Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease in which upper and lower motoneurons degenerate leading to muscle wasting, paralysis and eventually death from respiratory failure. Several studies indicate that skeletal muscle contributes to disease progression; however the molecular mechanisms remain elusive. Fibrosis is a common feature in skeletal muscle under chronic damage conditions such as those caused by muscular dystrophies or denervation. However, the exact mechanisms of fibrosis induction and the cellular bases of this pathological response are unknown. We show that extracellular matrix (ECM) components are augmented in skeletal muscles of symptomatic hSOD1G93A mice, a widely used murine model of ALS. These mice also show increased TGF-β1 mRNA levels, total Smad3 protein levels and p-Smad3 positive nuclei. Furthermore, platelet-derived growth factor receptor-α (PDGFRα), Tcf4 and α-smooth muscle actin (α-SMA) levels are augmented in the skeletal muscle of symptomatic hSOD1G93A mice. Additionally, the fibro/adipogenic progenitors (FAPs), which are the main producers of ECM constituents, are also increased in these pathogenic conditions. Therefore, FAPs and ECM components are more abundant in symptomatic stages of the disease than in pre-symptomatic stages. We present evidence that fibrosis observed in skeletal muscle of symptomatic hSOD1G93A mice is accompanied with an induction of TGF-β signaling, and also that FAPs might be involved in triggering a fibrotic response. Co-localization of p-Smad3 positive cells together with PDGFRα was observed in the interstitial cells of skeletal muscles from symptomatic hSOD1G93A mice. Finally, the targeting of pro-fibrotic factors such as TGF-β, CTGF/CCN2 and platelet-derived growth factor (PDGF) signaling pathway might be a suitable therapeutic approach to improve muscle function in several degenerative diseases.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by progressive degeneration of upper and lower motoneurons. The loss of motoneurons leads to muscle atrophy, spasticity, paralysis and death from respiratory failure, usually within 3–5 years of diagnosis [1, 2]. Most ALS cases (~90%) are sporadic, whereas the remaining ~10% corresponds to familial cases (fALS). Mutations in the superoxide dismutase 1 gene (SOD1) account for 10–20% of all fALS cases. Recently, a hexanucleotide repeat expansion in the C9ORF72 gene was found to explain more than 50% of fALS cases [3, 4]. It has been shown that overexpression of the mutant human SOD1 gene in mice reproduces the ALS phenotype observed in humans [5]. This model was useful to identify pathological alterations in motoneurons such as mitochondrial dysfunction, glutamate excitotoxicity, hyper-excitability, axonal transport deficits and protein aggregation, among others [6]. However, the mechanism by which mutant SOD1 becomes toxic remains elusive.
ALS is partially considered a non-cell autonomous disease, and several studies have shown that non-neuronal cells can contribute to disease progression. For instance, astrocytes that express mutant SOD1, and more recently mutant TDP-43, selectively kill motoneurons by a non-cell-autonomous mechanism [7–11]. Other studies suggest that microglia have enhanced redox stress and induce motoneurons injury when they are incubated in presence of extracellularly added mutant SOD1G93A [12, 13]. Nevertheless, whether skeletal muscle contributes to motoneuron degeneration and disease progression is not well established to date and the available evidence is still controversial. It has been shown that suppression of SOD1G93A by gene transfer in skeletal muscle does not delay disease progression nor does improve muscle function [14], suggesting that skeletal muscle is not a primary target in ALS. However, other studies have shown that the skeletal muscle is in fact, a primary target of SOD1G93A toxicity and can induce degeneration of motoneurons, contributing to disease onset [15, 16]. However, the molecular mechanisms by which skeletal muscle could be triggering neurodegeneration are not yet understood.
Fibrosis is a process that underlies several chronic disorders and involves the replacement of functional tissue by excessive non-functional connective tissue composed mainly by extracellular matrix (ECM), which affects normal cell physiology [17–19]. The fibrotic process in skeletal muscle has been well studied in the mdx mice, a murine model of Duchenne Muscular Dystrophy (DMD) [20–22]. It is known that several factors are involved in skeletal muscle fibrosis [23–25] and one of the best studied molecules is the transforming growth factor type-β (TGF-β), a central pro-fibrotic factor that is up-regulated in skeletal muscles of mdx mice [26–30]. The canonical TGF-β or Smad-dependent signaling pathway involves the direct phosphorylation of Smad2/3 by TGF-β receptor I kinase. Phosphorylated Smad2/3 binds to Smad4 and translocate into the nucleus [31, 32]. Several studies show elevated TGF-β1 levels in the serum and plasma from ALS patients [33, 34]. Intraperitoneal TGF-β2 injections improve motoneuron health and motor performance, however, they do not prevent motoneuron degeneration and mouse health deterioration [35]. A recent study shows strong evidence that TGF-β1 secreted by astrocytes from the spinal cord has a key role in the neuroprotective inflammatory response in ALS mice. However, excess of TGF-β1 from glia appears to have a negative role in neuroinflammation and blocking the TGF-β signaling pathway in a cell type-specific manner could be a therapeutic approach for neurodegenerative disorders [36]. It has been shown that TGF-β and Smad expression in skeletal muscle correlates with disease progression and could be a strong marker for ALS [37]. However, the role of TGF-β and ECM deposition in skeletal muscle from ALS mice models has not been studied.
Fibro/adipogenic progenitors (FAPs) are interstitial populations of mesenchymal cells that participate directly in fatty and fibrous connective tissue deposition in the skeletal muscle [38, 39]. These mesenchymal progenitors specifically express PDGFRα and Tcf4 [40, 41], and are increased in numbers in different models of chronic muscle damage [42–45]. Moreover, FAPs play a supporting role in healthy muscle regeneration [41, 46, 47].
In this work, we describe that elevated fibrosis correlates with enhanced TGF-β signaling in skeletal muscle of symptomatic hSOD1G93A mice. We also found that several fibro/adipogenic progenitor (FAPs) markers are increased, and that they could be responsible for ECM deposition observed in the skeletal muscle from symptomatic hSOD1G93A mice. Understanding the factors and cell types involved in exacerbated ECM synthesis and deposition is critical to further comprehend this devastating pathology.
Materials and methods
2.1 Animals and tissue collection
All protocols were conducted in strict accordance and with the formal approval of the Animal Ethics Committee of the Pontificia Universidad Católica de Chile. The hemizygous transgenic mice carrying the mutant human SOD1G93A (B6SJL-Tg (SOD1*G93A) 1Gur/J) gene were originally obtained from Jackson Laboratories (Bar Harbor, USA). Wild-type (B6SJL) and hSOD1G93A age-matched male mice (60 and 120 days old) were anesthetized with 3.0% isoflurane gas in pure oxygen and sacrificed by cervical dislocation. The gastrocnemius muscle was then dissected and removed. Tissues for western blot analysis were rapidly frozen in liquid nitrogen. Tissue samples for cryosectioning were frozen in liquid nitrogen cooled-isopentane (Merck, Darmstadt, GE) and stored at -80°C until processing [27].
