to 15 days with little or no change in metabolic activity, or in protein and RNA synthesis. With renewal of cell medium, death is slightly delayed and is preceded by a burst in RNA synthesis followed by a notable increase in protein synthesis. Cycloheximide inhibition of protein synthesis is transient and its effect on cell longevity variable. Nonetheless, in all instances cell death is preceded by a burst in protein synthesis. Actinomycin D (1.6 micromolar) did not significantly affect protein synthesis but delayed RNA synthesis and cell death. The possible roles of auxin, osmoticum, and macromolecular synthesis in cellular senescence and death are discussed.
Full text
PDF
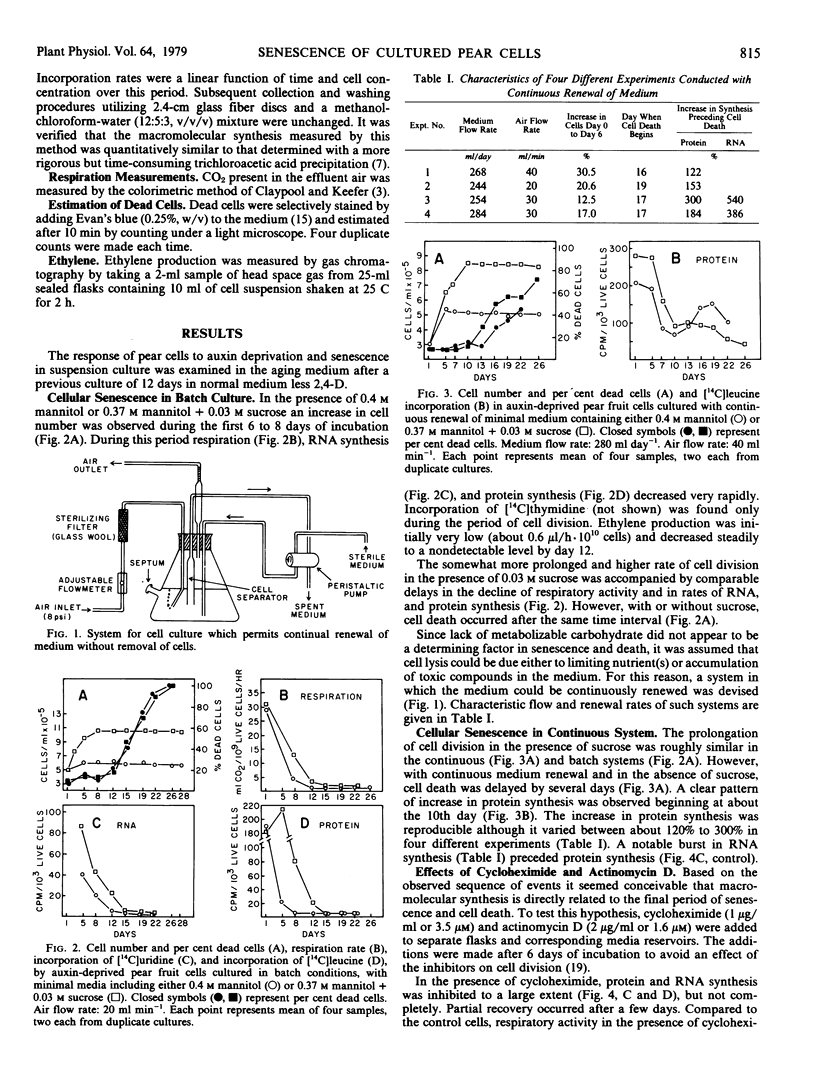
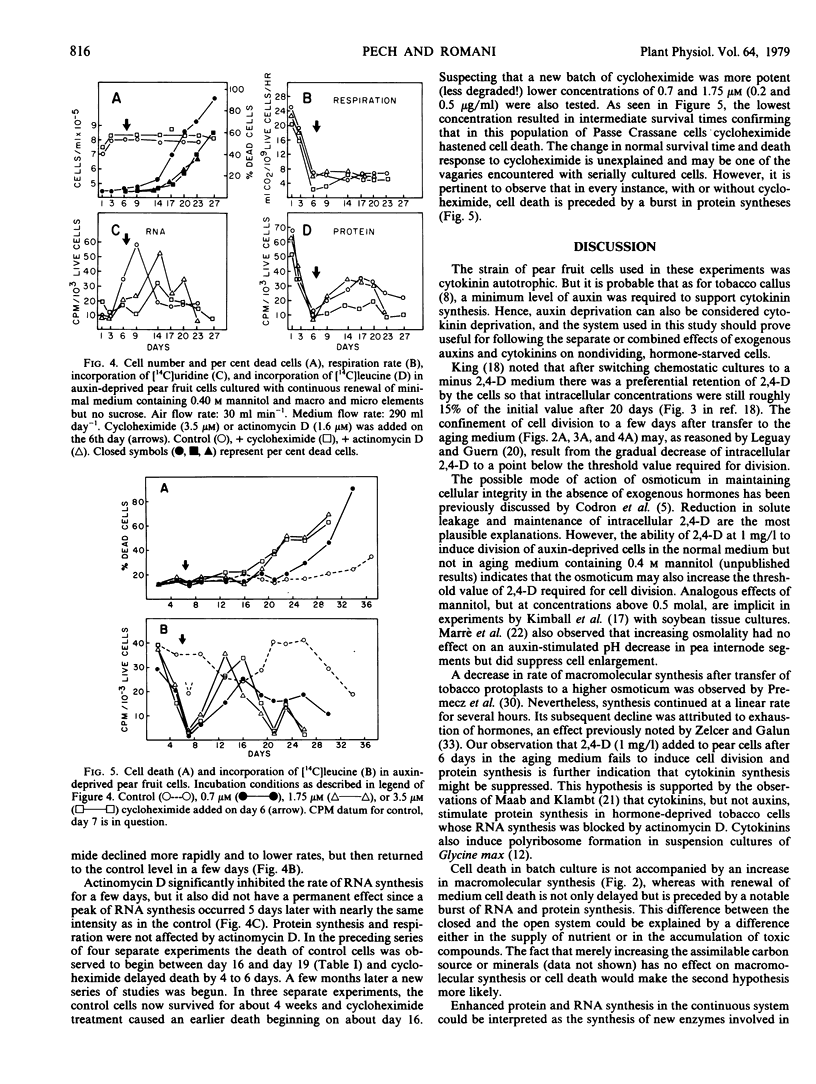

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E., Exworth C. P. Transient inhibition by cycloheximide of protein synthesis in cultured plant cell suspensions: a dose response paradox. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1075–1080. doi: 10.1016/0006-291x(73)91516-7. [DOI] [PubMed] [Google Scholar]
- Devilliers O. T., Ashton F. M. Metabolic Activity of Isolated Leaf Cells of Phaseolus vulgaris in Relation to Leaf Development. Plant Physiol. 1977 Jun;59(6):1072–1075. doi: 10.1104/pp.59.6.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W. Two effects of cytokinin on the auxin requirement of tobacco callus cultures. Plant Physiol. 1977 Jan;59(1):45–47. doi: 10.1104/pp.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Widholm J. M. A simple, rapid, and sensitive method for estimation of DNA, RNA, and protein synthesis in carrot cell cultures. Anal Biochem. 1973 Dec;56(2):346–352. doi: 10.1016/0003-2697(73)90200-5. [DOI] [PubMed] [Google Scholar]
