Abstract
Importance
As men age, they experience decreased serum testosterone concentrations, decreased bone mineral density (BMD), and increased risk of fracture.
Objective
To determine whether testosterone treatment of older men with low testosterone increases volumetric BMD (vBMD) and estimated bone strength.
Design, Setting, and Participants
Placebo-controlled, double-blind trial with treatment allocation by minimization at 9 US academic medical centers of men 65 years or older with 2 testosterone concentrations averaging less than 275 ng/L participating in the Testosterone Trials from December 2011 to June 2014. The analysis was a modified intent-to-treat comparison of treatment groups by multivariable linear regression adjusted for balancing factors as required by minimization.
Interventions
Testosterone gel, adjusted to maintain the testosterone level within the normal range for young men, or placebo gel for 1 year.
Main Outcomes and Measures
Spine and hip vBMD was determined by quantitative computed tomography at baseline and 12 months. Bone strength was estimated by finite element analysis of quantitative computed tomography data. Areal BMD was assessed by dual energy x-ray absorptiometry at baseline and 12 months.
Results
There were 211 participants (mean [SD] age, 72.3 [5.9] years; 86% white; mean [SD] body mass index, 31.2 [3.4]). Testosterone treatment was associated with significantly greater increases than placebo in mean spine trabecular vBMD (7.5%; 95% CI, 4.8% to 10.3% vs 0.8%; 95% CI, −1.9% to 3.4%; treatment effect, 6.8%; 95% CI, 4.8%-8.7%; P < .001), spine peripheral vBMD, hip trabecular and peripheral vBMD, and mean estimated strength of spine trabecular bone (10.8%; 95% CI, 7.4% to 14.3% vs 2.4%; 95% CI, −1.0% to 5.7%; treatment effect, 8.5%; 95% CI, 6.0%-10.9%; P < .001), spine peripheral bone, and hip trabecular and peripheral bone. The estimated strength increases were greater in trabecular than peripheral bone and greater in the spine than hip. Testosterone treatment increased spine areal BMD but less than vBMD.
Conclusions and Relevance
Testosterone treatment for 1 year of older men with low testosterone significantly increased vBMD and estimated bone strength, more in trabecular than peripheral bone and more in the spine than hip. A larger, longer trial could determine whether this treatment also reduces fracture risk.
As men age, they experience decreases in serum testosterone concentration.1,2 They also experience decreases in areal bone mineral density (aBMD),3-5 volumetric bone mineral density (vBMD),6 and estimated strength6 and an increase in fractures.7 When men of any age develop severely low testosterone due to known disease, their BMD decreases8-11 and fractures increase.12,13 In men who are frankly hypogonadal, testosterone treatment improves BMD,14-16 trabecular architecture,17 and mechanical properties.18
Prior studies of the effect oftestosterone treatment on bone in older men, however, have not been conclusive.19-22 In 1 placebo-controlled study, testosterone treatment did not improve spine BMD overall, but in a regression model, lower serum testosterone predicted a significantly greater effect of testosterone treatment on spine BMD.19 Another study demonstrated a significant increase in spine and hip BMD in testosterone-treated men, but supraphysiologic doses of testosterone were used.21
We report here the results of the Bone Trial of the Testosterone Trials (T-Trials), a group of 7 coordinated trials of the effects of testosterone treatment of older men with low testosterone concentrations.23,24 The purpose of the Bone Trial was to determine whether testosterone treatment would improve vBMD and estimated bone strength.
Methods
Study Design
The T-Trials were conducted at 12 US sites; 9 of them participated in the Bone Trial. The study design has been described.23 To enroll in the T-Trials overall, participants had to qualify for at least 1 of the 3 main trials.24 If they qualified, they could participate in the Bone Trial. Participants were randomly assigned to receive testosterone or placebo gel double-blindly for 1 year. This report describes the efficacy results for the Bone Trial.
The protocol (Supplement 1) was approved by the institutional review boards of all participating institutions. All men provided written, informed consent. A data safety monitoring board approved the protocol and monitored unblinded safety data.
