Abstract
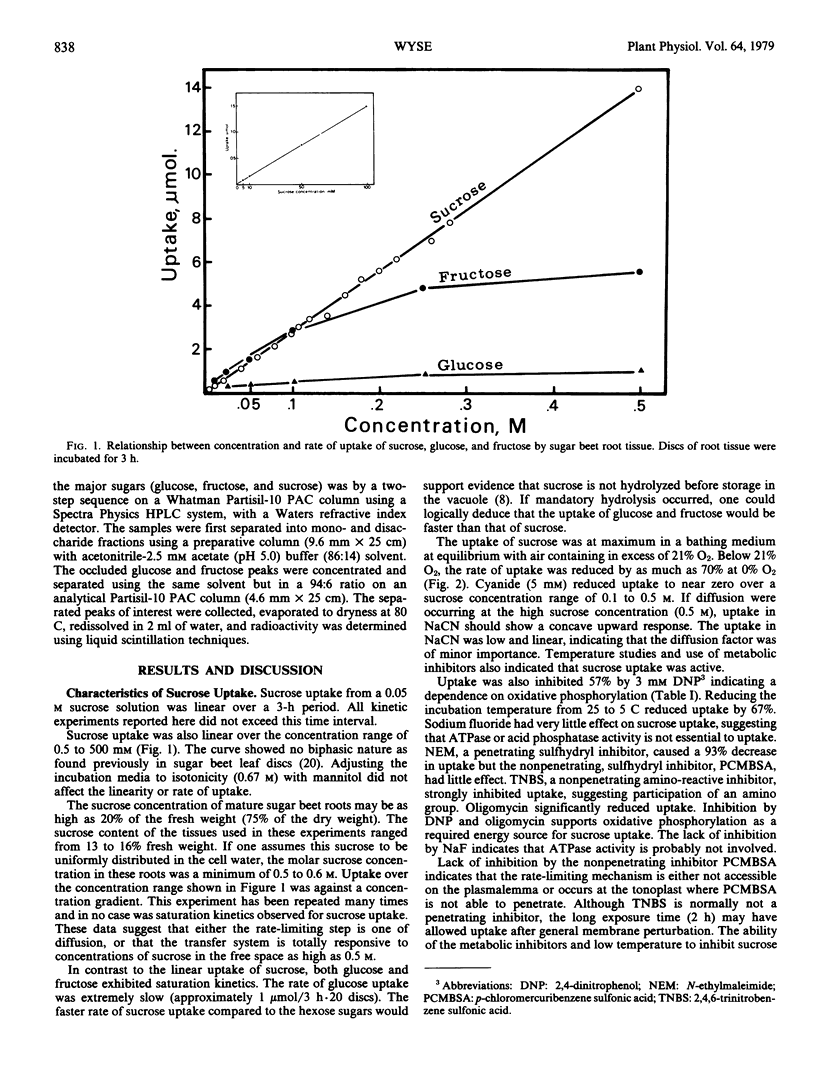
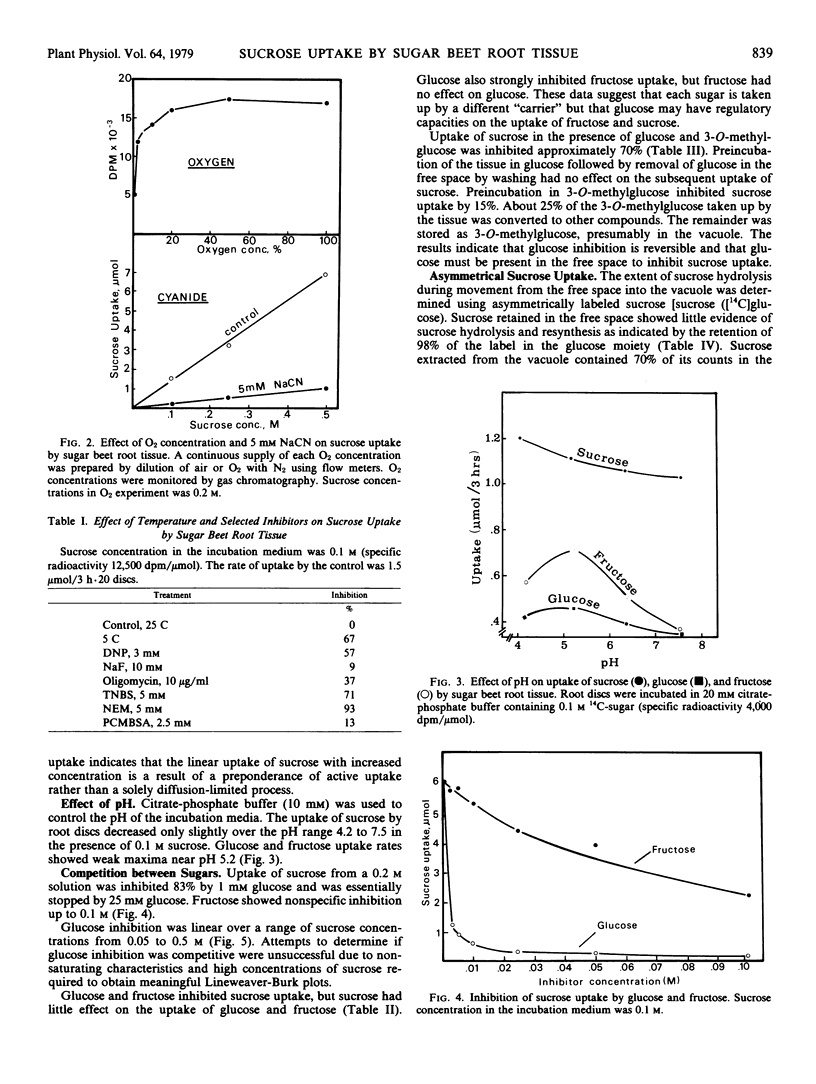
Sucrose uptake by discs of mature sugar beet root tissue incubated in [14C]-sucrose exhibited nonsaturating kinetics over the concentration range of 1 to 500 millimolar. Uptake was inhibited by dinitrophenol, sodium cyanide, low O2, and penetrating sulfhydryl inhibitors. ATPase inhibitors, sodium fluoride, and oligomycin reduced uptake by 20 and 40%, respectively. Uptake as asymmetrically labeled sucrose ([14C]glucose) occurred with approximately 80% retention of asymmetry, indicating a nonhydrolytic pathway. Uptake was against a concentration gradient and required metabolic energy.
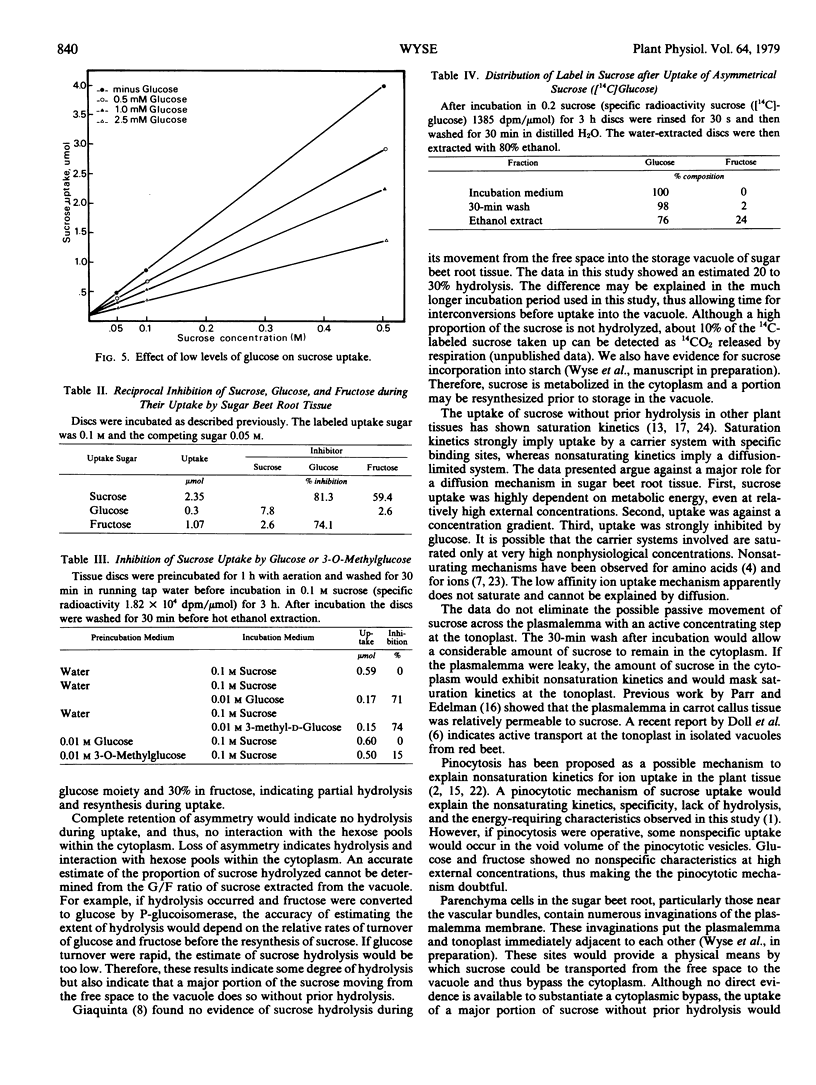
Glucose and fructose uptake exhibited typical saturation kinetics but rates of uptake were lower than that of sucrose, particularly at high concentration. Glucose strongly inhibited the uptake of sucrose and fructose but sucrose and fructose had little effect on the rate of glucose uptake. It is proposed that a major protion of the sucrose movement between its free space and vacuole occurs via a nonsaturating carrier at sites where the plasmalemma and tonoplast are appressed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT H. S. The concepts of membrane flow and membrane vesiculation as mechanisms for active transport and ion pumping. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):99–103. doi: 10.1083/jcb.2.4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H. N., Liang M. On the nature of the "non-saturable" migration of amino acids into Ehrlich cells and into rat jejunum. Bibl Laeger. 1966 Mar 14;112(3):524–531. doi: 10.1016/0926-6585(66)90255-x. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Poole R. J. Chloride accumulation by mung bean root tips: a low affinity active transport system at the plasmalemma. Plant Physiol. 1972 Nov;50(5):603–607. doi: 10.1104/pp.50.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Sucrose Hydrolysis in Relation to Phloem Translocation in Beta vulgaris. Plant Physiol. 1977 Sep;60(3):339–343. doi: 10.1104/pp.60.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. R., Beevers H. Absorption of Sugars by Plant Tissues. Plant Physiol. 1964 Jan;39(1):78–85. doi: 10.1104/pp.39.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriedemann P., Beevers H. Sugar uptake and translocation in the castor bean seedling I. Characteristics of transfer in intact and excised seedlings. Plant Physiol. 1967 Feb;42(2):161–173. doi: 10.1104/pp.42.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A., Hatch M. D., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. III. Physical & Metabolic Aspects of Cycle in Immature Storage Tissues. Plant Physiol. 1963 May;38(3):348–354. doi: 10.1104/pp.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J. A. The regulation of sugar uptake and accumulation in bean pod tissue. Plant Physiol. 1966 Jan;41(1):181–189. doi: 10.1104/pp.41.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J. C. Movement of C-Labeled Assimilates into Kernels of Zea mays L: II. Invertase Activity of the Pedicel and Placento-Chalazal Tissues. Plant Physiol. 1972 Feb;49(2):203–206. doi: 10.1104/pp.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovonick S. A., Geiger D. R., Fellows R. J. Evidence for active Phloem loading in the minor veins of sugar beet. Plant Physiol. 1974 Dec;54(6):886–891. doi: 10.1104/pp.54.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Laties G. G. Dual mechanisms of ion uptake in relation to vacuolation in corn roots. Plant Physiol. 1966 May;41(5):863–870. doi: 10.1104/pp.41.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. Enzymes Involved in the Postharvest Degradation of Sucrose in Beta vulgaris L. Root Tissue. Plant Physiol. 1974 Mar;53(3):507–508. doi: 10.1104/pp.53.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]


