Abstract
Background
The purpose of this review was to summarize the published literature on the association of childhood, adulthood and life course socio-economic status (SES) with obesity between January 1990 and June 2015.
Methods
The major medical electronic databases were searched to identify studies that examined SES over the life-course in relation to obesity. A total of 219 studies were identified through the initial search, and 35 qualified for full review. Of these, 14 publications met our inclusion criteria for the meta-analysis, all from developed or upper-middle income countries.
Results
There was a consistent association between lower life course SES and obesity among women (summary OR: 1.35, 95% CI: 1.04, 1.76), but not among men (summary OR: 0.92, 95% CI: 0.60, 1.40). Overall, mean BMI was higher among individuals with lower life course SES compared with those with higher life course SES (summary mean BMI difference: 0.65, 95% CI: 0.59, 0.71). Mean waist circumference (WC) was higher among women with lower life course SES compared with those with higher life course SES (summary mean WC: 4.67, 95% CI: 4.15, 5.20), but lower among men (summary mean WC difference: -0.10, 95% CI: -0.11, -0.08).
Conclusion
The inverse relationship between life course SES and obesity among women was consistent, based mostly on studies in developed countries. Nevertheless, critical information gaps remain in relation to the impact of childhood and life course SES on obesity in developing countries.
Background
Although the global prevalence of obesity nearly doubled between 1980 and 2008, obesity rates have risen faster in low- and middle- income countries (LMICs) compared with high income countries [1]. Recent transitions in nutrition and lifestyle in many LMICs have led to increased life expectancy, but also to increased consumption of high-fat and high-calorie diets and physical inactivity, mirroring trends observed in high-income countries several decades ago[2]. Renewed attention has been paid to the importance of social inequalities in health, and a recent Institute of Medicine report recognized that chronic disease risk factors, such as obesity, are likely shaped over the life course during critical windows of development from childhood to adolescence [3]. Scientific evidence suggests that social class affects health [4–9], and cumulative stress beginning in infancy predicts risk factors, leading to development of chronic diseases [10]. These observations, coupled with evidence of increasing global prevalence of obesity and obesity-associated chronic diseases, which are also socio-economically patterned, highlight the importance of understanding the socio-economic patterns of obesity over the life course.
Current recommendations from the World Cancer Research Fund (WCRF) state that median adult body mass index (BMI) be maintained between 21-23kg/m2, based on population-specific normal ranges [11]. As countries transition from low to middle income, understanding the demographic and socio-economic patterns of obesity across the life course will be critical for avoiding the obesity trend observed as middle-income countries transition to high-income[2]. Progress on reducing obesity and obesity-associated chronic diseases requires a thorough understanding of risk factors starting in early life in order to inform obesity prevention strategies that have the best chance of success. In this systematic review and meta-analysis, we summarize the existing literature on the link between life course SES and obesity, and assessed similarities and differences between studies from developed and developing countries.
Methods
Data sources and literature search
We conducted a systematic review of previously published studies between January 1990-June 2015 through the PUBMED, MEDLINE, EMBASE and CINAHL databases, following established PRISMA guidelines. We used relevant text words and medical subject heading (MESH) terms to identify relevant studies, including all iterations of ‘Life course’, ‘socioeconomic’, ‘social class’, ‘social accumulation’, ‘father’s’, ‘mother’s’ combined with any of the following keywords: ‘body mass index’, ‘obesity’, ‘adiposity’, and ‘weight change’. Included studies were those focused on adults and published in the English language. Data for this study was obtained from published articles, and was therefore exempt from ethical review.
Study selection and data extraction
A flowchart depicting the process of study selection according to PRISMA guidelines is detailed in S1 Fig. Two authors (SN and TA) independently reviewed study titles, abstracts and full text, and discrepancies in selected articles were resolved following a discussion by both authors. Studies were included in the analysis if they reported a quantitative estimate (e.g. odds ratio (OR), mean BMI, prevalence) and standard errors (SE or 95% confidence interval) between life course SES and a measure of obesity (weight, BMI, waist-to-hip ratio, adiposity). If there was no data on confidence interval or standard error of an estimate presented, the sample size of each group had to be present to be included in our analysis. Studies were excluded if they met at least one of the following criteria: (1) no relevant data presented; (2) non-English language; (3) other outcomes; (4) not original research; (5) no life course SES; or (6) published outside the range of 1990-June 2015. Data from each selected study was abstracted into an electronic database and independently verified against the original articles. Study data extracted included: author name and year of publication, country of the study, study design, study population demographics, sample size, SES construct, BMI or obesity measure and study covariates. We extracted estimates and 95% CI or standard errors for the two most extreme categories of life course SES- comparing the highest SES categories over the life course with the lowest.
Statistical analysis
We calculated the mean BMI, mean waist circumference (WC) and odds ratios (ORs) for BMI comparing the lowest life course SES category with the highest life course SES category for each study. When relative estimates were reported comparing the opposite contrast i.e. highest SES compared with lowest, we calculated the inverse to correspond to lowest SES vs. highest SES. We estimated summary rate ratios and mean BMI estimates comparing low life course SES with high life course SES using random-effects models. We assessed heterogeneity across studies using the Cochran Q-statistic, and used the I2 statistic to approximate the proportion of total variation in the estimates due to between-study heterogeneity. We conducted subgroup analysis by gender, (males, females, all genders), and tests of heterogeneity between subgroups were estimated using meta-regression analysis. Potential publication bias was assessed via visual inspection of funnel plots as well as with the Egger’s test. We present here the results of the Egger’s test. All statistical analyses were performed using STATA software, version 12.0 (Stata Corp, College Station, Texas USA).
