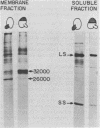
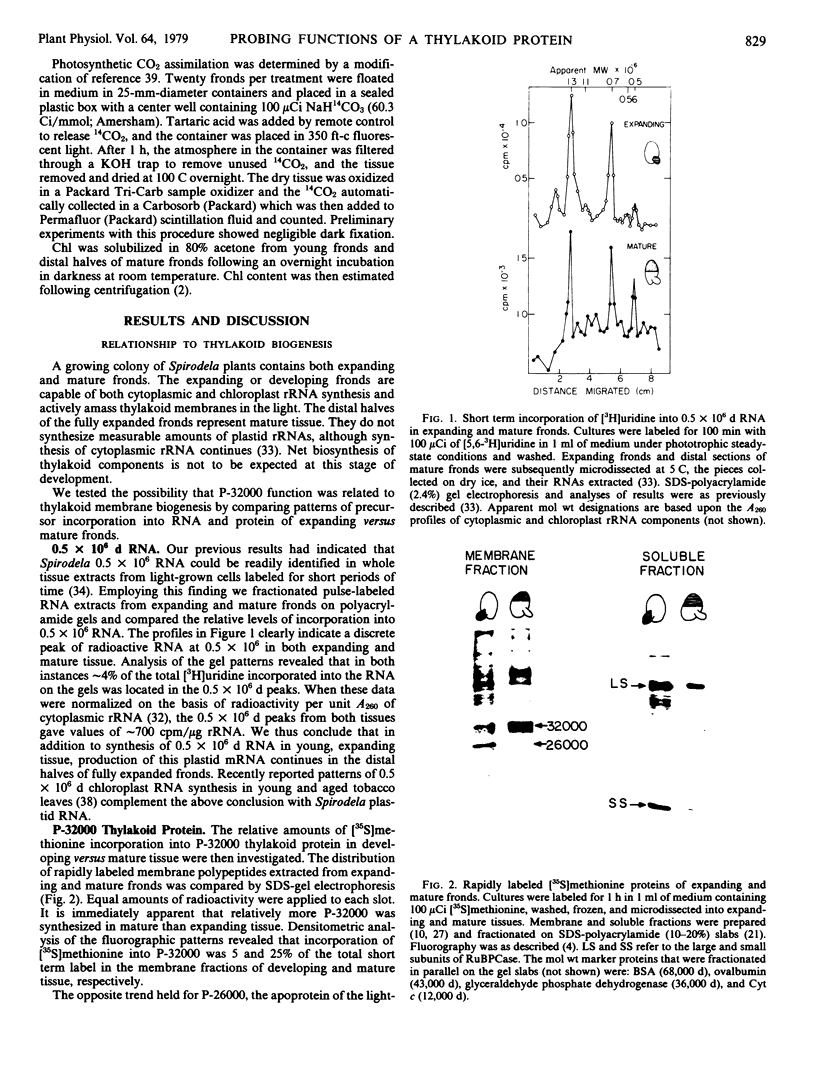
Abstract
The rapidly turning over, photoinduced thylakoid protein, P-32000, is the main pulse-labeled membrane polypeptide in the chloroplasts of Spirodela oligorrhiza, yet little is known of its physiological function. Two hypotheses are tested: that P-32000 synthesis is necessary for thylakoid biogenesis; that it directly participates in photosynthesis. Spirodela cultures were dissected into expanding and fully mature tissue. Fronds from both developmental stages transcribed a 0.5 × 106 dalton RNA likely to be the message for P-32000. As to the protein itself, synthesis occurred in both types of tissue but was considerably enhanced in the fully mature state. Thus, a purely transient, developmental function for P-32000 during thylakoid biogenesis appears ruled out. Low concentrations of d-threo-chloramphenicol severely suppressed P-32000 synthesis but not its turnover. As a result, fronds depleted in P-32000 were obtained. However, photoassimilation of CO2 remained at 86% of normal in tissue > 80% depleted of P-32000. Thus, P-32000 did not appear to be rate-limiting, suggesting that it does not serve as a direct, integral part of the photosynthetic pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Dickmann D. I., Gordon J. C. Incorporation of C-Photosynthate into Protein during Leaf Development in Young Populus Plants. Plant Physiol. 1975 Jul;56(1):23–27. doi: 10.1104/pp.56.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J. [Chloroplast ribosomes: stereospecificity of inhibition by chloramphenicol]. Science. 1969 Jan 31;163(3866):477–478. doi: 10.1126/science.163.3866.477. [DOI] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Steinback K. E., Bogorad L. Comparison of the Molecular Weights of Proteins Synthesized by Isolated Chloroplasts with Those Which Appear during Greening in Zea mays. Plant Physiol. 1979 Mar;63(3):436–439. doi: 10.1104/pp.63.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson D., Loening U. Ribosomal RNA precursors and the synthesis of chloroplast and cytoplasmic ribosomal ribonucleic acid in leaves of Phaseolus aureus. Eur J Biochem. 1974 May 15;44(2):501–507. doi: 10.1111/j.1432-1033.1974.tb03508.x. [DOI] [PubMed] [Google Scholar]
- Hartley M. R., Ellis R. J. Ribonucleic acid synthesis in chloroplasts. Biochem J. 1973 May;134(1):249–262. doi: 10.1042/bj1340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler J. J., Mendiola-Morgenthaler L. Synthesis of soluble, thylakoid, and envelope membrane proteins by spinach chloroplasts purified from gradients. Arch Biochem Biophys. 1976 Jan;172(1):51–58. doi: 10.1016/0003-9861(76)90046-1. [DOI] [PubMed] [Google Scholar]
- Patterson B. D., Smillie R. M. Developmental changes in ribosomal ribonucleic Acid and fraction I protein in wheat leaves. Plant Physiol. 1971 Feb;47(2):196–198. doi: 10.1104/pp.47.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz H., Reisfeld A., Sagher D., Edelman M. Ribulose Diphosphate Carboxylase from Autotrophic Euglena gracilis. Plant Physiol. 1975 Sep;56(3):345–350. doi: 10.1104/pp.56.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner A., Gressel J., Jakob K. M. Discoordination of ribosomal RNA metabolism during metabolic shifts of Spirodela plants. Biochim Biophys Acta. 1977 Feb 3;474(3):386–397. doi: 10.1016/0005-2787(77)90268-4. [DOI] [PubMed] [Google Scholar]
- Rosner A., Jakob K. M., Gressel J., Sagher D. The early synthesis and possible function of a 0.5 X 10(6) Mr RNA after transfer of dark-grown Spirodela plants to light. Biochem Biophys Res Commun. 1975 Nov 3;67(1):383–391. doi: 10.1016/0006-291x(75)90327-7. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Ellis R. J. Protein synthesis in chloroplasts. Characteristics and products of protein synthesis in vitro in etioplasts and developing chloroplasts from pea leaves. Biochem J. 1975 Mar;146(3):675–685. doi: 10.1042/bj1460675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J., Grierson D. Isolation and characterisation of 14-S RNA from spinach chloroplasts. Biochim Biophys Acta. 1978 Dec 21;521(2):619–633. doi: 10.1016/0005-2787(78)90303-9. [DOI] [PubMed] [Google Scholar]
- Swanson C. A., Hoddinott J. Effect of light and ontogenetic stage on sink strength in bean leaves. Plant Physiol. 1978 Sep;62(3):454–457. doi: 10.1104/pp.62.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]