Abstract
Chewing insects cause severe yield losses in crop production worldwide. Crop plants counteract chewing insects by transcriptionally promoting a repertoire of defense gene products that are either toxic to, or attractive to the natural enemies of, pest insects. However, the complexity of the transcriptional reprogramming in plant defense response against chewing insects is still not well understood. In this study, the genome-wide early responses in maize seedlings to Asian corn borer (ACB, Ostrinia furnacalis) and also to jasmonic acid(JA), the pivotal phytohormone controlling plant defense response against herbivory, were transcriptionally profiled by RNA-Seq. Clustering of differentially expressed genes (DEGs) along with functional enrichment analysis revealed important biological processes regulated in response to ACB infestation and/or jasmonic acid. Moreover, DEGs with distinct expression patterns were differentially enriched with diverse families of cis-elements on their promoters. Multiple inventories of differentially expressed transcription factors (DETFs) in each DEG group were also analyzed. A transient expression assay using transfected maize protoplastswas established to examine the potential roles of DETFs in maize defense response and JA signaling, and this was used to show that ZmNAC60, an ACB- and JA-inducible DETF, represented a novel positive regulator of JA and defense pathway genes. This study provided a comprehensive transcriptional picture for the early dynamics of maize defense responses and JA signaling, and the identification of DETFs offered potential targets for further functional genomics investigation of master regulators in maize defense responses against herbivory.
Introduction
The plant defense response against chewing insects is bipartite [1]. One branch of the defense system is termed direct defense, in which plants produce toxins to fight herbivores. These toxins can be proteinaceous, such as the protease Mir1-CP [2, 3], ribosome-inactivating proteins (RIPs) [4], and protease inhibitors [5]. They can also be secondary metabolites, such as DIMBOA [6], glucosinolates [7], pyrrolizidine alkaloids [8], and phenolics [9]. In addition to direct defense, plants also evolved another layer of defense response termed indirect defense, in which plants produce volatiles to attract the natural enemies of herbivores [10, 11].
Most of the defense mechanisms mentioned above are absent or only present at basal levels in plants without herbivore attack, but strongly activated upon herbivore infestation [12]. This inherently requires coherent signal transmission and integration within plants for coordinated transcriptional reprogramming of numerous genes in direct or indirect defense responses. To achieve this, several critical steps are required, with the first step being perception of herbivory. Mechanical damage can be adopted by plants as a sign of herbivore invasion to induce the expression of several defense genes [13]. Plants also recognize herbivore-derived compounds termed herbivore-associated molecular patterns (HAMPs) with unknown receptors [14–18]. Perception of herbivory triggers a number of extreme-early downstream events, such as generation of reactive oxygen species and depolarization of cell membrane [19], calcium signaling [20], and activation of MAPK cascades [21]. These events collectively induce the biogenesis and mobilization of JA [22].
Among all phytohormones, JA plays the most pivotal role in the transcriptional reprogramming in plant defense response to herbivores. The importance of JA signaling in defense response against herbivory has been demonstrated by the fact that deficiency in JA biosynthesis or signaling leads to severely hampered defense response [23, 24]. At the transcriptome level, a large portion of transcriptional reprogramming occurred during mechanical damage or herbivory is mediated by JA-signaling [25, 26]. The central components in JA signaling have been identified and characterized mainly in model species Arabidopsis and rice, including COI1, JAZ, and transcription factors downstream of JAZ such as MYCs [27, 28]. In addition to MYCs, a large number of transcription factors were identified as crucial positive or negative regulators in JA-mediated defense response, such as ORA59[29], ERF1 [30], RIM1 [31], bHLH3/13/14/17 [32], JAM1/2/3 [33], JAV1 [34], and AtERF4 [35]. In another study, more than 40 transcription factors were found induced by Spodoptera littoralis in Arabidopsis, with at least 9 of them playing significant roles in resistance to S. littoralis herbivory [36].
Although the transcriptomic changes and transcriptional regulators of plant defense response against chewing insects have been extensively investigated in Arabidopsis and rice, less is known on the transcriptional control in maize herbivore resistance. Previous studies have investigated maize defense response against Spodoptera frugiperda [37], Diabrotica undecimpunctata howardi [38], and Ostrinia furnacalis [39]. In this study, the genome-wide early responses in maize seedlings to Asian corn borer (ACB, Ostrinia furnacalis) and JA, were transcriptionally profiled. A comparative transcriptome investigation was conducted, with emphasis on functional enrichment analysis, differential usage of exons, cis-element enrichment analysis, and differentially expressed transcription factors (DETFs). We also established a transient expression assay to examine potential roles of DETFs identified in this study in maize defense response and JA signaling, and this was used to show that ZmNAC60, an ACB- and JA-inducible DETF, represents a novel positive regulator of JA and defense pathway genes. This study provided a comprehensive transcriptional picture for the early dynamics of maize defense responses and JA signaling, and the identification of DETFs offered potential targets for further functional genomics investigation of master regulators in maize defense responses to herbivory.
Materials and methods
Plant materials, growth conditions, and treatments
B73 maize plants were grown in a growth room with 12 h light/12 h dark cycles at 24°C for 3 weeks until reaching the V3 stage. On the day of treatment, plants were randomly divided into three groups: Control group, Ostrinia furnacalis treatment group, and jasmonic acid treatment group, with each group containing 30 seedlings. The three groups were then moved into separate growth chambers with identical growth conditions, considering that jasmonate is highly volatile, and also that plant volatiles from one group may change gene expression in other groups. Treatments started 2 hours after the lights were on. For the herbivory treatment, three two instar larvae were placed into the whorl of each plant. For jasmonic acidtreatment, each plant was sprayed with a fine mist of 100 μM jasmonic acid (Sigma, USA) suspended in 5 ml distilled water. JA was sprayed on whole plants. Control and ACB-treated groups were also mock-treated with a fine mist of distilled water. Eight hours after the onset of the treatment, the third and forth leaves were harvested and frozen in liquid nitrogen for RNA-Seq analysis. Each treatment contained three biological replicates, with 10 plants pooled for each replicate. To test for systemic gene expression after mechanical wounding, maize seedlings were scratched with a razor blade once every hour across the midrib in the middle of the third leaves. To test for systemic gene expression after ACB damage, two two instar larvae were enclosed in a small cage made out of Eppendorf tube caps attached on the surface of the third leaves. Third or fourth leaves were harvested at indicated time points, with 3 biological replicates for each time point and 6 seedlings for each replicate.