Results
Literature search and included studies
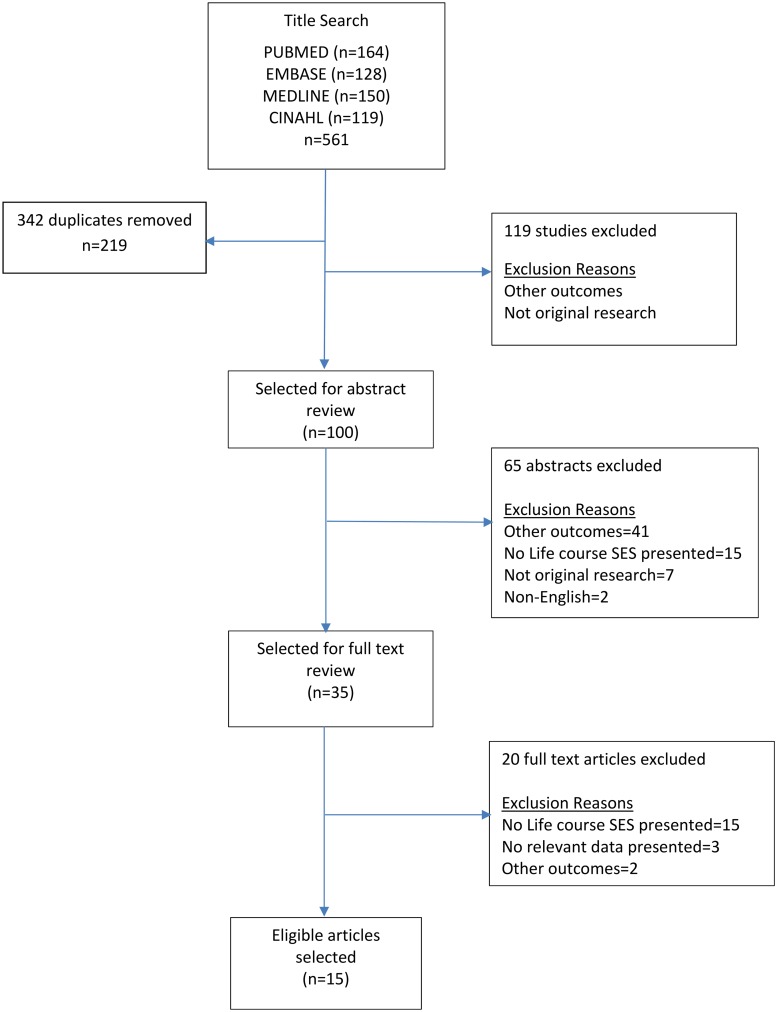
We identified 219 unique studies through our literature search, and reviewed 35 studies in full (Fig 1). After full-text review, 15 studies were eligible for inclusion in Table 1[4, 12–25]. One study did not report data required for calculating confidence intervals, and therefore was not included in the calculation of summary estimates using meta-analysis but was included in the systematic review since it met the study inclusion criteria[25]. Most of the studies were published between 2003 and 2014[4, 12–24]. Three out of the 15 were based on US populations [19, 20, 22], while others included study populations from the UK[13, 17, 18, 23], Denmark[15], Brazil[12, 21], Singapore[16], Scotland[14, 25], Australia[4], and Spain[24]. Seven studies were longitudinal in design [4, 12, 13, 15, 17, 19, 21], and eight were cross-sectional [14, 16, 18, 20, 22–25]. Life course SES was evaluated using self-reported data in only two studies [4, 15], while the majority used a combination of self-reported and primary data [12–14, 16–20, 22–25]. Data source was not reported in one study [21]. Eight studies reported life course SES based on father’s (or the primary caregiver’s) occupation [13, 14, 17, 18, 22–25], others reported life course SES based on childhood family income [12, 16, 21], while others used a combination of parental occupation, family income and/or parental education [4, 15, 19, 20]. One study [25] obtained data from males only, three studies [4, 22, 23] on females only, while 11 studies [12–21, 24] obtained data from both male and female participants. Nine studies [14–16, 18–20, 22, 23, 25] provided estimates of the association between life course SES and measures of obesity that were adjusted for confounders, while six studies [4, 12, 13, 17, 21, 24] reported only unadjusted estimates.
Fig 1. Publication search and selection results.
Table 1. Studies reviewing life course SES and obesity, published 1990–2015.
| Author, year | Country | Design | Population | Measure of Lifecourse SES | N | Estimate Low vs. High SES | Covariates | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Boylan, 2014[15] | Denmark | Longitudinal | Male and female teenagers born 1964–1969 | Father's occupation, father's education+own occupation, own education | 786 | OR (CI): M: 1(.3–2.9); F: 2.8(.9–8.3) | adult energy intake, physical activity, smoking, adolescent/parental BMI, adult/adolescent SES | Women in the stable low lifecourse SES group were more likely to be obese compared with those in the stable high SES. Men's BMI remain unchanged between the groups. |
| Aitsi-Selmi, 2013[12] | Brazil | Longitudinal | Males and females recruited at birth in 1978/79 | Childhood family income+current family income | 2063 | Mean BMI (SD): M: 24.8(4.6) vs. 25.4(4.7); F: 24.6(5.8) vs. 22.6(4.1); Mean WC (SD): M: 86.8(12.0) vs. 88.9(12.4); F: 79.4(13.1) vs. 73.9(9.7); Mean Waist-Hip Ratio (SE): M: .86(.06) vs .86(.06); F:.78(.06) vs. .74(.06) | None | Men in the persistently low lifecourse SES group had a slightly lower BMI on average than men in the persistently high lifecourse SES group, however women in the persisently low lifecourse SES group had a higher BMI than those in the high group. |
| Malhotra, 2013[16] | Singapore | Cross-sectional | Males and females aged 60+ | Family financial status+own education | 3566 | OR (CI): M: .68(.38–1.22); F: 1.32(.88–1.99) | age | Men were less likely to be obese if they were in low lifecourse SES, while results for women were opposite but non-significant. |
| Murray, 2011[17] | UK | Longitudinal | Males and females recruited at birth, 1946 | Father's occupation+own occupation | 3035 | Mean BMI (SE): M: 27.9(4.2) vs. 26.7(3.8); F: 28.6(6.3) vs. 26(4.4) | None | Men and women in the persistent low lifecourse SES groups had higher BMI than those in the persistent high lifecourse SES groups |
| Heraclides, 2010[18] | England | Cross-sectional | Males and females aged 44–69 | Father's occupation+own education, own occupation | 4598 | OR(CI): M: 1.25 (1.0–1.55)); F: 2.61 (1.79–3.78) | age | Women and men with low lifecourse SES were more likely to be obese compared with those of high lifecourse SES. |