2.2 Histology and Sirius red staining
Gastrocnemius muscle cryosections (7μm) were placed onto glass slides. Hematoxylin and eosin staining was performed to assess muscle architecture and histology. Briefly, tissue sections were incubated for 10 minutes in formalin (10% v/v), then washed with water, incubated for 5 minutes with diluted hematoxylin (Merck, Darmstadt, GE; 25% v/v in H2O) and washed in H2O. Eosin was added for 30 seconds and then dehydration with ethanol was performed. Finally, Entellan was added to the slices [27]. Total collagen content was evaluated by staining with 1% Sirius red in picric acid [28]. Briefly, sections were fixed in 100% ethanol at 4°C for 30 minutes, washed with H2O, incubated in picric acid at 50°C for 1 hour, washed with H2O at least three times and incubated for 5 minutes with a solution of direct Red plus picric acid protected from light. Sections were then washed briefly with acetic acid 2% v/v, ethanol 50% v/v plus picric acid 50% v/v in ethanol. A final dehydration was performed and xylol was added. Sections were imaged using bright field microscopy on a Nikon Eclipse E600 [48].
2.3 Immunofluorescence microscopy
For skeletal muscle immunofluorescence, cryosections (7μm) were fixed in 4% paraformaldehyde (Merck, Darmstadt, GE), blocked for 1 hour in blocking buffer (1% BSA, BM-0150, Winkler, Santiago, CL), 1% gelatin from cold water fish skin (G7765, Sigma, St. Louis, MO, USA), 0,01% Triton X-100 (X100-1L, Sigma, MO, St. Louis, USA) in PBS, and incubated overnight at 4°C with the following antibodies: anti-fibronectin (Sigma-Aldrich, St. Louis, MO, USA), anti-collagen-I (Abcam, Cambridge, UK), anti-p-Smad3 (Cell Signaling, Danvers, MA, USA), anti-Tcf4 (Cell Signaling, Danvers, MA, USA), anti-PDGFRα (R&D Systems, Minneapolis, MN, USA) anti-laminin (Sigma-Aldrich, St. Louis, MI, USA). The corresponding Alexa Fluor 568 or 488-conjugated anti IgGs (Invitrogen, Carlsbad, CA, USA) were used as secondary antibodies. For nuclear staining, sections were incubated with 1 μg/mL Hoechst 33258 in PBS for 10 minutes [42]. Slices were then washed in water and mounted in fluorescent mounting medium (DAKO, Santa Clara, CA, USA). Sections were visualized on a Nikon Eclipse E600 epifluorescence microscope or a Nikon Eclipse C2 si confocal spectral microscope using NIS-Elements software v4.20, 32 bit.
2.4 Immunoblot analysis
Muscles were homogenized in 10 volumes of Tris-EDTA buffer pH7.4 with 1mM phenylmethylsulfonyl fluoride (PMSF). Then, one volume of buffer containing 2% glycerol, 4% SDS and 0.125 M Tris pH 6.8 were added to the homogenates as described previously [27]. Protein concentration was determined in aliquots of muscle extracts using the Bicinchoninic Acid Protein Assay kit (Pierce, Rockford, IL, USA) with BSA as the standard. Aliquots (50μg) were subjected to sodium dodecyl sulphate gel electrophoresis (SDS-PAGE) in 9% polyacrylamide gels, electrophoretically transferred onto PVDF membranes (Millipore, Billerica, MA, USA) and probed with the following antibodies at 4°C overnight: anti-fibronectin (Sigma-Aldrich, St. Louis, MO, USA), anti-collagen-III (Rockland, Limerick, PA, USA), anti-Smad3 (Cell Signaling, Danvers, MA, USA), anti-PDGFRα R&D Systems, Minneapolis, MN, USA (), anti-Tcf4 (Cell Signaling, Danvers, MA, USA), α-SMA (Sigma-Aldrich, St. Louis, MO, USA), anti-TGF-β(1, 2, 3) (R&D Systems, USA), anti-CTGF (Santa Cruz, USA) and anti-GAPDH (Millipore, Billerica, MA, USA). Primary antibodies were detected with horseradish-peroxidase-conjugated secondary antibodies after 1h incubation at room temperature. All immunoreactions were visualized by enhanced chemiluminescence (Pierce, Rockford, IL, USA). Densitometric analysis and quantification were performed using the ImageJ software (NIH, USA).
2.5 RNA isolation, reverse transcription and quantitative real-time PCR
Total RNA was isolated from gastrocnemius muscle using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA (2μg) was reverse-transcribed into cDNA using random hexamers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). TaqMan quantitative real-time PCR reactions were performed in duplicate on an Eco Real-Time PCR System (Illumina, San Diego, CA, USA) using predesigned primer sets for mouse transforming growth factor-β1 gene (TGF- β1; Mm01178820_m1) and the housekeeping gene GAPDH Mm99999915_g1; TaqMan Assays-on-Demand, Applied Biosystems, Foster City, CA, USA) or using primer set for rat-mouse PDGFRα (F.P: 5’ CGACTCCAGATGGGAGTTCCC 3’, R.P: 5’ TGCCATCCACTTCACAGGCA 3’), mouse Zfand5 (F.P: 5’ CAGAGCGTCAGTCGTCGGGATC 3’, R.P: 5’ GGTAGGACTGTTGGAACCACTAGC 3’), mouse Schip1 (F.P: 5’ GCACAATGGCAACGTGGTGGTAGC 3’, R.P: 5’ CCGTCTTACTGTCATCTGCATCGCTG 3’) and 18S (F.P: 5’ TGACGGAAGGGCACCACCAG 3’, R.P: 5’ CACCACCACCCACGGAATCG 3’). (mRNA expression was quantified with the comparative dCt method (2-ddCt), using GAPDH or 18S as reference genes. The mRNA levels were expressed relative to the mean expression in the wild-type mice.
2.6 Determination of fiber diameter
Fresh-frozen gastrocnemius muscles were sectioned and cryosections (7 μm) were placed on glass slides, fixed in 4% paraformaldehyde (Merck, Darmstadt, GE) and incubated with Alexa Fluor 594 conjugated-wheat germ agglutinin (WGA) (Thermo Fisher, Waltham, MA, USA) in PBS for 2 hours. Fiber size was calculated using the ImageJ software (NIH, USA) on reconstructed images of each gastrocnemius muscle. Fibers were manually selected and the minimal Feret diameter of each fiber was computed by the software [48].