Participants
Participants were recruited and screened as described.25 Respondents were screened first by telephone interview and then during 2 clinic visits. To be included in the T-Trials, men had to be at least 65 years old, have subjective and objective evidence of impaired sexual or physical function or reduced vitality, and have a serum testosterone concentration on 2 morning specimens that averaged less than 275 ng/dL (to convert to nanomoles per liter, multiply by 0.0347). Potential participants were excluded if they were at increased risk of conditions that testosterone treatment might exacerbate. Potential participants for the Bone Trial were also excluded if they were taking a medication known to affect bone, except for calcium and over-the-counter vitamin D preparations; if they did not have at least 1 evaluable lumbar vertebra; or if they had a dual-energy x-ray absorptiometry (DXA) T-score at any site of less than −3.0.
Treatment
We allocated participants to receive testosterone or placebo gel by minimization.26,27 Balancing variables included participation in each of the main trials, clinical site, testosterone concentration greater than or less than 200 ng/dL, and age older or younger than 75 years. The testosterone preparation was AndroGel 1% in a pump bottle (AbbVie). Placebo gel was similar. The initial dose was 5 g daily. Serum testosterone concentration was measured at months 1, 2, 3, 6, and 9 in a central laboratory (Quest Clinical Trials), and the dose of testosterone gel was adjusted after each measurement to attempt to keep the concentration within the normal range for young men. To maintain blinding when the dose was adjusted in a participant taking testosterone, the dose was changed simultaneously in a participant taking placebo by a staff person in the Data Coordinating Center according to a prespecified algorithm; no site personnel knew the treatment allocation.
All participants were given and instructed to take 1 tablet containing 600 g of elemental calcium and 400 units of vitamin D3 twice a day with meals.
Assessments
At the end of the trials, the serum concentrations of testosterone and estradiol were measured by liquid chromatography and tandem mass spectroscopy and free testosterone by equilibrium dialysis in the Brigham Research Assay Core Laboratory, Boston, Massachusetts.24 Samples from baseline and months 3, 6, 9, and 12 from each participant were measured in the same assay run.
Efficacy outcomes in the Bone Trial were assessed at baseline and after 12 months of treatment. The primary efficacy outcome was percent change from baseline in vBMD of trabecular bone in the lumbar spine, as assessed by means of quantitative computed tomography (QCT). Volumetric BMD was chosen as the main method of assessment rather than aBMD by DXA because it is not artifactually influenced by osteophytes and aortic calcification4,28 and because it can distinguish between trabecular bone, which testosterone affects primarily, and cortical bone.18 Secondary outcomes were vBMD of peripheral bone and whole bone of the lumbar spine and trabecular, peripheral, and whole bone of the hip; estimated strength of the same sites by finite element analysis (FEA) from computed tomographic (CT) data; and aBMD of the spine and hip by DXA.
All T-Trials participants were asked about fractures every 3 months during treatment and at 6 and 12 months afterwards. An independent adjudicator reviewed radiographic reports of all reported fractures and adjudicated without knowledge of treatment allocation.
Computed tomographic scans of the lumbar spine and the hip were performed at baseline and month 12. The QCT reading center trained the technicians at each of the 9 clinical sites to ensure a consistent imaging technique. The spine scan extended from mid-T12 to mid-L4; L1 and L2 measurements were used preferentially, but if not assessable, L3 was used; the values of 2 vertebrae were averaged. The hip scan extended from 1 cm above the femoral heads to 2 cm below the lesser trochanters; both hips were used if assessable and the results averaged. Each image included an external bone mineral phantom (Mindways Software) beneath the participant for calibration. A second phantom (Mindways) was scanned monthly to detect any field nonuniformity or scanner drift. The mean (range) coefficient of variation for all scanners was 0.23% (0.13%-0.29%). This phantom was also used for cross-calibration for the 12 participants at 2 sites whose scans were acquired using different scanners at the baseline and 12-month visits. A third phantom (European Spine Phantom, QRM GmbH) was used to verify cross-calibration.
Image processing, vBMD measurements, and finite element strength analyses were performed at a central site (O.N. Diagnostics), blinded to treatment group, by analysis of the CT scans using VirtuOst software. O.N. Diagnostics also maintained quality control of the CT data collection. Construction of the finite element models has been described.29-31 For the vertebrae, trabecular vBMD was measured using an elliptical region of interest in the trabecular centrum (eFigure 1 in Supplement 2). Whole bone and peripheral vBMD were defined, respectively, as the mean vBMD for the whole vertebral body, and the outer 2 mm of bone, which included the cortex and neighboring trabecular bone. To measure vertebral strength, uniform axial compression was applied virtually to the finite element model through a layer of bone cement (eFigure 1 in Supplement 2); the whole bone strength was defined as the force at 2% deformation. Trabecular strength was similarly measured after removing the outer 2 mm of bone, and peripheral strength was calculated as whole-bone strength minus trabecular strength.