| Scharoun-Lee, 2009[19] | US | Longitudinal | M and F adolescents in grades 7–12 | Parental material endowments, skills, knowledge, material, human, and social capital+own of above | 12940 | RRR obesity (BMI) incidence (CI): M: 1.18(.82–1.7); F: 3.01(1.95–4.66); RRR obesity (BMI) persistence (CI): M: 1.98(1.25–3.15); F: 3.56(2.01–6.3) | age | Men were slightly more likely to be obese, and women were 3 times more likely to be obese if they were in the persistent low lifecourse SES compared to high lifecourse SES. |
| Hart, 2008[14] | Scotland | Cross-sectional | Males and females aged 30–59 | Parental occupation+own occupation | 2338 | PR(CI): M: 1.28(1.09–1.51); F: 1.92(1.63–2.26); Mean WC (SE): M: 93.3(11.9) vs. 93.4(10); F: 83(13.2) vs. 78.9(12.3) | age | Men and women in the stable low SES group had higher BMI than those in the stable high SES group |
| Bennett, 2007[20] | US | Cross-sectional | African American males and females aged 25–50 at baseline | Parental occupation, material household conditions+own education, occupation, employment status, homeowner | 1178 | Mean BMI at baseline (SE): M: 25.7 (0.3) vs. 26.6(.8); F: 30.0(.4) vs. 27.3(1); Mean BMI at followup (SE): M: 28.7(.4) vs. 30.6 (1.0); F: 34.5(.5) vs. 33.9(1.3); Mean BMI change (SE): M: 3.1(.3) vs. 4.0(.7); F: 4.5(.3) vs. 6.6(.9) | age | Men who were in the low lifecourse SES group had lower BMI, however women in the low lifecourse SES group had higher BMI at baseline compared with those in the high lifecourse SES. |
| Ball, 2006[4] | Australia | Longitudinal | Females aged 18–23 years at baseline | Father's education+own education; Mother's education+own education; Father's occupation+own occupation; Mother's occupation+own occupation | 8756 | Father edu. Mean BMI (SD): 24.3(5.2) vs. 22.8 (4.2); Δweight (SE): 2.9(7.3) vs 2.0(5.9); Mother edu. Mean BMI (SD): 24.2 (5.2) vs 22.9(3.9); Δweight (SE): 2.9(7.3) vs 1.9(5.7); Father occ. Mean BMI (SD): 24.2(5.1) vs. 23(4.2); Δweight (SE): 2.6(6.9) vs 2.2(6.0); Mother occ. Mean BMI(SD): 24(5) vs. 23 (4.2); Δweight (SE): 2.5(6.9) vs 2.0(5.9) | none | Average BMI was higher among women in the low lifecourse SES groups compared to higher lifecourse SES groups based on both mother and father education or employment. |
| Barros, 2006[21] | Brazil | Longitudinal | Males and females recruited at birth, 1982 | Childhood family income+current family income | 1031 | PR (CI): M: .42(.36-.49); F: 1.4(1.2–1.63) | none | The prevalence of overweight was higher among women who were always poor compared with those who were never poor. The opposite was true for men. |
| James, 2006[22] | US | Cross-sectional | African American females aged 25–50 | Parental occupation+own education, occupation, employment status, housing status | 679 | OR (CI): 2.12(.75–6.0) | age, marital status, alcohol, smoking, childhood food insecurity, fruit/veg consumption, strenuous exercise | Women in the stable low lifecourse SES group had twice the odds of obesity compared to women in the stable high lifecourse SES group, but this was not statistically significant |
| Ebrahim, 2004[23] | UK | Cross-sectional | Females aged 60–79 years | Father's occupation+ own occupation | 2936 | PR (CI): 1.88(1.63–2.87) | age | The prevalence of obesity was higher among women with low lifecourse SES (adult and childhood manual occupation) compared with high lifecourse SES. |
| Regidor, 2004[24] | Spain | Cross-sectional | Males and females aged 60 and older | Father's occupation+own occupation | 4009 | General obesity PR (CI): M: 1.01 (.84–1.21); F: 1.21(1.06–1.38); Abdominal obesity PR (CI): M: 1.02(.9–1.16); F: 1.11(1.04–1.18) | none | The prevalence of general obesity was higher among women of low lifecourse SES (working class childhood and adulthood) compared with high lifecourse SES, but not among men |
| Langenberg, 2003[13] | UK | Longitudinal | Males and females recruited at birth in 1946 | Father's occupation+own occupation | 3035 | Mean BMI (SE): M: 27.7(.20) vs. 26.8(.18); F: 28.8(.28) vs. 26.1(.26); Mean Waist Hip Ratio (SE): M: 94.9(.31 vs. 92.5(.48); F: 81.9(.31) vs. 79.6(.31); Mean WC (SE): M: 98.5(.54) vs. 96.6(.51); F: 89(.66) vs. 83.1(.59) | none | Mean BMI was higher among men and women with low lifecourse SES (manual father and adult social class) compared with those with high lifecourse SES |