2.7 Statistical analyses
The statistical significance of the differences between the means of the experimental groups was evaluated using one-way ANOVA or two-way ANOVA when appropriate, with a post hoc Bonferroni multiple comparison test. A difference was considered statistically significant with a P-value < 0.05. Data analyses and statistical analysis for all experiments were performed using Prism 5 software (GraphPad Software, CA, USA).
Results
3.1 Symptomatic ALS skeletal muscle shows significant atrophy and increased fibrosis
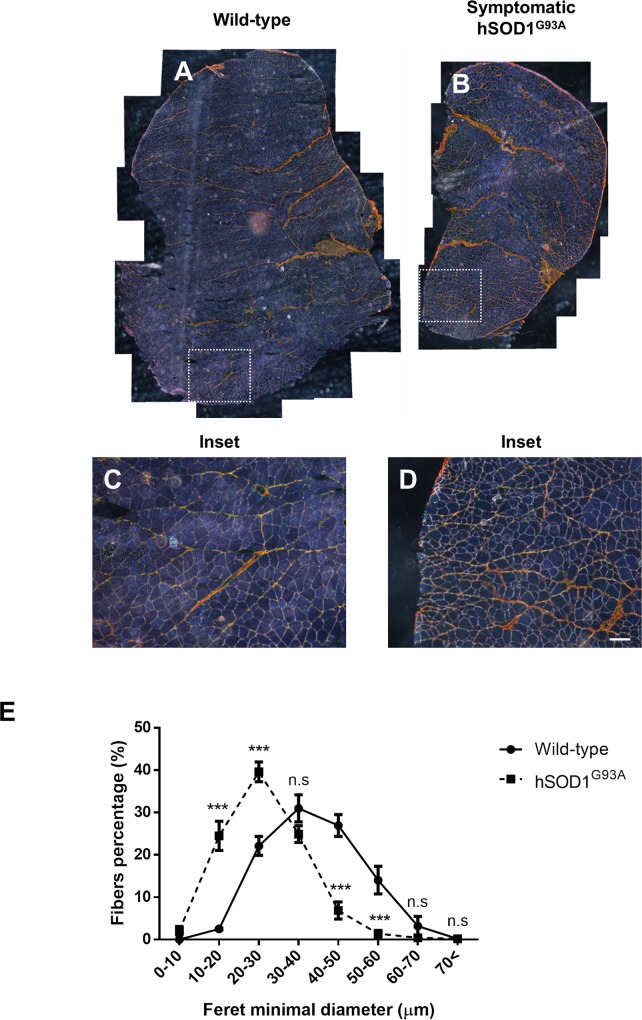
Skeletal muscle sections obtained from hSOD1G93A mice (ALS animal model) gastrocnemius muscle during symptomatic stages (120 days old), and from age matched wild type littermates, were analyzed by sirius red staining, a technique used to estimate total collagen. The samples were observed under polarized light. Fig 1A and 1C shows the normal architecture of wild-type mice skeletal muscle. In contrast, skeletal muscles from symptomatic hSOD1G93A mice show substantial atrophy with high variation in fiber size and accumulation of collagen evidencing a fibrotic process (Fig 1B and 1D). The atrophy of myofibers was further determined by analyzing the frequency of muscle fibers size. Fig 1E shows the analysis of gastrocnemius myofibers diameter indicating a significant decrease in the Feret minimal diameter in symptomatic hSOD1G93A mice compared to wild type littermates. This result suggests significant skeletal muscle atrophy in symptomatic hSOD1G93A mice. Gastrocnemius muscle extracted from symptomatic (120 days old) and pre-symptomatic (60 days old) hSOD1G93A mice as well as age matched wild type littermates were analyzed by hematoxylin and eosin staining. Fig 2A–2D shows the normal architecture of skeletal muscle from pre-symptomatic hSOD1G93A mice compared to wild type mice. We observed fibers of homogeneous size with peripherally located nuclei that most likely correspond to fibers and interstitial cells. In contrast, skeletal muscle from symptomatic hSOD1G93A mice show a substantial increase in interstitial nuclei that are mainly located surrounding packages of muscle fibers at the perimysium and around individual fibers in the endomysium. We also observed thickening of perimysium and endomysium, suggesting an increase in ECM components. To evaluate this, skeletal muscle sections were stained with sirius red. Fig 2E–2H indicates that symptomatic hSOD1G93A gastrocnemius muscle shows increased sirius red staining and decreased fiber size.
Fig 1. Muscle architecture is altered in gastrocnemius muscle from symptomatic hSOD1G93A mice.
(A-D) Sirius red staining of gastrocnemius muscle from wild-type (120 days old) and symptomatic (120 days old) hSOD1G93A age-matched mice seen under polarized light in cross-sections. Bar corresponds to 100 μm. (E) Fiber diameter in wild-type (120 days old; black circles) and symptomatic hSOD1G93A (120 days old; black squares) mice, values correspond to the mean ± SEM of three animals for each experimental condition. Two-way ANOVA, *** p<0.001, n.s: not significant.
Fig 2. Extracellular matrix (ECM) components deposition in gastrocnemius muscle from symptomatic hSOD1G93A mice.
(A-D) H&E staining. (E-H) Sirius red staining. (I-L) fibronectin (red) and (M-P) collagen-I (green) were detected by indirect immunofluorescence in cross-sections of gastrocnemius muscle from wild-type (60 days old), hSOD1G93A pre-symptomatic (60 days old), wild-type (120 days old), and hSOD1G93A symptomatic (120 days old) age-matched mice. Bar corresponds to 50 μm. Representative images of three mice per condition.
In order to confirm the increased fibrosis observed in hSOD1G93A mice suggested by sirius staining (Fig 2E–2H), we immunostained muscle sections with specific antibodies against the ECM proteins fibronectin and collagen type I. Fig 2I–2P shows that these two fibrotic proteins are more abundant in muscle from symptomatic hSOD1G93A mice compared to age matched wild type and pre-symptomatic hSOD1G93A mice. The increased staining was observed either at the perimysium or endomysium. To further evaluate and quantify the increment of ECM proteins, skeletal muscle extracts obtained from each experimental condition were analyzed by western blot. Fig 3A shows a representative western blot for fibronectin and collagen type III, two ECM constituents. An increase in the amount of these two fibrotic proteins was observed only in the muscle samples obtained from symptomatic hSOD1G93A mice compared to age matched wild type animals. No increase in these proteins was observed in pre-symptomatic stages of hSOD1G93A mice. Fig 3B and 3C shows a quantitative analysis of the significant increase of these two proteins from symptomatic hSOD1G93A compared to pre-symptomatic mice. Taken together, these results show that in the skeletal muscle of symptomatic hSOD1G93A mice there is increased atrophy. This becomes evident by a shift to the left in the distribution curve of Feret minimal diameter of the myofibers, together with a significant increase in fibrosis. These changes were not observed in pre-symptomatic hSOD1G93A mice.