For the femur, whole-bone vBMD was measured as the mean density of the entire model. Each model was then divided into a trabecular compartment (all bone with an apparent density less than 1 mg/cm3 and more than 3 mm from the periosteum) and a peripheral compartment (all bone not in the defined trabecular compartment containing the cortex and some adjacent trabecular bone) (eFigure 1 in Supplement 2). Trabecular and peripheral vBMD were measured as the mean vBMD of their respective compartments. Femoral strength was measured by simulation of a sideways fall. Trabecular strength was similarly measured after assigning 2 reference densities to the peripheral compartment; and peripheral strength was measured after assigning a single reference density to the trabecular compartment.
Dual-energy x-ray absorptiometry scans of the lumbar spine and hip were obtained at the baseline and 12-month visits using Hologic densitometers. Quality control of DXA was centrally monitored by the University of California San Francisco Coordinating Center, DXA QA Group. The DXA operators at each of the 9 sites were certified at the beginning of the trial. Scans were analyzed locally, using the same software version at baseline and follow-up, and sent to the Coordinating Center for incorporation into a central database. Flagged scans and a random sample of scans were reviewed for quality. Longitudinal performance of densitometers was monitored with regular scanning of a spine phantom.
Statistical Analyses
Sample size was based on a prior study in hypogonadal men that showed a mean (SD) increase in trabecular vBMD of 14% (3%) over 18 months of testosterone treatment.14 We posited a 9% improvement over 12 months, assuming no change in the placebo group and the same standard deviation. To achieve 90% power with a 2-sided significance level of .05, we required 172 men; we targeted 200 men to compensate for non-adherence and dropout.
Analyses followed the intention-to-treat principle; men allocated to testosterone were compared with men allocated to placebo, regardless of adherence or T level achieved. All participants who had baseline and month 12 scans were included in the analyses. Each outcome reported here was prespecified. The effect of testosterone compared with placebo on percent change in bone outcomes was evaluated by multivariable linear regression, adjusted for balancing factors as required for the analysis of interventions allocated by minimization. Multiple imputation was used to assess the influence of missing month 12 scans on the primary outcome analysis. Imputation models included demographic and clinical variables listed in Table 1. The Markov chain Monte Carlo method was used to impute missing values. All analyses were conducted at a 2-sided significance level of .05.
Table 1. Baseline Characteristics of Participants in the Bone Trial.
| Characteristic | Testosterone (n = 110) | Placebo (n = 101) |
|---|---|---|
| Age, mean (SD), y | 72.3 (6.3) | 72.4 (5.5) |
| Race, No. (%) | ||
| White | 93 (84.5) | 88 (87.1) |
| African American | 6 (5.5) | 4 (4.0) |
| Other | 11 (10.0) | 9 (8.9) |
| Concomitant conditions, mean (SD) | ||
| BMI, mean (SD) | 30.7 (3.7)a | 31.8 (3.1) |
| Alcohol use, mean (SD), No. drinks/wk | 2.5 (3.5)a | 4.0 (5.3) |
| Smoking, No. (%) | ||
| Current smoker | 6 (5.5) | 7 (6.9) |
| Ever smoker | 70 (63.6) | 72 (71.3) |
| Diabetes | 43 (39.1) | 40 (39.6) |
| Serum steroid hormone, mean (SD) | ||
| Total testosterone, ng/dL | 229.6 (65.3) | 238.8 (64.0) |
| Free testosterone, pg/mL | 61.2 (20.0) | 64.5 (21.1) |
| Estradiol, pg/mL | 20.5 (6.7)a | 22.4 (6.4) |
| DXA areal BMD, mean (SD), g/cm2 | ||
| Lumbar spine | 1.2 (0.2) | 1.2 (0.2) |
| Total hip | 1.0 (0.2) | 1.0 (0.1) |
| Femoral neck | 0.8 (0.1) | 0.8 (0.1) |
| DXA BMD T-score,b mean (SD) | ||
| Lumbar spine | 1.3 (1.8) | 1.2 (1.8) |
| Total hip | 0.7 (1.2) | 0.6 (1.2) |
| Femoral neck | -0.3 (1.1) | -0.3 (1.2) |
Abbreviations: BMD, bone mineral density; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DXA, dual-energy x-ray absorptiometry.