| Blane, 1996[25] | Scotland | Cross-sectional | Males aged 35–64 | Father's occupation+own occupation | 5645 | Mean BMI: 25.3 vs. 24.9 | age | Men who remained in a low lifecourse SES had higher BMI compared to men who remained in a high lifecourse SES |
BMI, body mass index; M, males; F, females; WC, waist circumference; OR, odds ratio; CI, confidence interval; SE, standard error; RRR, relative risk ratio; PR, prevalence ratio; edu, education; occ, occupation
Association between life-course SES and mean BMI
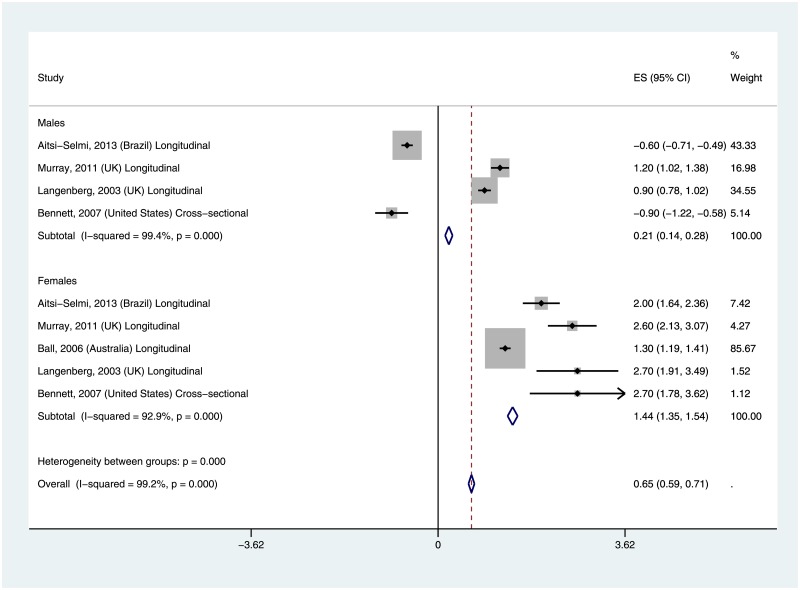
Five studies reported an association between life course SES and mean BMI[4, 12, 13, 17, 20], four included data from both males and females [12, 13, 17, 20], and one study included only females [4]. Four of the studies were conducted in the developed countries of US, UK and Australia [4, 13, 17, 20], while one study was conducted in Brazil [12]. The results of the meta-analysis examining the association between life course SES and adult BMI are displayed in Fig 2. Males with lower life course SES had slightly higher mean BMI compared with those of higher life course SES; the pooled estimate of the mean BMI difference was 0.21 (95% CI: 0.14–0.18). Among females, there was a larger mean BMI difference among those of lower life course SES compared with those of higher life course SES; the pooled estimate of the mean BMI difference was 1.44 (95% CI: 1.35–1.54). Overall when both genders were combined, mean BMI remained higher among participants with lower life course SES compared with those of higher life course SES: 0.65 (95% CI: 0.59–0.71). Results for females were consistent across all included studies, while two studies for males reported lower mean BMI among lower life course SES adults (63, 44). One of the two studies was a longitudinal study from Brazil[12] while the other was a cross-sectional study from the U.S.[20]. There was evidence of significant heterogeneity across the included studies (I2 = 99%, p-value = 0.00) overall, and for both males (I2 = 99%, p-value = 0.00) and females (I2 = 94%, p-value = 0.00). Sensitivity analysis excluding the only study with a cross-sectional design had minimal effects on the finding.
Fig 2. Mean BMI difference by life course SES.
Association between life course SES and BMI categories
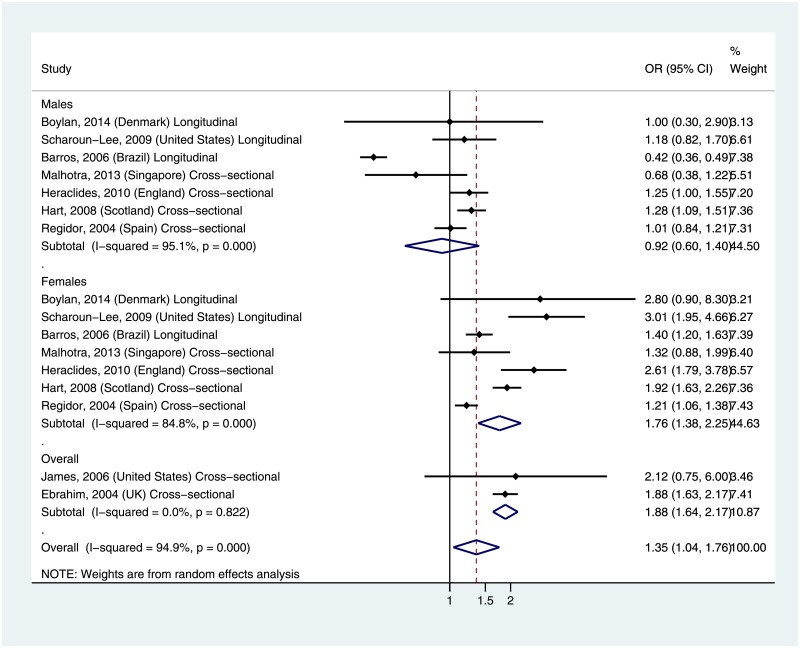
Seven studies examined life course SES in relation to the odds of obesity (Fig 3) among males and females [14–16, 18, 19, 21, 24], while two other studies examined the same association but results were not stratified by gender [22, 23]. The studies represented in the analyses were mostly from developed countries, including Denmark, US, Scotland, Spain, England, UK, Singapore, and one middle-income country- Brazil. There was no significant difference in the odds of obesity by life course SES among males (Summary OR: 0.92, 95% CI: 0.60–1.40). In contrast, females of lower life course SES had significantly higher odds of obesity compared with those of higher life course SES (Summary OR: 1.76, 95% CI: 1.38–2.25). Two studies reported estimates that were un-stratified by gender [22, 23], and also showed a higher summary odds ratio of obesity among lower life course SES compared with higher life course SES (Summary OR: 1.88, 95% CI: 1.6402.17). The overall summary OR across all included studies was 1.35 (1.04–1.76). While all of the included studies for females reported higher OR for obesity among lower life course SES participants, two out of the seven studies of males reported lower ORs among lower life course SES participants; a longitudinal study conducted in Brazil [21] and a cross-sectional study from Singapore [16]. There was evidence of significant heterogeneity across all of the included studies (I2 = 95%, p-value = 0.00) and for both males (I2 = 95%, p-value = 0.00) and females (I2 = 85%, p-value = 0.00). However, there was no evidence of heterogeneity in the two studies that were un-stratified by gender (I2 = 0.0%, p = 0.82). Both studies were cross-sectional designs, and based in the U.S. and U.K.
Fig 3. Summary odds ratio for obesity by life course SES.