Fig 3. Extracellular matrix (ECM) components are augmented in gastrocnemius muscle from symptomatic hSOD1G93A mice.
(A) Fibronectin and collagen-III were detected by western-blot in protein extracts from wild-type mice (60 days old), pre-symptomatic (60 days old) hSOD1G93A age-matched mice, wild-type (120 days old), and symptomatic (120 days old) hSOD1G93A age matched mice. GAPDH protein levels are shown as loading control. (B-C) Protein levels of fibronectin and collagen-III were quantified using densitometric analysis. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, ** p<0.05; *** p<0.001, n.s: not significant.
3.2 TGF-β signaling pathway is induced in gastrocnemius muscle from symptomatic hSOD1G93A mice
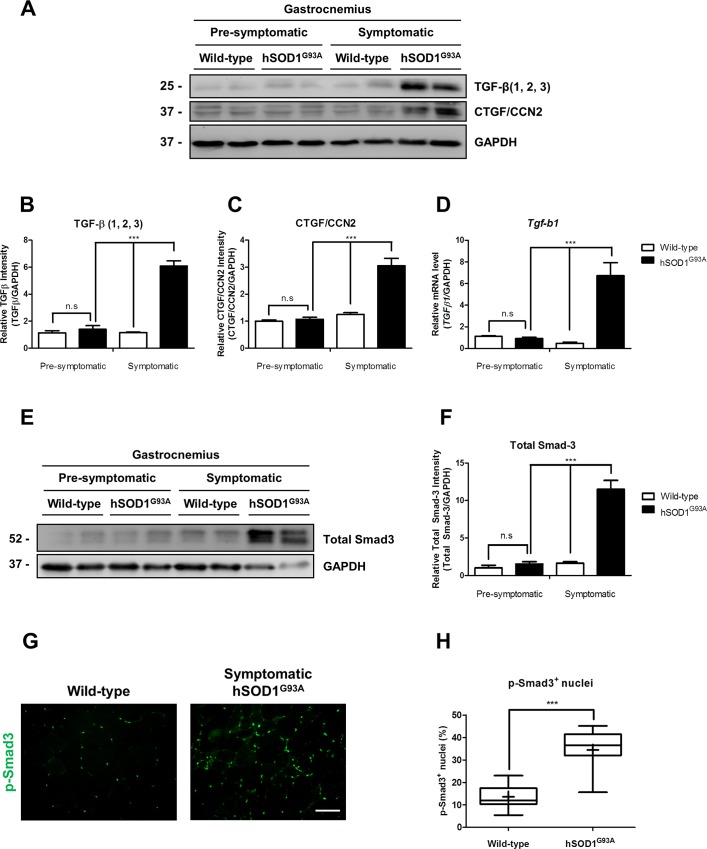
Since fibrosis was clearly augmented in symptomatic hSOD1G93A mice, we decided to evaluate the state of TGF-β signaling pathway, a well characterized pro-fibrotic pathway under these experimental conditions. Fig 4A shows a representative western blot for the three isoforms of TGF-β and CTGF/CCN2, a pro-fibrotic factor acting downstream TGF-β signaling pathway. We observed an increase of these two pro-fibrotic factors only in symptomatic hSOD1G93A mice. Quantitative analysis showed a 6-fold induction of TGF-β (1, 2, 3) and 3-fold induction of CTGF/CCN2 in symptomatic hSOD1G93A mice compared to pre-symptomatic animals (Figs 4B and 1C). Fig 4D shows a significant increase in TGF-β1 mRNA transcripts in skeletal muscle obtained from hSOD1G93A mice; in contrast, there was no significant difference in TGF-β1 mRNA levels in the pre-symptomatic stage. Next, we showed that there was no increase in total Smad3 in pre-symptomatic hSOD1G93A (Fig 4E) compared to the age-matched wild type. In contrast, we found a significant increase in Smad3 in skeletal muscle from hSOD1G93A mice compared to pre-symptomatic animals. Quantitative analyses indicate that there was a 10-fold increase in Smad3 in symptomatic hSOD1G93A mice (Fig 4F). To evaluate if TGF-β signaling pathway was active, we determined the amount of phosphorylated Smad3 (p-Smad3) in skeletal muscle from symptomatic hSOD1G93A and age-matched wild type mice by indirect immunofluorescence. Fig 4G shows a significant increase of nuclei positive for p-Smad3 in symptomatic hSOD1G93A mice. Quantitative analysis shows that 36% of total nuclei were positive for p-Smad3 in symptomatic hSOD1G93A mice compared to 14% in wild-type mice. These results suggest that TGF-β signaling pathway is activated in skeletal muscle of symptomatic hSOD1G93A mice.
Fig 4. TGF-β signaling pathway is induced in gastrocnemius muscle from symptomatic hSOD1G93A mice.
(A) TGF-β (1, 2, 3), CTGF/CCN2 was detected by western-blot in protein extracts under non-reducing condition from wild-type (60 days old), pre-symptomatic (60 days old) hSOD1G93A age-matched mice, wild-type (120 days old) and symptomatic (120 days old) hSOD1G93A age-matched mice. GAPDH protein levels are shown as loading control. (B-C) Protein levels of TGF-β (1, 2, 3) and CTGF/CCN2 were quantified using densitometric analysis. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, *** p<0.001. (D) Tgf-b1 expression levels were analyzed by quantitative PCR comparing wild-type (60 days old), pre-symptomatic (60 days old) hSOD1G93A age-matched mice, wild-type (120 days old) and symptomatic (120 days old) hSOD1G93A age-matched mice. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, *** p<0.001. (E) Total Smad3 was detected by western-blot in protein extracts from wild-type (60 days old), pre-symptomatic (60 days old) hSOD1G93A age matched mice, wild-type (120 days old), and symptomatic (120 days old) hSOD1G93A age-matched mice. GAPDH protein levels are shown as loading control. (F) Protein levels of total Smad3 were quantified using densitometric analysis. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, *** p<0.001. (F) p-Smad3 (green) was detected by indirect immunofluorescence in cross-sections of gastrocnemius muscle from wild-type (120 days old) and hSOD1G93A symptomatic (120 days old) age-matched mice, bar corresponds to 50 μm. (G) p-Smad3-positive nuclei were quantified in gastrocnemius muscle from wild-type (120 days old) and symptomatic (120 days old) hSOD1G93A age-matched mice, values correspond to the mean ± SEM of three animals for each experimental condition. One-way ANOVA, *** p<0.001, n.s: not significant.