RNA-seq library construction, sequencing and analysis
RNA-seq libraries were generated from 5 μg total RNA and size-selected for 250–300 bp inserts for paired-end sequencing (100 bp for each end). Libraries were quantified on an Agilent bioanalyzer (Agilent, CA, USA) and sequenced using the Illumina HiSeq2000 system according to standard Illumina protocols (Illumina Inc., CA, USA). Raw reads were trimmed and filtered using Sickle (version 1.33). The Tophat2-Cufflinks pipeline [40] was employed in the updating of gene models in the RNA-Seq analysis. Reads were aligned to the maize reference genome (AGP v3.23) by Tophat2 (version 2.1.0), followed by optimization of existing gene models and also the identification of novel transcripts identified in this study using Cufflinks (version 2.2.1). Differentially expressed genes were identified using the HTSeq-DESeq pipeline [41]. Aligned reads were counted by HTSeq according to the updated genome annotation. Gene-level expression values were represented as Fragments per Kilobase per Million (FPKM). Differentially expressed genes were identified using the DESeq package [42]. A False Discovery Rate (FDR) corrected p-value≤0.05 and a threshold fold change≥2 was used to call differentially expressed genes. Differential usage of exons was detected using DEXSeq [43] with the criteria FDR≤0.05 and threshold fold change≥2. Principal component analysis (PCA) of samples were carried out by the prcomp function implemented in the stats package of R (version 3.3.1), and only the first two components were plotted. Hierarchical clustering of samples was carried out using the hclust function implemented in the stats package of R (version 3.3.1). To determine whether ACB and JA lead to similar or contrasting transcriptomic changes, differentially expressed genes (DEGs) in at least one of the two treatments were determined, followed by evaluation of their fold changes in response to each treatment. After that, the pearson correlation coefficient between the two treatments was calculated using ACB- and JA-induced fold changes of DEGs by the cor function implemented in the stats package of R (version 3.3.1).
Quantitative RT–PCR
Total RNA was isolated using Trizol (Invitrogen, CA, USA), followed by first-strand cDNA synthesis using SuperScript III reverse transcriptase (Invitrogen, CA, USA). The primer sequences are listed in S1 Table. PCR was performed in a 25 μL reaction mixture with Maxima SYBR Green/ROX qPCR Master Mix (Invitrogen, cat. no. K0221) using the following program: 95°C for 3 min, 40 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, followed by one cycle of 95°C for 1 min, 55°C for 30 s and 95°C for 30 s. The instrument used for qPCR was the StepOnePlus system (Applied Biosystems, CA, USA). Target gene expression was normalized using EF1alpha as an internal control. Three biological replicates were included in quantitative RT-PCR analysis.
Functional classification
Maize gene annotations using GO (Gene Ontology) terms were acquired using the BLAST2GO pipeline [44], combined with existent GO annotation retrieved from Gramene BioMart. Enriched GO terms were determined by using Fisher’s exact tests followed by Benjamini-Hochberg multiple-test correction.
Cis-regulatory motif enrichment analysis
To determine enriched cis-regulatory elements on promoters of co-expressed genes, proximal promoter was defined as 2 kbp upstream and 500 bp downstream of transcription start site (TSS) since this region is adequate to capture the 5’UTR and the first intron of most maize gene models. A comprehensive collection of plant position weight matrices (PWMs) [45] and also PWMs deposited in the JASPAR database were used. Promoters were scanned for significantly enriched cis-elements using the PWMEnrich package in the Bioconductor project.
Maize protoplast isolation and transient transactivation assay
Maize protoplast preparation and PEG-mediated transfection was performed as previously described [46]. A modified version of pCAMBIA3300, referred to as pCAMBIA3300m, was constructed by inserting into it a fragment containing an Ubiquitin promoter, a multiple cloning site, and a nos terminator. ZmNAC60 CDS alone, or ZmNAC60 fused at its C-terminus with SRDX (transcriptional repression domain) or VP16 (transcriptional activation domain) were cloned into pCAMBIA3300m. The constructs were transiently transformed into maize protoplasts. The transformed protoplasts were treated with or without 10 μM jasmonic acid for 3h before harvest. In order to normalize transformation efficiency, a construct containing GUS CDS driven by the CaMV 35S promoter was used as a transfection control reporter. RNA extraction, first-strand cDNA synthesis, and quantitative PCR were conducted as described above. Expression of marker genes was calculated using the conventional 2−ΔΔCt method [47], followed by normalization by GUS activity for each sample.
Results and discussion
Establishment of experimental conditions to investigate the maize-Ostrinia furnacalis interaction
This study aimed to investigate the maize defense response to ACB. Maize plants were grown under a controlled environment in the growth chamber since numerous biotic or abiotic factors influencing maize-ACB interaction in field conditions would confound proper interpretation of data. Early-season ACB attack occurs at early or midwhorl stage as young larvae begin feeding within the whorl. However, as whorl stage maize is too large to grow healthily in growth chambers, we used three-week-old B73 seedlings. The third and forth leaves, which are preferred by ACB compared to the other two leaves, were harvested at various time points. The expression of a marker gene of ACB attack, maize proteinase inhibitor (mpi) [48], was monitored. The expression level of mpi was found significantly induced within 1h and reached steady state level at 8h (S1a Fig). At 8 h, around 5% of leaf surface, mostly distributed at leaf base area, was destructed (Fig 1a). Longer ACB attack caused extensive destruction of xylem and wilting of leaves, thus samples were harvested at 8h for RNA-Seq analysis. To conduct a comparative transcriptome analysis for ACB treatment and JA treatment, the expression of allene oxide synthase1 (aos1), a marker gene for maize JA signaling [49], was monitored on JA-treated seedlings. Expression of aos1 peaked at 6h and gradually decreased afterward (S1b Fig), suggesting that samples harvested at 8h were adequate to capture JA-induced gene expression. Another reason for simultaneous tissue harvest for control, ACB- and JA-treated samples was to eliminate circadian rhythm-caused differences in gene expression.
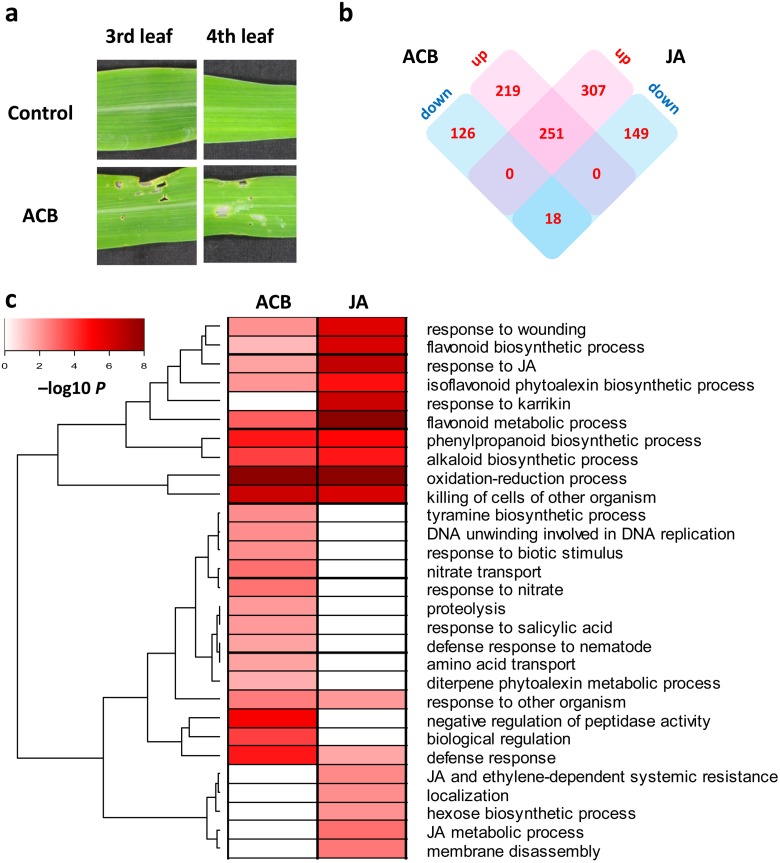
Fig 1. Dynamic reprogramming of maize transcriptome in response to ACB infestation and JA treatment.
a V3 stage B73 maize seedlings were used in ACB treatment. After 8h ACB infestation, around 5% leaf surface were damaged. b A Venn diagram showing the number of DEGs with specific expression patterns in response to treatments. c A heatmap showing enriched biological processes in ACB- and JA-induced DEGs. Color was scaled based on log transformed false discovery rates, and darker colors indicated that the genes in the corresponding biological processes were more highly enriched in their respective DEG categories.