Association between life course SES and waist circumference
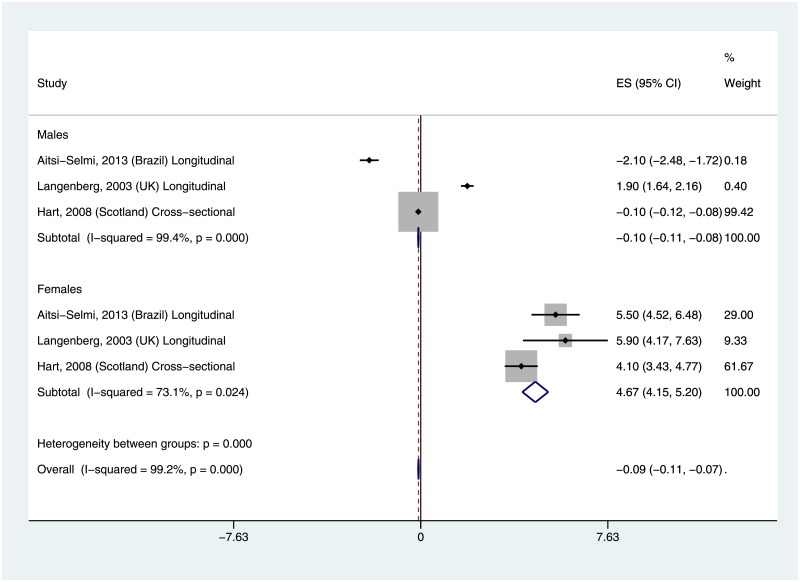
Three studies examined life course SES in relation to waist circumference (WC) among males and females [12–14]. The three studies were conducted in Brazil, UK and Scotland. Two [12, 14] of the three studies showed lower mean WC among lower life course SES males, while the other study [13] showed higher mean WC among lower life course SES males (Fig 4). The summary mean WC comparing lower life course SES with higher life course SES among males was -0.10 (95% CI: -0.11, -0.08). All three studies showed significantly higher WC among lower life course SES compared with higher life course SES females, with summary mean WC of 4.67 (95% CI: 4.15, 5.20). Overall, the summary WC comparing lower life course SES adults with higher life course SES adults was -0.09 (-0.11, -0.07). There was evidence of significant heterogeneity across all of the included studies (I2 = 99%, p-value = 0.00) and for both males (I2 = 99%, p-value = 0.00) and females (I2 = 73%, p-value = 0.02).
Fig 4. Mean difference in waist circumference by life course SES.
Discussion
We focused our review on the influence of SES across the life course on obesity. Whereas we observed that there were somewhat consistent findings for the association between life course SES and obesity in developed countries[4, 13, 14, 16, 18–20, 22, 23, 26, 27], there was very little information available for developing countries. We conducted a systematic review and meta-analysis to synthesize the current evidence in this area and identify gaps in the literature, focusing on studies that directly examined SES change over the life course. The results of our meta-analysis were highly consistent with previous findings. Women with higher SES throughout their life have lower BMI, while findings among men were less consistent. One possible reason for the differing association is that females may have weight-related ideals that are easier to maintain with higher income[28], and that these ideals may not exist for men[29]. While another possible reason for this difference is the fact that low SES men might engage in higher levels of physical activity because they engage in more manual occupational labor[30], this does not take into account the effect of childhood SES.
Most of the studies were conducted in developed countries (13 out of 15), with the exception of two studies that were conducted in Brazil[12, 21], an upper-middle income country according to the World Bank Income Classification. Therefore, it was not surprising that findings from the Brazilian studies were similar to results of other developed countries, i.e. women were more likely to be obese if they had lower life course SES. This is consistent with Monteiro’s and Dinsa’s reviews indicating that the association between SES and obesity becomes inverted as countries transition into higher income [31, 32]. Singapore was an interesting inclusion in the meta-analysis, because the majority of the participants, all aged ≥ 60 years, would have spent all or most of their childhood during the period 1912–1965, during which time Singapore was considered to be a developing country [16]. While the study from Singapore revealed that men were more likely to be obese if they had higher life course SES, women were more likely to be obese if they had lower life course SES, similar to patterns observed in developed countries. Further research is needed to illuminate the socio-economic transition that occurred in Singapore, and the impact of obesity and associated chronic diseases in adulthood.
The studies included in this review sought to determine the influence of SES on obesity from childhood through adulthood, providing a comprehensive investigation of reported differences in both developed and developing countries. Although some studies find that the effects of childhood SES on adult obesity were attenuated when adult SES was accounted for[4, 6, 13, 14, 20, 22, 23, 27, 33–45], other studies nevertheless highlight the importance of considering the lasting effects of childhood social class, notably Tucker-Seeley et al.’s 2011 study (74) on multi-morbidity among older adults. In this study, participants who experienced childhood financial hardship were slightly more likely to suffer from chronic conditions in adulthood compared with those who did not report childhood financial hardship[46]. Another recent study linked childhood SES to breast cancer incidence and mortality[47], and a systematic review on the association between childhood SES and cause-specific mortality concluded that mortality risk for all causes was higher among those who experienced poor SES during childhood[48]. In the current review, the vast majority of studies have been in developed countries, with a few studies in middle-income countries where the association between childhood SES and adult obesity remains inconsistent. This highlights the need for more studies in developing countries, where chronic disease rates have risen significantly and are expected to outstrip infectious diseases as the major cause of death in a few decades. In all regions, further studies are needed to develop public health strategies aimed at mitigating the impact of low childhood SES on adult health.
Several publications from countries in both developed and developing countries suggest that there may be opposite, but consistent associations between adult SES and obesity which may also vary by gender. Several reviews of the literature have been conducted focusing on adult SES and obesity across countries, with most concluding that there are no consistent associations between obesity and SES among men [8, 31, 32]. High-income countries consistently show an inverse relationship between adult SES and obesity[4–9, 49]. Data from middle-income countries are somewhat less consistent, but this is most likely explained by the views of Monteiro (27) and Dinsa (31) in previous literature reviews, in which the authors concluded that the association between SES and obesity in women changes from a positive association to an inverse association as a country’s GDP increases [31, 32]. This view is supported by other studies[8, 50–52] that include LMICs. Older studies have characterized a positive, i.e. direct, association between BMI and SES[9, 53–55], while more recent studies suggest this link is complicated and changes as nations become more developed. The decreasing levels of obesity in higher SES women observed in more recent studies could be attributed to the availability of more variety in diet, including healthier options, and more chances for exercise[56, 57]. However, studies conducted in Brazil highlight the opposite shift in women, with low-income groups transitioning from excessive under-nutrition to obesity within 20 years[50]. Public health messages on obesity prevention and physical activity should target both higher SES adults as well as lower SES adults in low-income countries. This strategy will potentially prevent observed patterns from developed countries where, as high SES adults became more aware of the negative health impact of obesity, consumption of food with low dietary quality was reduced, but similar reductions were not observed among low SES adults, likely due to the greater convenience and lower cost of fast food options compared with higher quality diets[58, 59].