3.3 FAPs are augmented in symptomatic hSOD1G93A skeletal muscle
We previously showed that the number of FAPs is augmented in models of increased fibrosis [42]. Therefore, we evaluated their presence by western blot analyses using markers of activated FAPs. Fig 5A shows that the levels of PDGFRα, Tcf4 (transcription factor 7-like 2, Tcf7L2-Genome Mouse Browser) and α-SMA found in gastrocnemius muscle are significantly increased in symptomatic compared to pre-symptomatic hSOD1G93A mice. Fig 5B–5I shows an important increase of positive interstitial nuclei for Tcf4 in gastrocnemius muscle from symptomatic hSOD1G93A compared to age-matched wild type animals. Fig 5J, shows a quantitative analysis of the increment in cells positive for Tcf4 from symptomatic hSOD1G93A mice compared to age-matched wild type mice. We observed that PDGFRα positive-cells reside in the interstitial space between myofibers located at the endomysium and perimysium of muscles from symptomatic hSOD1G93A mice (Fig 5K and 5L). Fig 5M and 5N shows that cells positive for PDGFRα co-localize with nuclei positive for p-Smad3 in immunostaining experiments. To test whether a PDGF dependent signaling pathway is activated in hSOD1G93A, we evaluated expression of two PDGF immediate early genes (IEG), Schip1 and Zfand5. We found an increase in mRNAs of these two genes in skeletal muscle of symptomatic hSOD1G93A mice (S1 Fig). These results clearly indicate that activated FAPs, likely responsible of the augmented fibrosis, are elevated in symptomatic hSOD1G93A mice compared to wild type littermates.
Fig 5. FAPs markers are increased in gastrocnemius muscle from symptomatic hSOD1G93A mice.
(A) PDGFRα, Tcf4 and α-SMA were detected by western-blot in protein extracts from wild-type (60 days old), pre-symptomatic (60 days old) hSOD1G93A age-matched mice, wild-type (120 days old), and symptomatic (120 days old) hSOD1G93A age-matched mice. GAPDH protein levels are shown as loading control. (B-I) Tcf4 (white and green) and laminin (white and red) were detected by indirect immunofluorescence in cross-sections of gastrocnemius muscle from wild-type (120 days old) and hSOD1G93A symptomatic (120 days old) age-matched mice, bar corresponds to 50 μm, nuclei were stained with Hoechst. Arrows show Tcf4-positive nuclei. (J) Tcf4-positive cells were quantified in gastrocnemius muscle from wild-type (120 days old) and symptomatic (120 days old) hSOD1G93A age-matched mice, values correspond to the mean ± SEM of three animals for each experimental condition. One-way ANOVA, ** p<0.005. (K-L) PDGFRα (green) and WGA (red) (M-N) PDGFRα and p-Smad3 (red) were detected by indirect immunofluorescence in cross-sections of gastrocnemius muscle from wild-type (120 days old) and hSOD1G93A symptomatic (120 days old) age-matched mice, bar corresponds to 50 μm, nuclei were stained with Hoechst. Arrows and asterisk show PDGFRα/p-Smad3 co-localization and PDGFRα-/p-Smad3+ cells, respectively.
Discussion
We have shown that skeletal muscle of symptomatic hSOD1G93A mice exhibits altered architecture, atrophy and increased interstitial space between myofibers. This pathological phenotype is accompanied by deposition of ECM molecules such as fibronectin and collagen I and III. The accumulation of these molecules indicates that a fibrotic process has been established in symptomatic stages and, therefore, functional tissue is being replaced by “non-functional” connective tissue.
Fibrosis is one of the key features in several disorders including muscular dystrophies such as DMD, where the absence of dystrophin leads to degeneration/regeneration cycles of the myofibers that will eventually be replaced by connective tissue and fat [22, 49]. The causes of fibrosis are not fully clear. However, mounting evidence shows that an inflammatory response is required to trigger the fibrotic process [50, 51]. We have also observed that the number of mononuclear cells residing in the interstitial space between the myofibers is increased. Recent work has shown that in hSOD1G93A rats, an active inflammatory response is occurring near the neuromuscular junction in skeletal muscle during symptomatic and end-stages. This inflammatory response is accompanied with an increase in CD11-b positive and CD68 positive macrophages and inflammatory cytokines IL1-β and TNF-α [52], suggesting that an inflammatory response might be preceding the fibrotic process in the skeletal muscle from ALS models.
We found an induction of the TGF-β signaling pathway in skeletal muscle of symptomatic hSOD1G93A mice that correlates with an increase in ECM molecules deposition. This data supports a recent study that proposes TGF-β1, 2 and 3 as a novel muscle biomarker for ALS [37]. It has been shown that intraperitoneal injections of TGF-β2 leads to transient improvement in motor performance without preventing motoneurons degeneration and disease progression [53]. One possible explanation is that, besides its neuroprotective role, TGF-β might also be deleterious in ALS. As reported recently, administration of SB-431542 (a strong inhibitor of the TGF-β type 1 receptor) delays ALS disease progression, indicating that the induction of TGF-β signaling has a detrimental effect [36].
Several studies have shown that TGF-β signaling pathway disturbances are associated with muscular dystrophies including Duchennes’s and Becker’s [54–56]. It has been shown that TGF-β signal inhibition using a TGF-β-neutralizing antibody or the angiotensin II type 1 receptor blocker losartan (an) reduces fibrosis levels and restores muscle architecture and function in two neuromuscular disease mouse models: the mdx mice and the fibrillin-1-deficient mice (Fbn1C1039G/+, Marfan Syndrome) [26]. We previously reported that TGF-β inhibition through angiotensin-1-7 administration reduces fibrosis and improves strength in dystrophic muscles of mdx mice [27]. The connective tissue growth factor (CTGF/CCN2) is a strong pro-fibrotic factor acting downstream of the TGF-β signaling pathway, and is found at highly elevated levels in several injured tissues such us skin, lung and liver [57–60]. In previous studies we demonstrated that CTGF over-expression induces a fibrotic response in healthy skeletal muscle [61] while CTGF inhibition improves the pathological phenotype and reduces fibrosis [48]. This evidence indicates that TGF-β, and other pro-fibrotic factors such as CTGF, play a crucial role in the fibrotic response observed in several myopathies. Therefore, taking into account that we have found an induction in TGF-β signaling, together with an increase in ECM molecules deposition in skeletal muscle of symptomatic hSOD1G93A mice, we speculate that targeting TGF-β and other pro-fibrotic factors may be a suitable therapeutic approach to restore, at least partially, muscle performance in ALS patients.