SI conversion factors: To convert testosterone to nanomoles per liter, multiply by 0.0347; to convert free testosterone to picomoles per liter, multiply by 3.47; to convert estradiol to picomoles per liter, multiply by 3.67.
P < .05 compared with placebo (t test).
Calculated from young female referent database.
Multivariable linear regression models with interactions of treatment and baseline factors were used to examine whether the magnitude of the effect of testosterone treatment differed according to baseline vBMD, total serum testosterone level, or estradiol level. Unadjusted linear regression was used to determine, in men in the testosterone arm, the association of the percent change in trabecular vBMD of the lumbar spine from baseline to month 12 with absolute change in total testosterone and estradiol from baseline to month 12.
Analyses did not adjust for multiple comparisons because the bone outcomes were likely highly correlated, making such adjustments overly conservative.
Results
Participants and Treatment
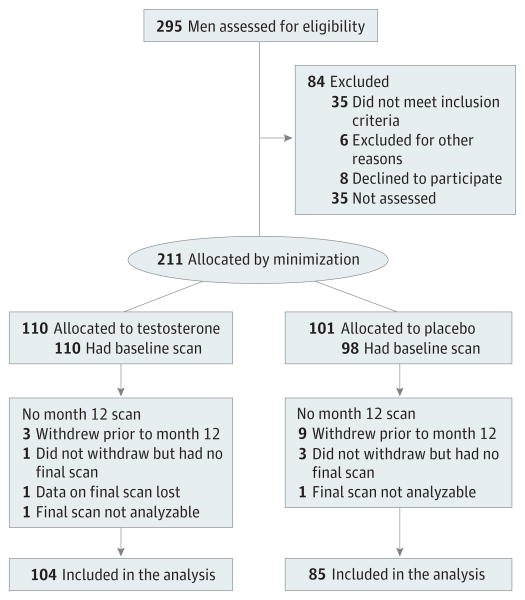
Recruitment began in December 2011. Targeted enrollment was completed in June 2013, and treatment was completed in June 2014. Of the 295 men who enrolled in one of the main T-Trials, at the 9 clinical trial sites from the inception of the Bone Trial, 211 met Bone Trial entry criteria and enrolled (Figure 1). Allocation to testosterone or placebo treatment was the same for each participant as in the T-Trials overall. One hundred eightynine participants (90%) completed 12 months of treatment and had analyzable baseline and 12-month scans. Noncompletion was more frequent among men in the placebo arm (16 [15.8%] placebo, 6 [5.5%] testosterone; P = .01); demographic characteristics, baseline hormone levels, and baseline bone strength and density measures did not differ between completers and noncompleters.
Figure 1. Screening and Retention of Participants.
At baseline, the participants had low serum testosterone concentrations for young men (Table 1). Baseline characteristics in the 2 treatment arms were similar, including total and free testosterone levels and aBMD. The mean T scores for the spine and hip were not low (Table 1). Mean body mass index, alcohol consumption, and serum estradiol level were slightly higher in the placebo-treated men.
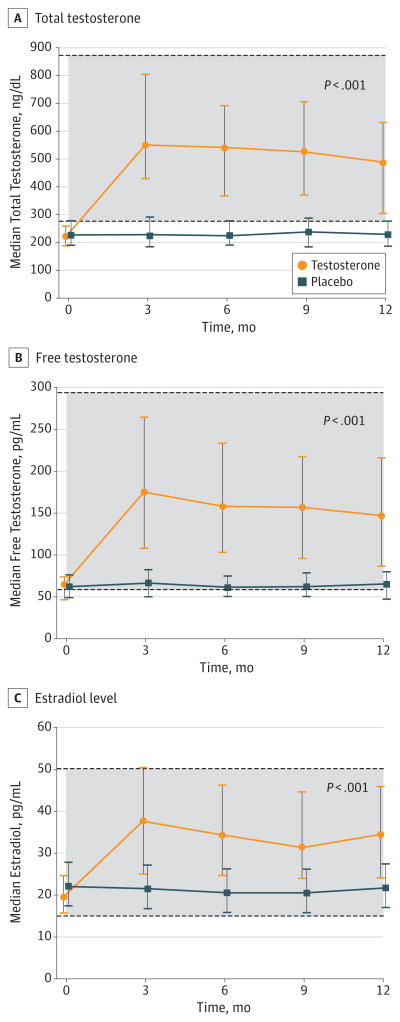
Treatment with testosterone increased the median serum concentrations of total testosterone, free testosterone, and estradiol to within the normal ranges for young men (Figure 2).