Strengths and limitations
Our systematic review and meta-analysis identified the lack of consensus among studies focusing on life course SES. The meta-analysis was strengthened by the inclusion of a large number of high-quality studies and participants. Some limitations of the studies included in this systematic review should also be noted. First, life course SES was frequently ascertained through self-reports, especially in cross-sectional studies, leading to potential recall bias. Nevertheless, the results were mostly consistent across cross-sectional and longitudinal studies. Second, given that all the included studies were conducted in developed and upper-middle income countries, the heterogeneity in dietary patterns across regions and countries may portend potentially limited generalizability of these findings. Third, differences in the definition of SES across included studies, with some studies using parental income and education, and others using material wealth, could be a source of heterogeneity in the observed associations. Finally, included studies differed in the type and number of confounders adjusted for in the analysis, raising the possibility of other unmeasured confounders of the associations. Nevertheless, these limitations are unlikely to significantly alter our conclusion since most of the included studies showed consistent associations.
Conclusion
At least 50% of NCD related deaths are preventable through prevention strategies focused on modifiable risk factors such as obesity, physical activity and nutrition [60–63]. Specifically, recent studies estimate that when started in early life, a large proportion of chronic diseases like cancer and cardiovascular diseases are preventable by reducing obesity and excess weight, and increasing physical activity [62, 63]. This systematic review and meta-analysis summarizes the existing literature on the association between SES and obesity, highlighting the need for public health strategies in population subgroups most vulnerable to obesity (due to easy access to cheap, unhealthy foods and lack of physical activity) and the need for effective strategies that have the best chance of reducing obesity at early ages. The lack of information regarding the association between childhood and life course SES and obesity in developing countries highlights the need for empirical studies to inform obesity prevention strategies in developing countries. Ethical Approval: This study was conducted using data from published studies and was exempt from ethical review.
Supporting information
(DOC)
Abbreviations
- BMI
Body Mass index
- HDI
Human Development Index
- HIV/AIDS
Human Immunodeficiency Virus/ Acquired Immune Deficiency Syndrome
- LMIC
Lower- Middle Income Countries
- NCD
Non Communicable Diseases
- OR
Odds Ratio
- SES
Socio-economic Status
- WC
Waist Circumference
- WCRF
World Cancer Research Fund
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organization. Obesity: Situation and trends http://www.who.int: WHO; 2015 [cited 2015 April 16]. http://www.who.int/gho/ncd/risk_factors/obesity_text/en/.
- 2.Steyn NP, McHiza ZJ. Obesity and the nutrition transition in Sub-Saharan Africa. Ann N Y Acad Sci. 2014;1311:88–101. 10.1111/nyas.12433 [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Breast Cancer and the Environment; A Life Course Approach. Washington, D.C.: Institute of Medicine of the National Academies, 2012. [Google Scholar]
- 4.Ball K, Mishra GD. Whose socioeconomic status influences a woman's obesity risk: her mother's, her father's, or her own? International journal of epidemiology. 2006;35(1):131–8. 10.1093/ije/dyi216 [DOI] [PubMed] [Google Scholar]
- 5.Burkert NT, Rasky E, Grossschadl F, Muckenhuber J, Freidl W. The Relation of Weight to Women's Health: A Matched Sample Study from Austria. Women & health. 2015;55(2):134–51. [DOI] [PubMed] [Google Scholar]
- 6.Chaparro MP, Koupil I. The impact of parental educational trajectories on their adult offspring's overweight/obesity status: a study of three generations of Swedish men and women. Social science & medicine. 2014;120:199–207. [DOI] [PubMed] [Google Scholar]
- 7.Senese LC, Almeida ND, Fath AK, Smith BT, Loucks EB. Associations between childhood socioeconomic position and adulthood obesity. Epidemiologic reviews. 2009;31:21–51. Epub 2009/08/04. 10.1093/epirev/mxp006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren L. Socioeconomic status and obesity. Epidemiologic reviews. 2007;29:29–48. 10.1093/epirev/mxm001 [DOI] [PubMed] [Google Scholar]
- 9.Stunkard AJ. Socioeconomic status and obesity. Ciba Foundation symposium. 1996;201:174–82; discussion 82–7, 88–93. Epub 1996/01/01. [PubMed] [Google Scholar]
- 10.Brunner E. Stress and the biology of inequality. BMJ (Clinical research ed). 1997;314(7092):1472–6. Epub 1997/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Cancer Research Fund. Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective. Washington, DC, USA: American Institute for Cancer Research, 2007. [Google Scholar]
- 12.Aitsi-Selmi A, Batty GD, Barbieri MA, Silva AAM, Cardoso VC, Goldani MZ, et al. Childhood socioeconomic position, adult socioeconomic position and social mobility in relation to markers of adiposity in early adulthood: evidence of differential effects by gender in the 1978/79 Ribeirao Preto cohort study. Int J Obes. 2013;37(3):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langenberg C, Hardy R, Kuh D, Brunner E, Wadsworth M. Central and total obesity in middle aged men and women in relation to lifetime socioeconomic status: evidence from a national birth cohort. J Epidemiol Community Health. 2003;57(10):816–22. Epub 2003/10/24. 10.1136/jech.57.10.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hart C, McConnachie A, Upton M, Watt G. Risk factors in the Midspan family study by social class in childhood and adulthood. International journal of epidemiology. 2008;37(3):604–14. Epub 2008/03/22. 10.1093/ije/dyn052 [DOI] [PubMed] [Google Scholar]