ACB attack and JA treatment triggered distinct yet overlapping transcriptional reprogramming
To analyze the dynamics of the maize response to ACB and JA, global gene expression profiles upon 8h exposure to each treatment were inspected. The number of clean reads and mapping rates were listed in S2 Table. The Tophat2-Cufflinks pipeline [40] was employed to optimize existing gene models in the v3.23 genome annotation (S3 Table), and also to identify new gene models (S4 Table). Gene expression values in FPKM were based on the updated genome annotation. Principal component analysis (PCA) and hierarchical clustering showed high degree of reproducibility among biological replicates within each group (S2 Fig). A total of 614 genes were identified as differentially regulated in response to ACB damage (absolute fold change ≥2 and FDR adjusted p-value ≤0.05), with 470 genes up-regulated and 144 down-regulated. Comparable numbers of DEGs were found in JA-induced responses, with 558 genes up-regulated and 167 down-regulated (Fig 1b). Due to partial overlap between the two DEG sets, in total 1070 DEGs early-responsive to ACB and/or JA were observed, representing 2.7% of all maize genes (S5 Table). We observed a high correlation between ACB- and JA-induced transcriptomic changes (R2 = 0.73), indicating that JA-signaling indeed played a significant role in maize-ACB interaction. Validation of the expression levels of 20 genes by qRT-PCR showed strong correlation (R2 = 0.92) between results from RNA-seq and qRT-PCR (S1 Table).
To gain an insight into the biological processes involved in maize response to ACB and JA, over-represented GO (Gene Ontology) terms were analyzed for DEGs in each treatment. As shown in Fig 1c, Several GO terms were over-represented in both treatments, including 'response to wounding', 'response to JA', and also terms related to flavonoid, isoflavonoid phytoalexin, phenylpropanoid, and alkaloid biosynthesis, suggesting that biosynthesis of secondary metabolites in maize direct defense response was largely mediated by JA. Several GO terms were found only enriched in ACB treatment, but not in JA treatment. Examples include the GO term 'response to biotic stimulus' containing multiple orthologs of Arabidopsispathogenesis-related genes (PR1, PR4, PR5, and PR10), and 'response to salicylic acid', indicating that salicylic acid played important regulatory roles on genes that were ACB-inducible but JA-unresponsive. GO terms in this catogory also included 'negative regulation of peptidase activity' (mostly protease inhibitors) and 'diterpene phytoalexin metabolic process' (mostly terpene synthases), suggesting that induction of these genes mainly depends on signaling events other than JA pathway. Notably, there were also several GO terms specifically enriched in JA treatment, but not ACB treatment (Fig 1c), such as ‘JA metabolic process’ and‘JA and ethylene-dependent systemic resistance’, suggesting that corresponding genes might not be required for ACB resistance.
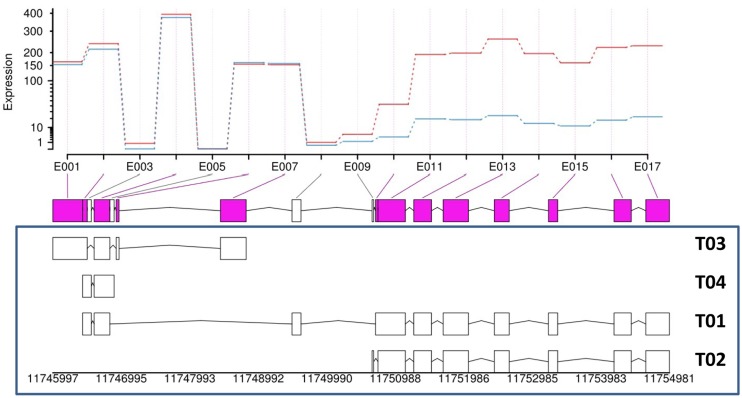
Alternative splicing has been reported to cause functional consequences. For example, production of JAZ repressors that are more stable at the protein level by alternative splicing prevents hyperactivation of the JA response in Arabidopsis [50]. In this study, differentially used exons (DUEs) in response to ACB and/or JA treatment were examined using DEXSeq. In total 16 DUEs from 8 genes were identified (S6 Table). Alternative usage of exons was not detected in any maize JAZ genes, suggesting that maize may adopt strategies different from that of Arabidopsis to control hyperactivation of the JA pathway. Notably, among the 16 DUEs, 8 were in gene GRMZM2G177098, which encodes sesquiterpene cyclase1 (stc1), a maize gene that responds to caterpillar herbivory by producing a chemical defense signal to attract natural enemies of the herbivore [15]. There are four alternative splicing variants for stc1 (designated T01~T04), and the elevated usage of the last 8 exons of stc1 in response to JA treatment indicated preferential induction of T02, a shorter version of the full-length stc1 (Fig 2). It would be interesting to investigate whether differential usage of exons of stc1 would cause biochemical or physiological consequences.
Fig 2. Differential usage of exons in stc1 in response to JA treatment.
The lower panel is a schematic representation of the four isoforms (T01~T04) of stc1. The middle panel is a collapsed view of all exons from the four isoforms. The upper panel represents mean expression levels of each exon in control (blue lines) and JA-treated (red lines) samples.