Figure 2. Median Serum Concentrations of Total Testosterone, Free Testosterone, and Estradiol From Months 0 to 12 in Men Treated With Testosterone or Placebo.
The P values indicate the significance of the difference in serum concentrations in men in the testosterone arm compared with men in the placebo arm. The shaded areas represent the normal ranges for healthy young men. Error bars indicate interquartile ranges.
SI conversion factors: To convert testosterone to nanomoles per liter, multiply by 0.0347; to convert free testosterone to picomoles per liter, multiply by 3.47; to convert estradiol to picomoles per liter, multiply by 3.67.
Efficacy
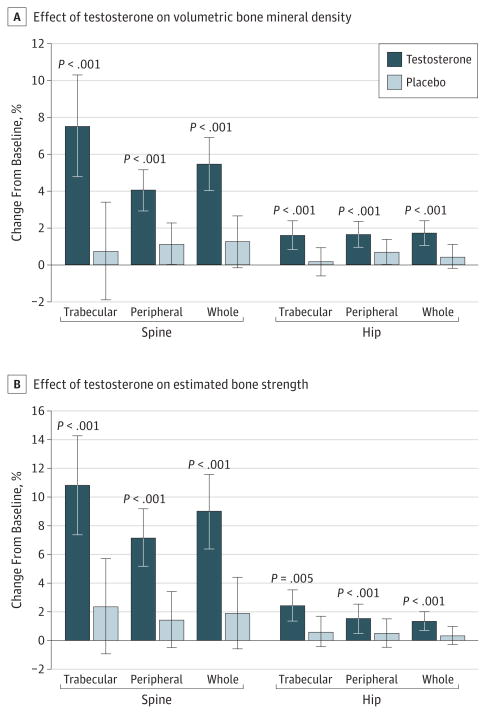
Testosterone treatment increased mean lumbar spine trabecular vBMD (primary outcome) by 7.5% (95% CI, 4.8% to 10.3%), compared with 0.8% (95% CI, −1.9% to 3.4%) by placebo (Figure 3A and Table 2), a difference of 6.8% (95% CI, 4.8% to 8.7%; P < .001; r2 = 0.26). The mean difference was somewhat less (4.0%; 95% CI, 3.0% to 5.0%) in sensitivity analyses for missing month 12 scans but still significant (P < .001).
Figure 3. Effects of Testosterone or Placebo Treatment for 12 Months on Volumetric Bone Mineral Density and Estimated Bone Strength of Trabecular, Peripheral, and Whole Bone of the Spine and Hip, as Assessed by Quantitative Computed Tomography.
Bars indicate means, and error bars, standard deviations. The P values indicate the significance of the difference in change in percent volumetric bone mineral density or estimated strength from baseline to 12 months for men in the testosterone arm compared with the placebo arm.
Table 2. Bone Trial Primary and Secondary Outcomes.