- 15.Boylan SM, Gill TP, Hare-Bruun H, Andersen LB, Heitmann BL. Associations between adolescent and adult socioeconomic status and risk of obesity and overweight in Danish adults. Obes Res Clin Pract. 2014;8(2):e163–71. Epub 2014/04/20. 10.1016/j.orcp.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 16.Malhotra R, Malhotra C, Chan A, Ostbye T. Life-course socioeconomic status and obesity among older Singaporean Chinese men and women. The journals of gerontology Series B, Psychological sciences and social sciences. 2013;68(1):117–27. Epub 2012/11/20. 10.1093/geronb/gbs102 [DOI] [PubMed] [Google Scholar]
- 17.Murray ET, Mishra GD, Kuh D, Guralnik J, Black S, Hardy R. Life course models of socioeconomic position and cardiovascular risk factors: 1946 birth cohort. Ann Epidemiol. 2011;21(8):589–97. Epub 2011/07/09. 10.1016/j.annepidem.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heraclides A, Brunner E. Social mobility and social accumulation across the life course in relation to adult overweight and obesity: the Whitehall II study. J Epidemiol Community Health. 2010;64(8):714–9. Epub 2009/09/10. 10.1136/jech.2009.087692 [DOI] [PubMed] [Google Scholar]
- 19.Scharoun-Lee M, Kaufman JS, Popkin BM, Gordon-Larsen P. Obesity, race/ethnicity and life course socioeconomic status across the transition from adolescence to adulthood. J Epidemiol Community Health. 2009;63(2):133–9. Epub 2008/11/04. 10.1136/jech.2008.075721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett GG, Wolin KY, James SA. Lifecourse socioeconomic position and weight change among blacks: The Pitt County study. Obesity (Silver Spring). 2007;15(1):172–81. Epub 2007/01/18. [DOI] [PubMed] [Google Scholar]
- 21.Barros AJ, Victora CG, Horta BL, Goncalves HD, Lima RC, Lynch J. Effects of socioeconomic change from birth to early adulthood on height and overweight. International journal of epidemiology. 2006;35(5):1233–8. Epub 2006/08/24. 10.1093/ije/dyl160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James SA, Fowler-Brown A, Raghunathan TE, Van Hoewyk J. Life-course socioeconomic position and obesity in African American Women: the Pitt County Study. American journal of public health. 2006;96(3):554–60. Epub 2006/02/02. 10.2105/AJPH.2004.053447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebrahim S, Montaner D, Lawlor DA. Clustering of risk factors and social class in childhood and adulthood in British women's heart and health study: cross sectional analysis. BMJ (Clinical research ed). 2004;328(7444):861. Epub 2004/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regidor E, Banegas JR, Gutierrez-Fisac JL, Dominguez V, Rodriguez-Artalejo F. Socioeconomic position in childhood and cardiovascular risk factors in older Spanish people. International journal of epidemiology. 2004;33(4):723–30. Epub 2004/03/27. 10.1093/ije/dyh105 [DOI] [PubMed] [Google Scholar]
- 25.Blane D, Hart CL, Smith GD, Gillis CR, Hole DJ, Hawthorne VM. Association of cardiovascular disease risk factors with socioeconomic position during childhood and during adulthood. BMJ (Clinical research ed). 1996;313(7070):1434–8. Epub 1996/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N, Chen JT, Selby JV. Class inequalities in women's health: combined impact of childhood and adult social class—a study of 630 US women. Public Health. 2001;115(3):175–85. Epub 2001/06/29. 10.1038/sj/ph/1900754 [DOI] [PubMed] [Google Scholar]
- 27.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, et al. Association between children's experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360(9346):1640–5. Epub 2002/11/30. 10.1016/S0140-6736(02)11602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeffery RW, French SA. Socioeconomic status and weight control practices among 20- to 45-year-old women. American journal of public health. 1996;86(7):1005–10. Epub 1996/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H, Harris KM, Gordon-Larsen P. Life Course Perspectives on the Links Between Poverty and Obesity During the Transition to Young Adulthood. Population research and policy review. 2009;28(4):505–32. Epub 2010/02/18. 10.1007/s11113-008-9115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang VW, Lauderdale DS. Income disparities in body mass index and obesity in the United States, 1971–2002. Archives of internal medicine. 2005;165(18):2122–8. Epub 2005/10/12. 10.1001/archinte.165.18.2122 [DOI] [PubMed] [Google Scholar]
- 31.Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bulletin of the World Health Organization. 2004;82(12):940–6. Epub 2005/01/18. doi: /S0042-96862004001200011 [PMC free article] [PubMed] [Google Scholar]
- 32.Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2012;13(11):1067–79. Epub 2012/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dundas R, Leyland AH, Macintyre S, Leon DA. Does the primary school attended influence self-reported health or its risk factors in later life? Aberdeen Children of the 1950s Study. International journal of epidemiology. 2006;35(2):458–65. Epub 2005/11/15. 10.1093/ije/dyi239 [DOI] [PubMed] [Google Scholar]
- 34.Giskes K, van Lenthe FJ, Turrell G, Kamphuis CB, Brug J, Mackenbach JP. Socioeconomic position at different stages of the life course and its influence on body weight and weight gain in adulthood: a longitudinal study with 13-year follow-up. Obesity (Silver Spring). 2008;16(6):1377–81. Epub 2008/03/22. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez D, Nazmi A, Victora CG. Childhood poverty and abdominal obesity in adulthood: a systematic review. Cadernos de saude publica. 2009;25 Suppl 3:S427–40. Epub 2010/01/21. [DOI] [PubMed] [Google Scholar]
- 36.Hardy R, Wadsworth M, Kuh D. The influence of childhood weight and socioeconomic status on change in adult body mass index in a British national birth cohort. Int J Obes Relat Metab Disord. 2000;24(6):725–34. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 37.Huurre T, Aro H, Rahkonen O. Well-being and health behaviour by parental socioeconomic status: a follow-up study of adolescents aged 16 until age 32 years. Soc Psychiatry Psychiatr Epidemiol. 2003;38(5):249–55. Epub 2003/04/30. 10.1007/s00127-003-0630-7 [DOI] [PubMed] [Google Scholar]