We found that FAPs markers PDGFRα and Tcf4 were highly expressed in skeletal muscles of symptomatic hSOD1G93A mice and we also observed an increase in the number of Tcf4+ and PDGFRα cells mainly in the interstitial space between the myofibers. We have previously demonstrated that the number of connective tissue cells expressing FAPs markers are increased in three different fibrotic models: mdx mice, BaCl2-induced chronic damage and sciatic nerve denervation [42], suggesting a role of these cells in fibrosis associated to skeletal muscle. It seems that FAPs cells are required for correct regeneration in skeletal muscle. However, under conditions where persistent damage occurs, these cells appear to play a rather deleterious role [62]. Current evidence suggests that mesenchymal progenitors that reside in connective tissue are probably the major contributors of ECM deposition in skeletal muscle, and that expression of fibrotic genes including different types of collagens, CTGF and α-SMA, take place in PDGFRα+ cells [42, 44]. We showed that PDGFRα+ cells co-localize with p-Smad3+ nuclei, suggesting that these cells are sensitive to TGF-β and might be responsible of ECM deposition in muscles of hSOD1G93A mice. Thus, FAPs seems to be a principal actor in fibrosis observed in hSOD1G93A mice and focusing on these cells might be a suitable therapeutic approach. The exact source of TGF-β to induce this response in FAPs in hSOD1G93A mice has to be determined. It has been shown in mdx mice, that infiltrating macrophages express elevated level of TGF-β avoiding apoptosis of FAPs by a TNF mediated mechanism [63]. Moreover, we evaluated expression of Schip1 and Zfand5, two specific IEG of the PDFG signaling pathway [64, 65]. We found an increase in expression of these two genes, suggesting an induction of PDGF signaling pathway in skeletal muscle of symptomatic hSOD1G93A mice. The induction of PDGF signaling is probably contributing to the fibrotic process [47, 66] together with TGF-β signaling.
Another work shows that Adam12+ cells differentiate into α-SMA-expressing myofibroblasts under TGF-β stimulation [67]. This result suggests that FAPs cells might differentiate into α-SMA-expressing myofibroblasts to ultimately secrete a plethora of ECM molecules, thus, contributing to a fibrotic process under muscle insults.
We demonstrated that TGF-β-dependent signaling is enhanced in skeletal muscle in symptomatic hSOD1G93A mice compared to pre-symptomatic animals; this was determined by elevated levels of TGF-β1 transcripts, elevated protein levels of TGF-β(1, 2, 3),total Smad3 protein and increased positive nuclei for phosphorylated Smad3. We and others [37] have found augmented levels of TGF-β (three isoforms) in symptomatic hSOD1G93A mice. The pro-fibrotic effect of TGF-β1 have been well-studied, however, controversial evidence regarding TGF-β3 exist. It has been shown that TGF-β3 is required for a suitable wound healing [68]. Other study showed that TGF-β3 is a poor inducer of pro-fibrotic molecules and differentiation to myofibroblast in vocal fold fibroblasts, when compared to TGF-β1 and TGF-β2. Furthermore, TGF-β3 administration ameliorates scar formation in vocal fold acute injury [69]. However, the role of TGF-β3 in ALS has not been evaluated to the date. Further studies are needed to understand the role of the three different isoforms of TGF-β in neurodegenerative diseases such as ALS.
As discussed above, FAPs are increased in skeletal muscle interstitial space in symptomatic hSOD1G93A mice, and they are probably secreting fibrotic proteins. It is plausible to suggest that TGF-β dependent fibrotic signals could be inducing an increase in FAPs number and in the secretion of ECM constituents. CTGF is a potential candidate to play this pro-fibrotic signaling, since is expressed and secreted by muscle cells [23] and could be exerting its effects in the pro-fibrotic FAPs present at the interstitial space.
In conclusion, our work provides evidence showing a fibrotic phenotype in skeletal muscles of symptomatic hSOD1G93A mice accompanied by the enhancement of the TGF-β signaling pathway. We have shown that FAPs cells are increased in this fibrotic context and could be involved in ECM deposition observed in symptomatic stages. Finally, targeting pro-fibrotic factors such as TGF-β might be a novel therapeutic approach to improve muscle function in several degenerative diseases.
Supporting information
RT-PCR analysis of (A) Pdgfra, (B) Schip1 and (C) Zfand5 expression in skeletal muscle from wild-type and symptomatic hSOD1G93A age-matched mice, 18S is shown as housekeeping gene. (D) Schip1 and (E) Zfand5 expression levels were analyzed by quantitative PCR in skeletal muscle from wild-type and symptomatic hSOD1G93A age-matched mice. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, * p<0.05, n.s: not significant.
(TIF)
Acknowledgments
The authors are grateful to Victor Troncoso, Darling Vera and Lina Correa for technical support. This study received financial support from Fondecyt grant #1150106 and CARE-PFB-12/2007 grant to E.B. Fondecyt grant #3140357 to D.R. and Conicyt Doctoral Fellowship grants to D.G. and O.C. This work was supported by the ALS Therapy Alliance-2014-F-034, and DRI USA 2013–0030 to BvZ.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- α-SMA
α-smooth muscle actin
- CTGF/CCN-2
Connective Tissue Growth Factor
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- fALS
Familial ALS
- FAPs
Fibro/adipogenic progenitors
- PDGF
Platelet-derived growth factor
- PDGFRα
Platelet-derived growth factor receptor-α
- SOD1
Superoxide dismutase 1
- TGF-β
Transforming growth factor type-β
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study received financial support from FONDECYT, grant #1150106 to E.B., a CARE-PFB-12/2007 grant, FONDECYT grant #3140357 to D.L.R., CONICYT, 21130854, PhDc David Gonzalez and CONICYT Doctoral Fellowship grants to D.G. and O.C. This work was supported by the ALS Therapy Alliance-2014-F-034, and DRI USA 2013-0030 to B.v.Z. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mancuso R, Navarro X. Amyotrophic lateral sclerosis: Current perspectives from basic research to the clinic. Prog Neurobiol. 2015;133:1–26. doi: 10.1016/j.pneurobio.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 2.Pansarasa O, Rossi D, Berardinelli A, Cereda C. Amyotrophic lateral sclerosis and skeletal muscle: an update. Mol Neurobiol. 2014;49(2):984–90. doi: 10.1007/s12035-013-8578-4 [DOI] [PubMed] [Google Scholar]