Differentially enriched cis-elements in distinct ACB- and JA-regulated gene clusters
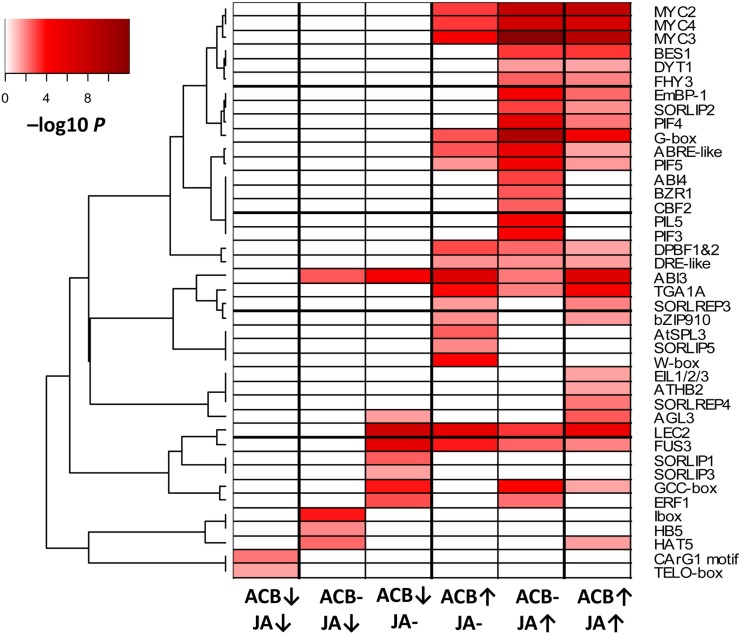
The 1070 DEGs were categorized into 6 groups according to their expression patterns shown in the Venn diagram in Fig 1b. The 6 groups were named ACB-up/JA-up, ACB-up/JA-normal, ACB-normal/JA-up, ACB-down/JA-down, ACB-down/JA-normal, and ACB-normal/JA-down, respectively. Genes with similar expression patterns generally share common regulatory cis-elements in their promoters, thus the enrichment of cis-elements for each DEG group was analyzed. This led to the identification of a total of 41 cis-elements enriched in at least one DEG group (Fig 3). Notably, the ACB-up/JA-up, ACB-normal/JA-up, and ACB-up/JA-normal group, although containing mutually exclusive sets of DEGs, shared a large number of enriched common cis-elements in their promoters, including MYC2/3/4 binding sites, G-box, ABRE-like motif, PIF5 binding site, DPBF1&2 motif, DRE-like motif, and also binding motifs for ABI3, TGA1A, LEC2 and FUS3. MYC2/3/4 are among the most pivotal master regulators in JA-signaling [51, 52]. ABRE-like elements, DRE-like elements, and DPBF1&2 binding sites were reported to be responsible for ABA and drought-responsive gene regulation [53–55]. Enrichment of these motifs implied an important role of ABA-signaling in maize response to ACB and/or JA. Moreover, over-representation of TGA1A binding sites, which were important for pathogen resistance responses [56], was consistent with enrichment of pathogenesis-related genes in our DEG sets. LEC2, FUS3 and ABI3 were reported to coordinately control biosynthesis of storage compounds including storage proteins and fatty acids [57]. In addition, A number of cis-elements were found only enriched in one or two of the ACB-up/JA-up, ACB-normal/JA-up, and ACB-up/JA-normal group, such as EIL1/2/3, BES1, BZR1, PIF3/4 binding sites, suggesting involvement of ethylene, BR and GA signaling in maize ACB and/or JA response. Interestingly, for ACB-down/JA-down, ACB-down/JA-normal, and ACB-normal/JA-down groups, cis-elements involved in plant development and light signaling were enriched, such as TELO-box, CArG1 motif, SORLIP1, SORLIP3, HAT5, and HB-5 binding sites. TELO-boxes were found enriched in the 5’ region of Arabidopsis genes encoding components of the translational apparatus [58, 59]. CArG1 box have been identified as targets of MADS domain transcription factors required for normal plant development [60–62]. These results suggested that corresponding transcription factors may play suppressive roles on their downstream target genes in maize herbivore defense response and JA response.
Fig 3. Enriched cis-elements in promoters of each DEG group.
DEGs were grouped based on their expression pattern shown in Fig 1b. Cis-elements over-represented in at least one DEG group promoters were listed. Color was scaled to represent log transformed false discovery rates.
Distinct inventories of transcription factors in ACB and JA response
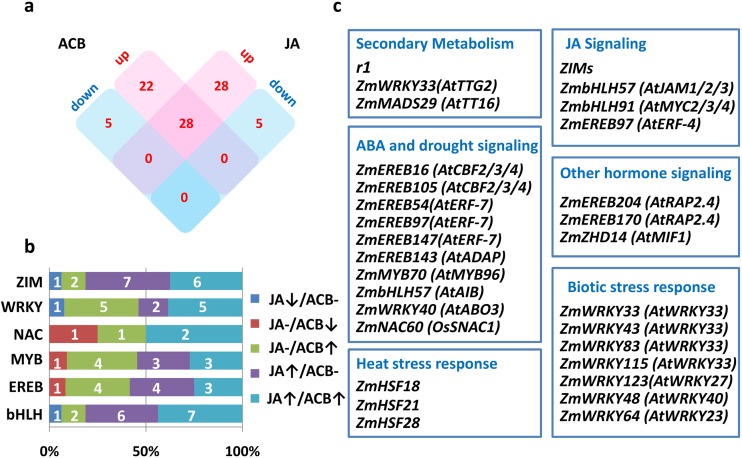
Among all categories of DEGs, special attention was paid to TFs that are transcriptionally regulated, and are therefore potentially involved, in herbivore defense and JA signaling. To identify TFs in our DEG sets, DEGs with homology to known TFs were combined with information from the GRASSIUS database, followed by manual removal of mis-annotated genes in GRASSIUS, leading to the annotation of in total 88 TFs (summarized in S5 Table). Among the 614 ACB-responsive DEGs, 50 up-regulated and 5 down-regulated differentially expressed TFs (DETFs) were identified. 56 up-regulated and 5 down-regulated DETFs were identified in response to JA (Fig 4a). The two sets of DETFs partially overlapped (28 DETFs inducible by both ACB and JA treatments), a scenario observed for DEGs. The 88 DETFs belonged to 17 families, with only 6 families with more than 3 members. The distribution of the 6 families in different DEG groups was examined to identify family-specific expression trends (Fig 4b). We found that bHLH and ZIM family TFs largely belonged to the ACB-normal/JA-up group and ACB-up/JA-up group, while a larger proportion of WRKY, MYB and EREB family members belonged to the ACB-up/JA-normal group. In addition, transcription factors were absent in ACB-down/JA-down group.
Fig 4. Transcription factors differentially regulated in response to ACB infestation and/or JA treatment.
a A Venn diagram showing the number of DETFs in DEG groups defined in Fig 1b. b Distribution of TF families among DETF groups. c Representative functions and TFs differentially regulated in response to ACB and/or JA treatment. For clarity, functionally characterized Arabidopsis or rice orthologs were listed in parenthesis next to their maize counterparts.