- 38.Kittleson MM, Meoni LA, Wang NY, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of internal medicine. 2006;166(21):2356–61. Epub 2006/11/30. 10.1001/archinte.166.21.2356 [DOI] [PubMed] [Google Scholar]
- 39.Kivimaki M, Smith GD, Juonala M, Ferrie JE, Keltikangas-Jarvinen L, Elovainio M, et al. Socioeconomic position in childhood and adult cardiovascular risk factors, vascular structure, and function: cardiovascular risk in young Finns study. Heart. 2006;92(4):474–80. Epub 2005/09/15. 10.1136/hrt.2005.067108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laaksonen M, Sarlio-Lahteenkorva S, Lahelma E. Multiple dimensions of socioeconomic position and obesity among employees: The Helsinki Health Study. Obes Res. 2004;12(11):1851–8. Epub 2004/12/17. 10.1038/oby.2004.230 [DOI] [PubMed] [Google Scholar]
- 41.Lawlor DA, Batty GD, Morton SM, Clark H, Macintyre S, Leon DA. Childhood socioeconomic position, educational attainment, and adult cardiovascular risk factors: the Aberdeen children of the 1950s cohort study. American journal of public health. 2005;95(7):1245–51. Epub 2005/06/29. 10.2105/AJPH.2004.041129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lidfeldt J, Li TY, Hu FB, Manson JE, Kawachi I. A prospective study of childhood and adult socioeconomic status and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165(8):882–9. Epub 2007/02/08. 10.1093/aje/kwk078 [DOI] [PubMed] [Google Scholar]
- 43.Power C, Atherton K, Strachan DP, Shepherd P, Fuller E, Davis A, et al. Life-course influences on health in British adults: effects of socio-economic position in childhood and adulthood. International journal of epidemiology. 2007;36(3):532–9. Epub 2007/01/27. 10.1093/ije/dyl310 [DOI] [PubMed] [Google Scholar]
- 44.Power C, Manor O, Matthews S. Child to adult socioeconomic conditions and obesity in a national cohort. Int J Obes Relat Metab Disord. 2003;27(9):1081–6. Epub 2003/08/15. 10.1038/sj.ijo.0802323 [DOI] [PubMed] [Google Scholar]
- 45.Power C, Graham H, Due P, Hallqvist J, Joung I, Kuh D, et al. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. International journal of epidemiology. 2005;34(2):335–44. Epub 2005/01/22. 10.1093/ije/dyh394 [DOI] [PubMed] [Google Scholar]
- 46.Tucker-Seeley RD, Li Y, Sorensen G, Subramanian SV. Lifecourse socioeconomic circumstances and multimorbidity among older adults. BMC public health. 2011;11:313 Epub 2011/05/17. 10.1186/1471-2458-11-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pudrovska T, Anikputa B. The role of early-life socioeconomic status in breast cancer incidence and mortality: unraveling life course mechanisms. Journal of aging and health. 2012;24(2):323–44. Epub 2011/10/01. 10.1177/0898264311422744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62(5):387–90. Epub 2008/04/17. 10.1136/jech.2007.065508 [DOI] [PubMed] [Google Scholar]
- 49.Cohen AK, Rai M, Rehkopf DH, Abrams B. Educational attainment and obesity: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2013;14(12):989–1005. Epub 2013/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monteiro CA, Conde WL, Popkin BM. The burden of disease from undernutrition and overnutrition in countries undergoing rapid nutrition transition: a view from Brazil. American journal of public health. 2004;94(3):433–4. Epub 2004/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Addo J, Smeeth L, Leon DA. Obesity in urban civil servants in Ghana: association with pre-adult wealth and adult socio-economic status. Public Health. 2009;123(5):365–70. Epub 2009/04/14. 10.1016/j.puhe.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 52.Senekal M, Steyn NP, Nel JH. Factors associated with overweight/obesity in economically active South African populations. Ethnicity & disease. 2003;13(1):109–16. Epub 2003/05/02. [PubMed] [Google Scholar]
- 53.Sobal J, Stunkard AJ. Socioeconomic status and obesity: a review of the literature. Psychological bulletin. 1989;105(2):260–75. Epub 1989/03/01. [DOI] [PubMed] [Google Scholar]
- 54.Nube M, Asenso-Okyere WK, van den Boom GJ. Body mass index as indicator of standard of living in developing countries. European journal of clinical nutrition. 1998;52(2):136–44. Epub 1998/03/20. [DOI] [PubMed] [Google Scholar]
- 55.Gilberts EC, Arnold MJ, Grobbee DE. Hypertension and determinants of blood pressure with special reference to socioeconomic status in a rural south Indian community. J Epidemiol Community Health. 1994;48(3):258–61. Epub 1994/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization. Diet, nutrition and the prevention of chronic diseases. Geneva: WHO, 2003. [PubMed] [Google Scholar]
- 57.World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: WHO, 2000. [PubMed] [Google Scholar]
- 58.Ball K, Lamb KE, Costa C, Cutumisu N, Ellaway A, Kamphuis CB, et al. Neighbourhood socioeconomic disadvantage and fruit and vegetable consumption: a seven countries comparison. Int J Behav Nutr Phys Act. 2015;12:68 10.1186/s12966-015-0229-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jack D, Neckerman K, Schwartz-Soicher O, Lovasi GS, Quinn J, Richards C, et al. Socio-economic status, neighbourhood food environments and consumption of fruits and vegetables in New York City. Public Health Nutr. 2013;16(7):1197–205. 10.1017/S1368980012005642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Global status report on noncommunicable diseases 2010. Geneva, Switzerland: 2011.
- 61.Kelly BB, Fuster V. Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health: National Academies Press; 2010. [PubMed] [Google Scholar]
- 62.Colditz GA, Bohlke K. Priorities for the primary prevention of breast cancer. CA: a cancer journal for clinicians. 2014;64(3):186–94. [DOI] [PubMed] [Google Scholar]
- 63.Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. The American journal of clinical nutrition. 2012;96(1):150–63. 10.3945/ajcn.111.031674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.