Abstract
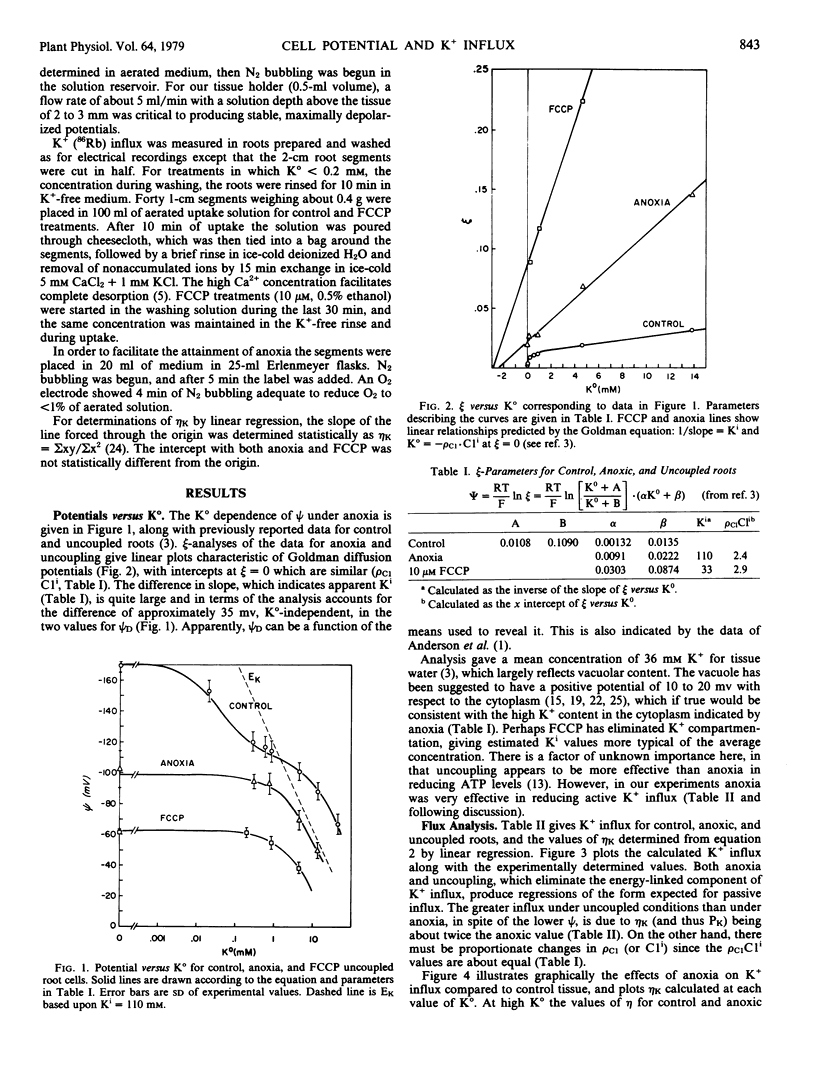
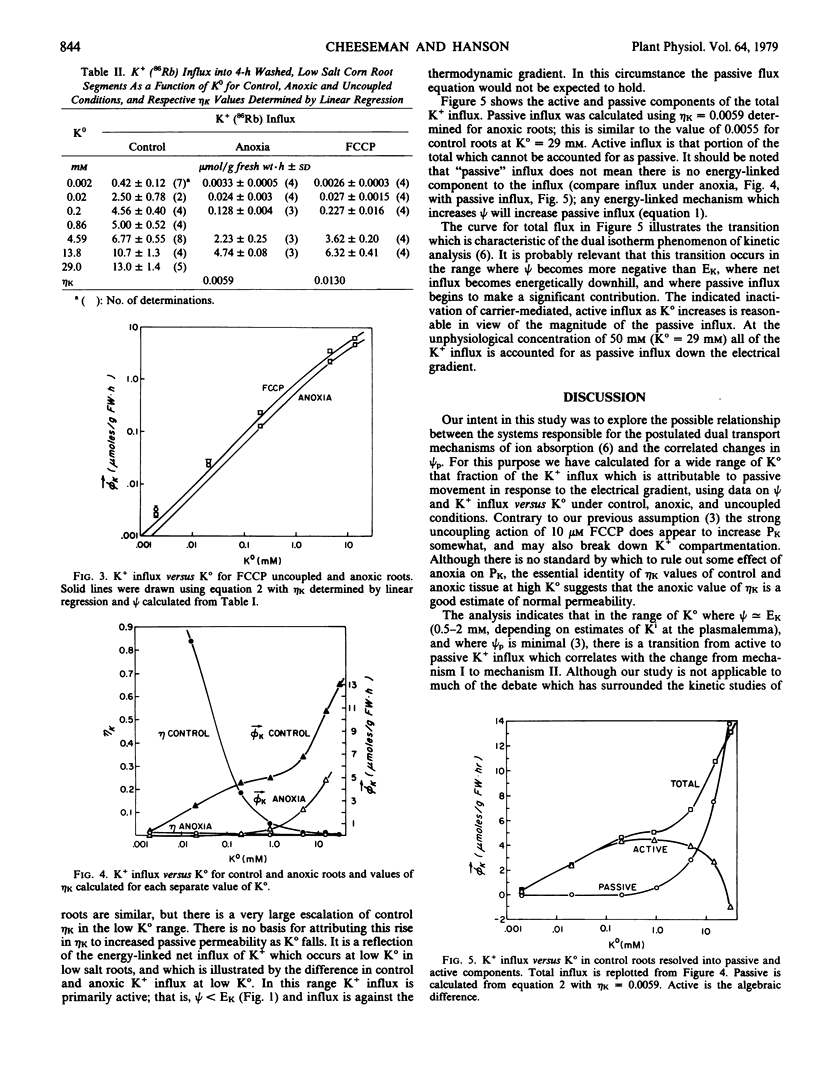
Cell potentials and K+ (86Rb) influx were determined for corn roots over a wide range of external K+ activity (K°) under control, anoxic, and uncoupled conditions. The data were analyzed using Goldman theory for the contribution of passive influx to total influx. For anoxic and uncoupled roots the K+ influx shows the functional relationship with K° predicted with constant passive permeability, although K+ permeability in uncoupled roots is about twice that of anoxic roots. In control roots the equation fails to describe K+ influx at low K°, but does so at high K°, with a gradual transition over the region where the electrical potential becomes equal to the equilibrium potential for K+ (ψ = EK). In the low K° range, where net K+ influx is energetically uphill, participation of an energy-linked K+ carrier is indicated. In the high K° range, K+ influx becomes passive down the electrical gradient established by the cell potential. Since the cell potential includes a substantial electrogenic component, anoxia or uncoupling reduces passive influx.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974 Nov;54(5):712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Hanson J. B. Mathematical analysis of the dependence of cell potential on external potassium in corn roots. Plant Physiol. 1979 Jan;63(1):1–4. doi: 10.1104/pp.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarty M., Morvan C., Thellier M. Exchange Properties of Isolated Cell Walls of Lemna minor L. Plant Physiol. 1978 Oct;62(4):477–481. doi: 10.1104/pp.62.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald J. W., Cheeseman J. M., Hanson J. B. Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiol. 1979 Feb;63(2):255–259. doi: 10.1104/pp.63.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Cell potentials, cell resistance, and proton fluxes in corn root tissue: effects of dithioerythritol. Plant Physiol. 1976 Sep;58(3):276–282. doi: 10.1104/pp.58.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz S. M., Higinbotham N. Transmembrane electropotential in barley roots as related to cell type, cell location, and cutting and aging effects. Plant Physiol. 1976 Feb;57(2):123–128. doi: 10.1104/pp.57.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce W. S., Higinbotham N. Compartments and Fluxes of K, NA, and CL in Avena Coleoptile Cells. Plant Physiol. 1970 Nov;46(5):666–673. doi: 10.1104/pp.46.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]