- Fletcher J. S. Control of amino Acid synthesis in tissue culture cells. Plant Physiol. 1975 Sep;56(3):450–451. doi: 10.1104/pp.56.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket D. E., Volk M. J., Goldsmith M. R. Polyribosome Formation in Relation to Cytokinin-induced Cell Division in Suspension Cultures of Glycine max [L.] Merr. Plant Physiol. 1977 Oct;60(4):554–562. doi: 10.1104/pp.60.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki R. I., Zaitlin M., Jensen R. G. Metabolism of Separated Leaf Cells: II. Uptake and Incorporation of Protein and Ribonucleic Acid Precursors by Tobacco Cells. Plant Physiol. 1971 Jul;48(1):14–18. doi: 10.1104/pp.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C., Klein I., Dilley D. R. Protein synthesis in relation to ripening of pome fruits. Plant Physiol. 1968 Jul;43(7):1146–1153. doi: 10.1104/pp.43.7.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Altman A., Galston A. W. Dual Mechanisms in Polyamine-mediated Control of Ribonuclease Activity in Oat Leaf Protoplasts. Plant Physiol. 1978 Jul;62(1):158–160. doi: 10.1104/pp.62.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. T. Cytokinin-controlled Indoleacetic Acid Oxidase Isoenzymes in Tobacco Callus Cultures. Plant Physiol. 1971 Feb;47(2):181–185. doi: 10.1104/pp.47.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguay J. J., Guern J. Quantitative Effects of 2,4-Dichlorophenoxyacetic Acid on Growth of Suspension-cultured Acer pseudoplatanus Cells: II. Influence of 2,4-D Metabolism and Intracellular pH on the Control of Cell Division by Intracellular 2,4-D Concentration. Plant Physiol. 1977 Aug;60(2):265–270. doi: 10.1104/pp.60.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]