DETFs identified in this study have not been functionally characterized, except for the ZmbHLH1/r1/colored1 gene, a regulator of the anthocyanin pathway [63], thus putative functions of DETFs were assigned by their homology with characterized TFs in model species Arabidopsis or rice (summarized in S5 Table and Fig 4c). In addition to r1, ZmWRKY33 and ZmMADS29, ortholog of Arabidopsis TRANSPARENT TESTA GLABRA 2 (TTG2) [64] and TRANSPARENT TESTA16 (TT16) [65], respectively, were potential regulators of proanthocyanidin biosynthesis in maize defense and JA responses. Notably, TFs involved in phytohormone-signaling were particularly enriched, especially JA and ABA signaling. JA-related TFs included 16 JAZ/ZIM family genes, ZmbHLH57 (an ortholog of ArabidopsisJAM1/2/3 [33]), ZmbHLH91 (an ortholog of Arabidopsis MYC2/3/4 [51, 52]), and ZmEREB97 (orthologous to ATERF-4, a negative regulator of JA-responsive defense gene expression [66]). DETFs potentially involved in ABA signaling and drought stress response include: two orthologs of CBF2/3/4 [67] (ZmEREB105 and ZmEREB16), three orthologs of ArabidopsisAtERF-7 [68] (ZmEREB54, ZmEREB97, ZmEREB147), ZmMYB70 orthologous to AtMYB96 [69], ZmEREB143 orthologous to ArabidopsisADAP [70], ZmbHLH57 orthologous to Arabidopsis ABA-inducible BHLH-type transcription factor (AIB) [71], ZmWRKY40 orthologous to Arabidopsis ABA overly sensitive mutant 3 (ABO3) [72], and ZmNAC60 orthologous to rice SNAC1 [73]. In addition to JA and ABA signaling, a number of DETFs were implicated in other phytohormone signaling as well, including ZmEREB204 and ZmEREB170, two orthologs of ArabidopsisRelated to AP2.4 (RAP2.4) that mediates light and ethylene signaling [74]; and ZmZHD14, an ortholog of Arabidopsis Mini zinc Finger 1 (MIF1) that mediates GA signaling [75]. There were also a large number of DETFs implicated in biotic or abiotic stress responses. Four orthologs of AtWRKY33 (ZmWRKY83, ZmWRKY33, ZmWRKY115 and ZmWRKY43), an ortholog of AtWRKY27 (ZmWRKY123), and an ortholog of AtWRKY40 (ZmWRKY48), were in the DETF list. AtWRKY33, AtWRKY27, and AtWRKY40 have been previously reported to regulate defense response against bacteria or fungi pathogens [76–78]. We also identified ZmWRKY64, an ortholog of AtWRKY23 that was involved in nematode feeding site establishment [79]. DETFs potentially involved in abiotic stress responses include ZmHSF18, ZmHSF28 and ZmHSF21 belonging to the heat shock transcription factor family.
ZmNAC60 was a novel positive regulator of maize JA signaling
Among all DETFs identified in this study, we were particularly interested in ZmNAC60, since (1) ZmNAC60 was among the most highly ACB- and JA-inducible genes; (2) Phylogenetic analysis of NAC family members from maize, rice and Arabidopsis revealed that ZmNAC60 belonged to the SNAC subfamily (S3 Fig), according to the classification of NAC genes in rice [80, 81], and the role of SNAC family members in plant defense response against herbivores has not been defined, although they have been implicated in diverse abiotic and biotic stress responses in Arabidopsis, rice and poplar [26, 82–84]. Interestingly, ZmNAC60 is on a small branch of the SNAC subfamily devoid of any Arabidopsis NAC genes, and the Arabidopsis NAC genes most closely related to ZmNAC60 are ATAF1/2, which do not share sequence similarity with ZmNAC60 outside the NAC domain (S3 Fig), suggesting that functionally equivalent counterparts of ZmNAC60 might be absent in Arabidopsis. The rice NAC gene most closely related to ZmNAC60 was SNAC1, a positive regulator of rice drought resistance [73, 85], and the role of SNAC1 in defense response against herbivory was undefined.
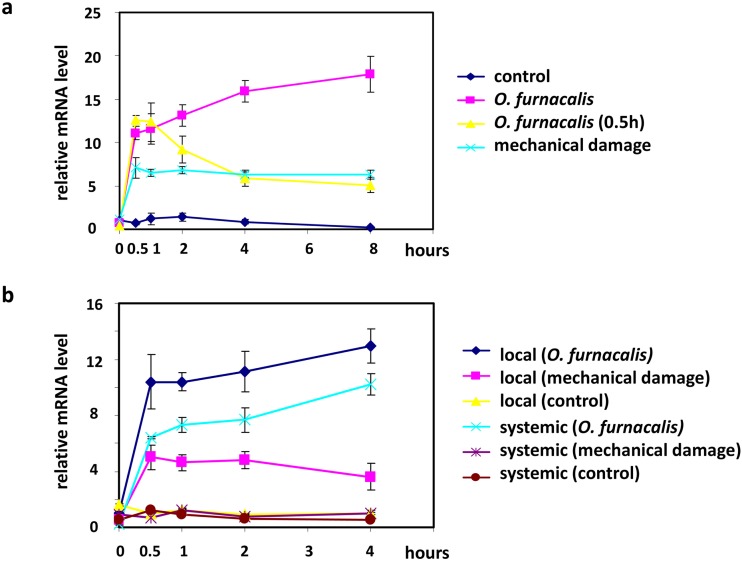
We first examined the expression patterns of ZmNAC60 in response to ACB and mechanical wounding that mimicked ACB damage. As shown in Fig 5a, both ACB herbivory and mechanical wounding swiftly induced ZmNAC60 expression within 0.5h, but ACB herbivory induced significantly higher levels of ZmNAC60 expression, compared with mechanical wounding alone. Moreover, prolonged ACB treatment after 0.5h, but not mechanical wounding, continuously elevated ZmNAC60 expression. These results indicated that mechanical wounding, albeit an important factor contributing to ZmNAC60 induction, did not completely explain the expression pattern of ZmNAC60. In addition, when ACBs were removed from maize plants at 0.5h, the expression level of ZmNAC60 decreased following ACB removal, indicating that hyper-accumulation of ZmNAC60 transcripts was dependent on continuous ACB infestation. The signal of herbivory can be transmitted systemically in plants through multiple mechanisms [86, 87]. To investigate whether systemic herbivore signal would induce ZmNAC60 expression, we restricted ACB damage and mechanical wounding on the third leaves of maize seedlings, and evaluated ZmNAC60 expression in the forth leaves. As shown in Fig 5b, ACB damage induced ZmNAC60 expression in systemic leaves, but the induction was not as strong as in local leaves. And mechanical damage failed to induce ZmNAC60 expression in systemic leaves in our experiment.
Fig 5. Time-series expression pattern of ZmNAC60 in response to ACB attack and mechanical wounding.
a Time-series expression pattern of ZmNAC60 in maize leaves subjected to continuous ACB damage, mechanical wounding, or short-term ACB damage (0.5h treatment). Untreated maize plants were used as control. b The maize third leaves were subjected to ACB damage or mechanical wounding, and expression of ZmNAC60 was quantified in the third (local tissue) as well as the forth leaves (systemic tissue). Error bars represent SEs from 3 biological replicates, with each replicate being a pooled sample containing leaves from 5~8 individual maize seedlings.
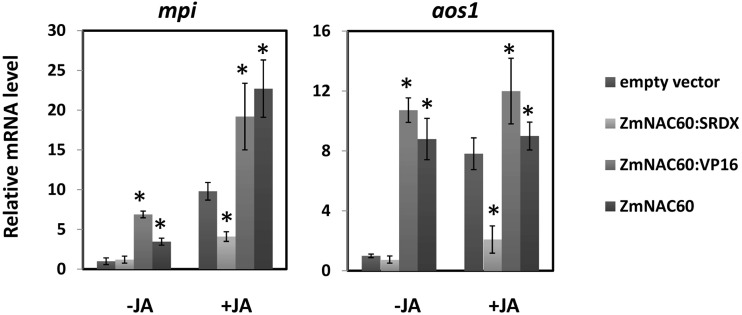
We then investigated whether ZmNAC60 functioned as a transcriptional regulator in maize defense response and JA-signaling. The full-length ZmNAC60 protein, or ZmNAC60 fused with the transcriptional suppressor SRDX or transcriptional activator VP16, were transiently expressed in maize protoplasts driven by the constitutive Ubiquitin promoter (Ubi). The transiently transformed protoplasts were treated either with or without JA. The validity of this experiment system was confirmed by the fact that for protoplasts transformed with the empty vector, those treated with JA displayed significantly higher expression of mpi and aos1 than those untreated with JA (Fig 6). In JA untreated groups, compared with protoplasts transformed with the empty vector, those transformed with Ubi::ZmNAC60:VP16 displayed higher expression of mpi and aos1, while the Ubi::ZmNAC60:SRDX construct did not significantly alter mpi and aos1 expression. In JA treated samples, Ubi::ZmNAC60:VP16 and Ubi::ZmNAC60:SRDX transformation lead to significant induction and suppression of marker genes, respectively. Moreover, Ubi::ZmNAC60 displayed effects on marker gene expression similar to that of Ubi::ZmNAC60:VP16 (Fig 6). These results collectively indicated that ZmNAC60 and ZmNAC60:VP16 were positive regulators, while ZmNAC60:SRDX functioned as negative regulators of, mpi and aos1 expression. Although it is still unknown whether ZmNAC60 functions directly or indirectly upstream of mpi and aos1, we propose that this transient expression assay could be adopted to screen DETFs for novel regulators in maize defense response and JA signaling.