| Outcome | Treatment | No. | Mean (SD) | Adjusted Change From Baseline, %a (95% CI) | Treatment Effect, %b (95% CI) | P Valuec | r2 d | |
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 12 | |||||||
| Volumetric BMD by QCT, mg/cm3 | ||||||||
| Primary outcome | ||||||||
| Spine trabecular bone | Testosterone | 110 | 102.4 (31.9) | 106.8 (32.4) | 7.5 (4.8 to 10.3) | 6.8 (4.8 to 8.7) | <.001 | 0.26 |
| Placebo | 97 | 99.4 (27.0) | 99.6 (27.1) | 0.8 (-1.9 to 3.4) | ||||
| Secondary outcomes | ||||||||
| Spine peripheral bone | Testosterone | 110 | 285.4 (42.5) | 292.9 (43.1) | 4.0 (2.9 to 5.2) | 2.9 (2.1 to 3.7) | <.001 | 0.29 |
| Placebo | 97 | 284.2 (43.3) | 288.4 (43.8) | 1.1 (0.0 to 2.2) | ||||
| Spine whole bone | Testosterone | 110 | 193.4 (37.2) | 199.6 (37.2) | 5.5 (4.0 to 6.9) | 4.2 (3.2 to 5.3) | <.001 | 0.32 |
| Placebo | 97 | 192.6 (34.9) | 194.6 (34.8) | 1.2 (-0.2 to 2.6) | ||||
| Hip trabecular bone | Testosterone | 103 | 185.4 (34.3) | 187.1 (35.0) | 1.6 (0.8 to 2.4) | 1.5 (0.9 to 2.0) | <.001 | 0.25 |
| Placebo | 88 | 180.7 (33.1) | 181.9 (32.9) | 0.1 (-0.6 to 0.9) | ||||
| Hip peripheral bone | Testosterone | 103 | 399.0 (46.4) | 402.8 (46.1 | 1.6 (0.9 to 2.3) | 1.0 (0.5 to 1.5) | <.001 | 0.22 |
| Placebo | 88 | 391.7 (50.2) | 395.7 (47.9) | 0.7 (-0.0 to 1.4) | ||||
| Hip whole bone | Testosterone | 103 | 248.8 (37.9) | 251.2 (38.6) | 1.7 (1.0 to 2.4) | 1.3 (0.8 to 1.7) | <.001 | 0.27 |
| Placebo | 88 | 243.1 (38.9) | 245.2 (37.8) | 0.4 (-0.2 to 1.1) | ||||
| Bone strength by finite element analysis, N | ||||||||
| Spine whole bone | Testosterone | 110 | 8258 (2491) | 8614 (2461) | 9.0 (6.4 to 11.6) | 7.1 (5.3 to 8.9) | <.001 | 0.31 |
| Placebo | 97 | 8106 (2256) | 8104 (2149) | 1.9 (-0.6 to 4.4) | ||||
| Spine trabecular bone | Testosterone | 110 | 4404 (1572) | 4618 (1547) | 10.8 (7.4 to 14.3) | 8.5 (6.0 to 10.9) | <.001 | 0.29 |
| Placebo | 97 | 4343 (1451) | 4313 (1332) | 2.4 (-1.0 to 5.7) | ||||
| Spine peripheral bone | Testosterone | 110 | 3855 (1015) | 3996 (1006) | 7.2 (5.2 to 9.2) | 5.7 (4.3 to 7.2) | <.001 | 0.30 |
| Placebo | 97 | 3763 (920.0) | 3791 (932.4) | 1.5 (-0.5 to 3.4) | ||||
| Hip whole bone | Testosterone | 103 | 4937 (1068) | 5008 (1090) | 2.5 (1.4 to 3.5) | 1.8 (1.1 to 2.6) | <.001 | 0.27 |
| Placebo | 88 | 4937 (1056) | 4967 (1031) | 0.6 (-0.4 to 1.7) | ||||
| Hip trabecular bone | Testosterone | 103 | 4848 (872.4) | 4892 (885.9) | 1.5 (0.5 to 2.5) | 1.0 (0.3 to 1.7) | .005 | 0.19 |
| Placebo | 88 | 4830 (877.6) | 4864 (870.3) | 0.5 (-0.5 to 1.5) | ||||
| Hip peripheral bone | Testosterone | 103 | 4751 (728.8) | 4782 (709.0) | 1.4 (0.7 to 2.0) | 1.0 (0.5 to 1.4) | <.001 | 0.25 |
| Placebo | 88 | 4756 (684.1) | 4776 (689.0) | 0.4 (-0.3 to 1.0) | ||||
| Areal BMD by DXA, g/cm2 | ||||||||
| Lumbar spine | Testosterone | 109 | 1.18 (0.19) | 1.20 (0.19) | 3.3 (2.01 to 4.56) | 1.2 (0.25 to 2.09) | .01 | 0.12 |
| Placebo | 101 | 1.17 (0.19) | 1.19 (0.20) | 2.1 (0.87 to 3.36) | ||||
| Total hip | Testosterone | 108 | 1.03 (0.15) | 1.03 (0.16) | 1.2 (0.19 to 2.17) | 0.7 (-0.01 to 1.36) | .052 | 0.13 |
| Placebo | 100 | 1.02 (0.14) | 1.02 (0.14) | 0.5 (-0.45 to 1.46) | ||||
| Femoral neck | Testosterone | 108 | 0.83 (0.14) | 0.83 (0.14) | 1.5 (0.02 to 2.97) | 0.56 (-0.45 to 1.58) | .27 | 0.06 |
| Placebo | 100 | 0.82 (0.14) | 0.82 (0.13) | 0.9 (-0.49 to 2.35) | ||||
Abbreviations: BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; QCT, quantitative computed tomography.