- 3.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–68. PubMed Central PMCID: PMCPMC3200438. doi: 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bros-Facer V, Krull D, Taylor A, Dick JR, Bates SA, Cleveland MS, et al. Treatment with an antibody directed against Nogo-A delays disease progression in the SOD1G93A mouse model of Amyotrophic lateral sclerosis. Hum Mol Genet. 2014;23(16):4187–200. doi: 10.1093/hmg/ddu136 [DOI] [PubMed] [Google Scholar]
- 5.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–5. [DOI] [PubMed] [Google Scholar]
- 6.Philips T, Rothstein JD. Rodent Models of Amyotrophic Lateral Sclerosis. Curr Protoc Pharmacol. 2015;69:5 67 1–21. PubMed Central PMCID: PMCPMC4562058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10(5):608–14. PubMed Central PMCID: PMCPMC3139463. doi: 10.1038/nn1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–22. PubMed Central PMCID: PMCPMC3799799. doi: 10.1038/nn1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz E, Izaurieta P, Weiss A, Mir FR, Rojas P, Gonzalez D, et al. Mutant SOD1-expressing astrocytes release toxic factors that trigger motoneuron death by inducing hyperexcitability. J Neurophysiol. 2013;109(11):2803–14. PubMed Central PMCID: PMCPMC3680799. doi: 10.1152/jn.00500.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas F, Cortes N, Abarzua S, Dyrda A, van Zundert B. Astrocytes expressing mutant SOD1 and TDP43 trigger motoneuron death that is mediated via sodium channels and nitroxidative stress. Front Cell Neurosci. 2014;8:24 PubMed Central PMCID: PMCPMC3916762. doi: 10.3389/fncel.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas F, Gonzalez D, Cortes N, Ampuero E, Hernandez DE, Fritz E, et al. Reactive oxygen species trigger motoneuron death in non-cell-autonomous models of ALS through activation of c-Abl signaling. Front Cell Neurosci. 2015;9:203 PubMed Central PMCID: PMCPMC4460879. doi: 10.3389/fncel.2015.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118(2):659–70. PubMed Central PMCID: PMCPMC2213375. doi: 10.1172/JCI34060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, et al. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58(2):231–43. PubMed Central PMCID: PMCPMC2784168. doi: 10.1002/glia.20919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, et al. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103(51):19546–51. PubMed Central PMCID: PMCPMC1748262. doi: 10.1073/pnas.0609411103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell metabolism. 2008;8(5):425–36. doi: 10.1016/j.cmet.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19(11):2284–302. PubMed Central PMCID: PMCPMC2865380. doi: 10.1093/hmg/ddq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. doi: 10.1002/path.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Lu H. Targeting fibrosis in Duchenne muscular dystrophy. J Neuropathol Exp Neurol. 2010;69(8):771–6. PubMed Central PMCID: PMC2916968. doi: 10.1097/NEN.0b013e3181e9a34b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandan E, Gutierrez J. Role of proteoglycans in the regulation of the skeletal muscle fibrotic response. The FEBS journal. 2013;280(17):4109–17. doi: 10.1111/febs.12278 [DOI] [PubMed] [Google Scholar]
- 20.Vainzof M, Ayub-Guerrieri D, Onofre PC, Martins PC, Lopes VF, Zilberztajn D, et al. Animal models for genetic neuromuscular diseases. J Mol Neurosci. 2008;34(3):241–8. doi: 10.1007/s12031-007-9023-9 [DOI] [PubMed] [Google Scholar]
- 21.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. The FEBS journal. 2013;280(17):4177–86. PubMed Central PMCID: PMCPMC4147949. doi: 10.1111/febs.12267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caceres S, Cuellar C, Casar JC, Garrido J, Schaefer L, Kresse H, et al. Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur J Cell Biol. 2000;79(3):173–81. doi: 10.1078/S0171-9335(04)70020-5 [DOI] [PubMed] [Google Scholar]
- 23.Vial C, Zuniga LM, Cabello-Verrugio C, Canon P, Fadic R, Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol. 2008;215(2):410–21. doi: 10.1002/jcp.21324 [DOI] [PubMed] [Google Scholar]
- 24.Cozzoli A, Nico B, Sblendorio VT, Capogrosso RF, Dinardo MM, Longo V, et al. Enalapril treatment discloses an early role of angiotensin II in inflammation- and oxidative stress-related muscle damage in dystrophic mdx mice. Pharmacol Res. 2011;64(5):482–92. PubMed Central PMCID: PMC3184479. doi: 10.1016/j.phrs.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorts A, Schwanekamp JA, Baudino TA, McNally EM, Molkentin JD. Deletion of periostin reduces muscular dystrophy and fibrosis in mice by modulating the transforming growth factor-beta pathway. Proc Natl Acad Sci U S A. 2012;109(27):10978–83. PubMed Central PMCID: PMC3390853. doi: 10.1073/pnas.1204708109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nature medicine. 2007;13(2):204–10. doi: 10.1038/nm1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, et al. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet. 2014;23(5):1237–49. doi: 10.1093/hmg/ddt514 [DOI] [PubMed] [Google Scholar]
- 28.Pessina P, Cabrera D, Morales MG, Riquelme CA, Gutierrez J, Serrano AL, et al. Novel and optimized strategies for inducing fibrosis in vivo: focus on Duchenne Muscular Dystrophy. Skeletal muscle. 2014;4:7 PubMed Central PMCID: PMCPMC4142391. doi: 10.1186/2044-5040-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharraz Y, Guerra J, Pessina P, Serrano AL, Munoz-Canoves P. Understanding the process of fibrosis in Duchenne muscular dystrophy. Biomed Res Int. 2014;2014:965631 PubMed Central PMCID: PMCPMC4024417. doi: 10.1155/2014/965631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quattrocelli M, Spencer MJ, McNally EM. Outside in: The matrix as a modifier of muscular dystrophy. Biochim Biophys Acta. 2017;1864(3):572–9. PubMed Central PMCID: PMCPMC5262521. doi: 10.1016/j.bbamcr.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- 32.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–27. doi: 10.1096/fj.03-1273rev [DOI] [PubMed] [Google Scholar]
- 33.Houi K, Kobayashi T, Kato S, Mochio S, Inoue K. Increased plasma TGF-beta1 in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2002;106(5):299–301. [DOI] [PubMed] [Google Scholar]
- 34.Ilzecka J, Stelmasiak Z, Dobosz B. Transforming growth factor-Beta 1 (tgf-Beta 1) in patients with amyotrophic lateral sclerosis. Cytokine. 2002;20(5):239–43. [DOI] [PubMed] [Google Scholar]
- 35.Katsuno M, Adachi H, Banno H, Suzuki K, Tanaka F, Sobue G. Transforming growth factor-beta signaling in motor neuron diseases. Curr Mol Med. 2011;11(1):48–56. [DOI] [PubMed] [Google Scholar]
- 36.Endo F, Komine O, Fujimori-Tonou N, Katsuno M, Jin S, Watanabe S, et al. Astrocyte-derived TGF-beta1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep. 2015;11(4):592–604. doi: 10.1016/j.celrep.2015.03.053 [DOI] [PubMed] [Google Scholar]