Fig 6. ZmNAC60 transcriptionally regulated marker genes in maize defense response and JA signaling.
ZmNAC60, ZmNAC60:SRDX, or ZmNAC60:VP16 were transiently expressed in maize protoplasts, driven by the constitutive Ubiquitin promoter, with empty vector used as control. The transiently transformed protoplasts were treated either with or without jasmonic acidfor 3 hours. Expression of mpi and aos1 was quantified by qRT-PCR, and expression levels were normalized by transfection efficiency using a transfection control reporter vector constitutively expressing a GUS gene driven by the CaMV 35S promoter. Error bars represent SEs from 3 biological replicates. Student's t-test was used for comparisons between each sample with corresponding control sample transformed with the empty vector. * P < 0.05.
Conclusions
In this study, a comparative analysis of transcriptome and cis-elements on ACB- and JA-treated maize revealed distinct yet overlapping signaling network for the two processes. Transcription factors responsive to ACB and/or JA treatments were identified, and a transient expression assay was established to screen for important regulators in maize ACB and JA response. As a proof of concept, we proved that ZmNAC60 was a novel positive regulator for maize herbivore resistance response and JA signaling.
Supporting information
V3 stage B73 maize seedlings were treated with ACB or JA, and leaves were harvested at designated time points. Error bars represent SEs from 3 biological replicates, with each replicate being a pooled sample of 10 individual plants. Student's t-test was used for comparisons of treated and untreated (0h) samples. *P < 0.05.
(TIF)
(TIF)
The amino acid sequences of NAC domains of SNAC subfamily members were obtained from PlantTFDB and GRASSIUS. The evolutionary history was inferred using the UPGMA method implemented in MEGA6. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) were shown next to the branches. The tree were drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Sunita Kumari and Dr. David Jackson at Cold Spring Harbor Laboratory for kindly providing a comprehensive collection of PWMs of cis-elements in plants. This work was supported by National Natural Science Foundation of China (Grant 31501325 to Hai Wang and Grant 31570272 to Zhihong Lang).
Data Availability
The datasets supporting the conclusions of this article are available in the SRA repository (accession SRP102375).
Funding Statement
This work was supported by National Natural Science Foundation of China (Grant 31501325 to Hai Wang and Grant 31570272 to Zhihong Lang). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annual Review of Plant Biology. 2002;53:299–328. 10.1146/annurev.arplant.53.100301.135207 [DOI] [PubMed] [Google Scholar]
- 2.Lopez L, Camas A, Shivaji R, Ankala A, Williams P, Luthe D. Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta. 2007;226(2):517–27. 10.1007/s00425-007-0501-7 [DOI] [PubMed] [Google Scholar]
- 3.Fescemyer HW, Sandoya GV, Gill TA, Ozkan S, Marden JH, Luthe DS. Maize toxin degrades peritrophic matrix proteins and stimulates compensatory transcriptome responses in fall armyworm midgut. Insect Biochemistry and Molecular Biology. 2013;43(3):280–91. 10.1016/j.ibmb.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Chuang WP, Herde M, Ray S, Castano-Duque L, Howe GA, Luthe DS. Caterpillar attack triggers accumulation of the toxic maize protein RIP2. New phytologist. 2014;201(3):928–39. 10.1111/nph.12581 [DOI] [PubMed] [Google Scholar]
- 5.Schluter U, Benchabane M, Munger A, Kiggundu A, Vorster J, Goulet MC, et al. Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. Journal of Experimental Botany. 2010;61(15):4169–83. 10.1093/jxb/erq166 [DOI] [PubMed] [Google Scholar]
- 6.Butron A, Chen YC, Rottinghaus GE, McMullen MD. Genetic variation at bx1 controls DIMBOA content in maize. Theoretical and Applied Genetics. 2010;120(4):721–34. 10.1007/s00122-009-1192-1 [DOI] [PubMed] [Google Scholar]
- 7.Manzaneda AJ, Prasad KV, Mitchell-Olds T. Variation and fitness costs for tolerance to different types of herbivore damage in Boechera stricta genotypes with contrasting glucosinolate structures. New Phytologist. 2010;188(2):464–77. 10.1111/j.1469-8137.2010.03385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macel M, Bruinsma M, Dijkstra SM, Ooijendijk T, Niemeyer HM, Klinkhamer PG. Differences in effects of pyrrolizidine alkaloids on five generalist insect herbivore species. Journal of Chemical Ecology. 2005;31(7):1493–508. [DOI] [PubMed] [Google Scholar]
- 9.Santiago R, Malvar RA, Baamonde MD, Revilla P, Souto XC. Free phenols in maize pith and their relationship with resistance to Sesamia nonagrioides (Lepidoptera: Noctuidae) attack. Journal of Economic Entomology. 2005;98(4):1349–56. [DOI] [PubMed] [Google Scholar]
- 10.Degenhardt J, Hiltpold I, Kollner TG, Frey M, Gierl A, Gershenzon J, et al. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13213–8. 10.1073/pnas.0906365106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnee C, Kollner TG, Held M, Turlings TC, Gershenzon J, Degenhardt J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(4):1129–34. 10.1073/pnas.0508027103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatehouse JA. Plant resistance towards insect herbivores: a dynamic interaction. New Phytologist. 2002;156(2):145–69. [DOI] [PubMed] [Google Scholar]
- 13.Mithofer A, Wanner G, Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. II. Continuous mechanical wounding resembling insect feeding is sufficient to elicit herbivory-related volatile emission. Plant Physiology. 2005;137(3):1160–8. 10.1104/pp.104.054460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mithofer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiology. 2008;146(3):825–31. 10.1104/pp.107.113118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen B, Zheng Z, Dooner HK. A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: characterization of wild-type and mutant alleles. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(26):14807–12. 10.1073/pnas.240284097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer M, Fischer C, Meldau S, Seebald E, Oelmuller R, Baldwin IT. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiology. 2011;156(3):1520–34. 10.1104/pp.111.173567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmelz EA, Huffaker A, Carroll MJ, Alborn HT, Ali JG, Teal PE. An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defenses. Plant Physiology. 2012;160(3):1468–78. 10.1104/pp.112.201061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH 3rd, Teal PE. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(2):653–7. 10.1073/pnas.0811861106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffei ME, Mithofer A, Arimura G, Uchtenhagen H, Bossi S, Bertea CM, et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiology. 2006;140(3):1022–35. 10.1104/pp.105.071993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecourieux D, Raneva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytologist. 2006;171(2):249–69. 10.1111/j.1469-8137.2006.01777.x [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Hettenhausen C, Meldau S, Baldwin IT. Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. The Plant Cell. 2007;19(3):1096–122. 10.1105/tpc.106.049353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JQ, Baldwin IT. New Insights into Plant Responses to the Attack from Insect Herbivores. Annual Review of Genetics, Vol 44. 2010;44:1–24. [DOI] [PubMed] [Google Scholar]