- 37.Si Y, Kim S, Cui X, Zheng L, Oh SJ, Anderson T, et al. Transforming Growth Factor Beta (TGF-beta) Is a Muscle Biomarker of Disease Progression in ALS and Correlates with Smad Expression. PLoS One. 2015;10(9):e0138425 PubMed Central PMCID: PMCPMC4574401. doi: 10.1371/journal.pone.0138425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12(2):153–63. PubMed Central PMCID: PMCPMC4580288. doi: 10.1038/ncb2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12(2):143–52. doi: 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- 40.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–84. PubMed Central PMCID: PMC3005608. doi: 10.1242/dev.057463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–37. PubMed Central PMCID: PMCPMC3152921. doi: 10.1242/dev.064162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Contreras O, Rebolledo DL, Oyarzun JE, Olguin HC, Brandan E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016;364(3):647–60. doi: 10.1007/s00441-015-2343-0 [DOI] [PubMed] [Google Scholar]
- 43.Lemos DR, Paylor B, Chang C, Sampaio A, Underhill TM, Rossi FM. Functionally convergent white adipogenic progenitors of different lineages participate in a diffused system supporting tissue regeneration. Stem cells. 2012;30(6):1152–62. doi: 10.1002/stem.1082 [DOI] [PubMed] [Google Scholar]
- 44.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124(Pt 21):3654–64. doi: 10.1242/jcs.086629 [DOI] [PubMed] [Google Scholar]
- 45.Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol. 2014;5:68 PubMed Central PMCID: PMCPMC3932482. doi: 10.3389/fphys.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–88. PubMed Central PMCID: PMC3663598. doi: 10.1016/j.cell.2013.02.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller AA, van Velthoven CT, Fukumoto KD, Cheung TH, Rando TA. Intronic polyadenylation of PDGFRalpha in resident stem cells attenuates muscle fibrosis. Nature. 2016;540(7632):276–9. doi: 10.1038/nature20160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, et al. Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet. 2013;22(24):4938–51. doi: 10.1093/hmg/ddt352 [DOI] [PubMed] [Google Scholar]
- 49.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, et al. Aberrant repair and fibrosis development in skeletal muscle. Skeletal muscle. 2011;1(1):21 PubMed Central PMCID: PMC3156644. doi: 10.1186/2044-5040-1-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R345–53. Epub 2005/01/08. doi: 10.1152/ajpregu.00454.2004 [DOI] [PubMed] [Google Scholar]
- 51.Cabrera D, Gutierrez J, Cabello-Verrugio C, Morales MG, Mezzano S, Fadic R, et al. Andrographolide attenuates skeletal muscle dystrophy in mdx mice and increases efficiency of cell therapy by reducing fibrosis. Skeletal muscle. 2014;4:6 PubMed Central PMCID: PMCPMC4021597. doi: 10.1186/2044-5040-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Dyke JM, Smit-Oistad IM, Macrandar C, Krakora D, Suzuki M. Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS). Exp Neurol. 2016;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Day WA, Koishi K, Nukuda H, McLennan IS. Transforming growth factor-beta 2 causes an acute improvement in the motor performance of transgenic ALS mice. Neurobiol Dis. 2005;19(1–2):323–30. doi: 10.1016/j.nbd.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 54.Burks TN, Cohn RD. Role of TGF-beta signaling in inherited and acquired myopathies. Skeletal muscle. 2011;1(1):19 Epub 2011/07/30. PubMed Central PMCID: PMC3156642. doi: 10.1186/2044-5040-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104(3):579–87. Epub 2007/11/23. doi: 10.1152/japplphysiol.01091.2007 [DOI] [PubMed] [Google Scholar]
- 56.Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280(19):18871–80. doi: 10.1074/jbc.M410762200 [DOI] [PubMed] [Google Scholar]
- 57.Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Grotendorst GR, et al. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol. 1995;105(2):280–4. [DOI] [PubMed] [Google Scholar]
- 58.Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Fujimoto M, et al. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J Invest Dermatol. 1996;106(4):729–33. [DOI] [PubMed] [Google Scholar]
- 59.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53(4):853–61. doi: 10.1111/j.1523-1755.1998.00820.x [DOI] [PubMed] [Google Scholar]
- 60.Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82(6):767–74. [DOI] [PubMed] [Google Scholar]
- 61.Morales G, Cabello-Verrugio C, Cabrera D, Goldschmeding R, Brandan E. CTGF/CCN-2 overexpression can directly induce features of skeletal muscle dystrophy. J Pathol. 2011;225 490–501. doi: 10.1002/path.2952 [DOI] [PubMed] [Google Scholar]
- 62.Judson RN, Zhang RH, Rossi FM. Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs? The FEBS journal. 2013;280(17):4100–8. doi: 10.1111/febs.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nature medicine. 2015;21(7):786–94. doi: 10.1038/nm.3869 [DOI] [PubMed] [Google Scholar]
- 64.Schmahl J, Raymond CS, Soriano P. PDGF signaling specificity is mediated through multiple immediate early genes. Nat Genet. 2007;39(1):52–60. doi: 10.1038/ng1922 [DOI] [PubMed] [Google Scholar]
- 65.Chen WV, Delrow J, Corrin PD, Frazier JP, Soriano P. Identification and validation of PDGF transcriptional targets by microarray-coupled gene-trap mutagenesis. Nat Genet. 2004;36(3):304–12. doi: 10.1038/ng1306 [DOI] [PubMed] [Google Scholar]
- 66.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16(2):303–13. PubMed Central PMCID: PMCPMC2664622. doi: 10.1016/j.devcel.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nature medicine. 2012;18(8):1262–70. doi: 10.1038/nm.2848 [DOI] [PubMed] [Google Scholar]
- 68.Le M, Naridze R, Morrison J, Biggs LC, Rhea L, Schutte BC, et al. Transforming growth factor Beta 3 is required for excisional wound repair in vivo. PLoS One. 2012;7(10):e48040 PubMed Central PMCID: PMCPMC3482237. doi: 10.1371/journal.pone.0048040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang Z, Kishimoto Y, Hasan A, Welham NV. TGF-beta3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis Model Mech. 2014;7(1):83–91. PubMed Central PMCID: PMCPMC3882051. doi: 10.1242/dmm.013326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR analysis of (A) Pdgfra, (B) Schip1 and (C) Zfand5 expression in skeletal muscle from wild-type and symptomatic hSOD1G93A age-matched mice, 18S is shown as housekeeping gene. (D) Schip1 and (E) Zfand5 expression levels were analyzed by quantitative PCR in skeletal muscle from wild-type and symptomatic hSOD1G93A age-matched mice. Values correspond to the mean ± SEM of four animals for each experimental condition. One-way ANOVA, * p<0.05, n.s: not significant.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.