- 23.Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305(5684):665–8. 10.1126/science.1096931 [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Allmann S, Wu J, Baldwin IT. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiology. 2008;146(3):904–15. 10.1104/pp.107.109264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reymond P, Bodenhausen N, Van Poecke RM, Krishnamurthy V, Dicke M, Farmer EE. A conserved transcript pattern in response to a specialist and a generalist herbivore. The Plant Cell. 2004;16(11):3132–47. 10.1105/tpc.104.026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. The Plant Cell. 2000;12(5):707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature. 2007;448(7154):661–5. 10.1038/nature05960 [DOI] [PubMed] [Google Scholar]
- 28.Ye M, Luo SM, Xie JF, Li YF, Xu T, Liu Y, et al. silencing COI1 in rice increases susceptibility to chewing insects and impairs inducible defense. PloS One. 2012;7(4):e36214 10.1371/journal.pone.0036214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Z, An F, Feng Y, Li P, Xue L, A M, et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(30):12539–44. 10.1073/pnas.1103959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao JL, Miao ZQ, Wang Z, Yu LH, Cai XT, Xiang CB. Arabidopsis ERF1 Mediates Cross-Talk between Ethylene and Auxin Biosynthesis during Primary Root Elongation by Regulating ASA1 Expression. PLoS Genetics. 2016;12(1):e1005760 10.1371/journal.pgen.1005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshii M, Yamazaki M, Rakwal R, Kishi-Kaboshi M, Miyao A, Hirochika H. The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling. The Plant Journal. 2010;61(5):804–15. 10.1111/j.1365-313X.2009.04107.x [DOI] [PubMed] [Google Scholar]
- 32.Song S, Qi T, Fan M, Zhang X, Gao H, Huang H, et al. The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genetics. 2013;9(7):e1003653 10.1371/journal.pgen.1003653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki-Sekimoto Y, Jikumaru Y, Obayashi T, Saito H, Masuda S, Kamiya Y, et al. Basic Helix-Loop-Helix Transcription Factors JASMONATE-ASSOCIATED MYC2-LIKE1 (JAM1), JAM2, and JAM3 Are Negative Regulators of Jasmonate Responses in Arabidopsis. Plant Physiology. 2013;163(1):291–304. 10.1104/pp.113.220129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu P, Zhou W, Cheng Z, Fan M, Wang L, Xie D. JAV1 controls jasmonate-regulated plant defense. Molecular Cell. 2013;50(4):504–15. 10.1016/j.molcel.2013.04.027 [DOI] [PubMed] [Google Scholar]
- 35.McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Maclean DJ, et al. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiology. 2005;139(2):949–59. 10.1104/pp.105.068544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweizer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P. Differential Contribution of Transcription Factors to Arabidopsis thaliana Defense Against Spodoptera littoralis. Frontiers in Plant Science. 2013;4:13 10.3389/fpls.2013.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ankala A, Kelley RY, Rowe DE, Williams WP, Luthe DS. Foliar herbivory triggers local and long distance defense responses in maize. Plant Science. 2013;199–200:103–12. 10.1016/j.plantsci.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 38.Lawrence SD, Novak NG, Kayal WE, Ju CJ, Cooke JE. Root herbivory: molecular analysis of the maize transcriptome upon infestation by Southern corn rootworm, Diabrotica undecimpunctata howardi. Physiologia Plantarum. 2012;144(4):303–19. 10.1111/j.1399-3054.2011.01557.x [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Zhang Y, Huang Q, Yin G, Pennerman KK, Yu J, et al. Analysis of key genes of jasmonic acid mediated signal pathway for defense against insect damages by comparative transcriptome sequencing. Scientific Reports. 2015;5:16500 10.1038/srep16500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7(3):562–78. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nature Protocols. 2013;8(9):1765–86. 10.1038/nprot.2013.099 [DOI] [PubMed] [Google Scholar]
- 42.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11(10):R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Research. 2012;22(10):2008–17. 10.1101/gr.133744.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–6. 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 45.Eveland AL, Goldshmidt A, Pautler M, Morohashi K, Liseron-Monfils C, Lewis MW, et al. Regulatory modules controlling maize inflorescence architecture. Genome Research. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheen J. Molecular mechanisms underlying the differential expression of maize pyruvate, orthophosphate dikinase genes. The Plant Cell. 1991;3(3):225–45. 10.1105/tpc.3.3.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature Protocols. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 48.Tamayo MC, Rufat M, Bravo JM, San Segundo B. Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of Spodoptera littoralis larvae. Planta. 2000;211(1):62–71. 10.1007/s004250000258 [DOI] [PubMed] [Google Scholar]
- 49.Yan Y, Christensen S, Isakeit T, Engelberth J, Meeley R, Hayward A, et al. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. The Plant Cell. 2012;24(4):1420–36. 10.1105/tpc.111.094151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung HS, Cooke TF, Depew CL, Patel LC, Ogawa N, Kobayashi Y, et al. Alternative splicing expands the repertoire of dominant JAZ repressors of jasmonate signaling. The Plant Journal. 2010;63(4):613–22. 10.1111/j.1365-313X.2010.04265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schweizer F, Fernandez-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, et al. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. The Plant Cell. 2013;25(8):3117–32. 10.1105/tpc.113.115139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, et al. The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. The Plant Cell. 2011;23(2):701–15. 10.1105/tpc.110.080788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, et al. AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. The Plant Cell. 2005;17(12):3470–88. 10.1105/tpc.105.035659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. The Plant Cell. 1994;6(2):251–64. 10.1105/tpc.6.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11632–7. 10.1073/pnas.190309197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kesarwani M, Yoo J, Dong X. Genetic interactions of TGA transcription factors in the regulation of pathogenesis-related genes and disease resistance in Arabidopsis. Plant Physiology. 2007;144(1):336–46. 10.1104/pp.106.095299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, et al. A network of local and redundant gene regulation governs Arabidopsis seed maturation. The Plant Cell. 2006;18(7):1642–51. 10.1105/tpc.105.039925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manevski A, Bertoni G, Bardet C, Tremousaygue D, Lescure B. In synergy with various cis-acting elements, plant insterstitial telomere motifs regulate gene expression in Arabidopsis root meristems. FEBS Letters. 2000;483(1):43–6. [DOI] [PubMed] [Google Scholar]
- 59.Tremousaygue D, Manevski A, Bardet C, Lescure N, Lescure B. Plant interstitial telomere motifs participate in the control of gene expression in root meristems. The Plant Journal. 1999;20(5):553–61. [DOI] [PubMed] [Google Scholar]
- 60.Tilly JJ, Allen DW, Jack T. The CArG boxes in the promoter of the Arabidopsis floral organ identity gene APETALA3 mediate diverse regulatory effects. Development. 1998;125(9):1647–57. [DOI] [PubMed] [Google Scholar]
- 61.Tang W, Perry SE. Binding Site Selection for the Plant MADS Domain Protein AGL15: AN IN VITRO AND IN VIVO STUDY. Journal of Biological Chemistry. 2003;278(30):28154–9. 10.1074/jbc.M212976200 [DOI] [PubMed] [Google Scholar]
- 62.Mendes MA, Guerra RF, Berns MC, Manzo C, Masiero S, Finzi L, et al. MADS Domain Transcription Factors Mediate Short-Range DNA Looping That Is Essential for Target Gene Expression in Arabidopsis. The Plant Cell. 2013;25(7):2560–72. 10.1105/tpc.112.108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cone KC, Burr FA, Burr B. Molecular analysis of the maize anthocyanin regulatory locus C1. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(24):9631–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pesch M, Dartan B, Birkenbihl R, Somssich IE, Hulskamp M. Arabidopsis TTG2 regulates TRY expression through enhancement of activator complex-triggered activation. The Plant Cell. 2014;26(10):4067–83. 10.1105/tpc.114.129379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nesi N, Debeaujon I, Jond C, Stewart AJ, Jenkins GI, Caboche M, et al. The TRANSPARENT TESTA16 locus encodes the ARABIDOPSIS BSISTER MADS domain protein and is required for proper development and pigmentation of the seed coat. The Plant Cell. 2002;14(10):2463–79. 10.1105/tpc.004127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pre M, Atallah M, Champion A, De Vos M, Pieterse CM, Memelink J. The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiology. 2008;147(3):1347–57. 10.1104/pp.108.117523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR. Abscisic Acid Induces CBF Gene Transcription and Subsequent Induction of Cold-Regulated Genes via the CRT Promoter Element. Plant Physiology. 2004;135(3):1710–7. 10.1104/pp.104.043562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song C-P, Agarwal M, Ohta M, Guo Y, Halfter U, Wang P, et al. Role of an Arabidopsis AP2/EREBP-Type Transcriptional Repressor in Abscisic Acid and Drought Stress Responses. The Plant Cell. 2005;17(8):2384–96. 10.1105/tpc.105.033043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, et al. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiology. 2009;151(1):275–89. 10.1104/pp.109.144220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SJ, Cho DI, Kang JY, Kim SY. An ARIA-interacting AP2 domain protein is a novel component of ABA signaling. Molecules and Cells. 2009;27(4):409–16. 10.1007/s10059-009-0058-3 [DOI] [PubMed] [Google Scholar]
- 71.Li H, Sun J, Xu Y, Jiang H, Wu X, Li C. The bHLH-type transcription factor AtAIB positively regulates ABA response in Arabidopsis. Plant Molecular Biology. 2007;65(5):655–65. 10.1007/s11103-007-9230-3 [DOI] [PubMed] [Google Scholar]
- 72.Ren X, Chen Z, Liu Y, Zhang H, Zhang M, Liu Q, et al. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. The Plant Journal. 2010;63(3):417–29. 10.1111/j.1365-313X.2010.04248.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, et al. Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):12987–92. 10.1073/pnas.0604882103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin RC, Park HJ, Wang HY. Role of Arabidopsis RAP2.4 in regulating light- and ethylene-mediated developmental processes and drought stress tolerance. Molecular Plant. 2008;1(1):42–57. 10.1093/mp/ssm004 [DOI] [PubMed] [Google Scholar]
- 75.Hu W, Ma H. Characterization of a novel putative zinc finger gene MIF1: involvement in multiple hormonal regulation of Arabidopsis development. The Plant Journal. 2006;45(3):399–422. 10.1111/j.1365-313X.2005.02626.x [DOI] [PubMed] [Google Scholar]
- 76.Pandey SP, Roccaro M, Schon M, Logemann E, Somssich IE. Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. The Plant Journal. 2010;64(6):912–23. 10.1111/j.1365-313X.2010.04387.x [DOI] [PubMed] [Google Scholar]
- 77.Mukhtar MS, Deslandes L, Auriac MC, Marco Y, Somssich IE. The Arabidopsis transcription factor WRKY27 influences wilt disease symptom development caused by Ralstonia solanacearum. The Plant Journal. 2008;56(6):935–47. 10.1111/j.1365-313X.2008.03651.x [DOI] [PubMed] [Google Scholar]
- 78.Zheng Z, Qamar SA, Chen Z, Mengiste T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. The Plant Journal. 2006;48(4):592–605. 10.1111/j.1365-313X.2006.02901.x [DOI] [PubMed] [Google Scholar]
- 79.Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler F, et al. A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiology. 2008;148(1):358–68. 10.1104/pp.108.119131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang Y, You J, Xie K, Xie W, Xiong L. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Molecular Genetics and Genomics. 2008;280(6):547–63. 10.1007/s00438-008-0386-6 [DOI] [PubMed] [Google Scholar]
- 81.Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, et al. Genome-wide analysis of NAC transcription factor family in rice. Gene. 2010;465(1–2):30–44. 10.1016/j.gene.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 82.Greve K, La Cour T, Jensen MK, Poulsen FM, Skriver K. Interactions between plant RING-H2 and plant-specific NAC (NAM/ATAF1/2/CUC2) proteins: RING-H2 molecular specificity and cellular localization. The Biochemical Journal. 2003;371(Pt 1):97–108. 10.1042/BJ20021123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delessert C, Kazan K, Wilson IW, Van Der Straeten D, Manners J, Dennis ES, et al. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis. The Plant Journal. 2005;43(5):745–57. 10.1111/j.1365-313X.2005.02488.x [DOI] [PubMed] [Google Scholar]
- 84.Nakashima K, Tran LS, Van Nguyen D, Fujita M, Maruyama K, Todaka D, et al. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. The Plant Journal. 2007;51(4):617–30. 10.1111/j.1365-313X.2007.03168.x [DOI] [PubMed] [Google Scholar]
- 85.Redillas MC, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, et al. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnology Journal. 2012;10(7):792–805. 10.1111/j.1467-7652.2012.00697.x [DOI] [PubMed] [Google Scholar]
- 86.Pena-Cortes H, Fisahn J, Willmitzer L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature. 2013;500(7463):422–6. 10.1038/nature12478 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
V3 stage B73 maize seedlings were treated with ACB or JA, and leaves were harvested at designated time points. Error bars represent SEs from 3 biological replicates, with each replicate being a pooled sample of 10 individual plants. Student's t-test was used for comparisons of treated and untreated (0h) samples. *P < 0.05.
(TIF)
(TIF)
The amino acid sequences of NAC domains of SNAC subfamily members were obtained from PlantTFDB and GRASSIUS. The evolutionary history was inferred using the UPGMA method implemented in MEGA6. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) were shown next to the branches. The tree were drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
The datasets supporting the conclusions of this article are available in the SRA repository (accession SRP102375).