SI conversion factor: To convert testosterone to nanomoles per liter, multiply by 0.034.
The adjusted changeis the within-arm mean percent change in bone outcomes between baseline and month 12 adjusted for balancing factors: baseline total testosterone greater or less than 200 ng/dL, age younger or older than 75 years, site, participation in main trials, use of antidepressants, and use of phosphodiesterase type 5 inhibitors.
The treatment effect is the mean difference in the change from baseline between testosterone and placebo arms.
The P value for the significance of the treatment effect was determined by multivariable linear regression adjusted for balancing factors.
r2 Describes the proportion of variability in the outcome that is explained by treatment and balancing factors.
The magnitude of the treatment effect on trabecular vBMD of the spine did not vary significantly by baseline total testosterone, estradiol, or vBMD. The magnitude of the percent increase in spine trabecular vBMD from baseline to month 12 in testosterone-treated men, however, was significantly associated with changes in total testosterone (β = 0.01, ρ = 0.25, P = .01) and estradiol (β = 0.17, ρ = 0.37, P < .001) (eAppendix 1 and eFigure 2 in Supplement 2). A 200 ng/dL increase in testosterone was associated with a 6.1% increase in trabecular vBMD, and a 15 pg/mL increase in estradiol was associated with a 6.3% increase.
Testosterone treatment also increased peripheral and whole-bone vBMD of the spine and trabecular, peripheral, and whole-bone vBMD of the hip (Figure 3A and Table 2). The magnitudes of the increases were less in the hip than in the spine but still statistically significant.
Based on FEA of QCT data, testosterone treatment also increased estimated bone strength. Testosterone treatment increased estimated strength of spine trabecular bone by 10.8% (95% CI, 7.4% to 14.3%), compared with 2.4% (95% CI, −1.0% to 5.7%) in placebo-treated men (Figure 3B and Table 2). The difference was 8.5% (95% CI, 6.0% to 10.9%; P < .001). Testosterone treatment also significantly increased estimated strength of peripheral and whole bone (Figure 3B and Table 2). The magnitudes of the effects of testosterone treatment on estimated hip strength were less than those on the spine, but still significant (Figure 3B and Table 2).
By DXA, testosterone treatment increased mean aBMD (3.3%; 95% CI, 2.01% to 4.56%) more than placebo (2.1%; 95% CI, 0.87% to 3.36%; P = .01, r2 = 0.12) (Table 2). In the total hip, testosterone treatment was associated with a mean increase of 1.2% (95% CI, 0.19% to 2.17%) compared with 0.5% (95% CI, −0.45% to 1.46%) for placebo (P = .052, r2 = 0.13). In the femoral neck, testosterone treatment was associated with a mean increase of 1.5% (95% CI, 0.02% to 2.97%) and placebo of 0.9% (95% CI, −0.49% to 2.35%; P = .27, r2 = 0.06).
Adjusting the analyses for the variables in which the 2 arms differed at baseline (body mass index, alcohol use, and estradiol level) did not change appreciably any of the QCT or DXA results.
During the treatment year, 6 fractures were reported and confirmed in each treatment arm (eTable 1 in Supplement 2). During the subsequent year of observation, 3 fractures were reported and confirmed in the testosterone arm and 4 in the placebo arm.
Adverse Events
In the entire TTrial population of 790, testosterone treatment was not associated with a greater incidence of prostate or cardiovascular adverse events than placebo treatment.24
Discussion
Testosterone treatment for 1 year of older men with low testosterone concentrations improved all aspects of sexual function and improved somewhat mood and depressive symptoms.24 The results reported here show that testosterone treatment of these men also significantly increased the vBMD and estimated bone strength, more so in the spine than the hip and more so in trabecular bone than cortical-rich peripheral bone.
These results are unequivocal compared with prior studies of the effect of testosterone treatment on bone in older men,19-22 in spite of treatment limited to 1 year, perhaps because the mean pretreatment testosterone level was lower and the sample size larger than in prior studies and because the primary outcome in this trial was vBMD by QCT. This technique avoids the artifactual increases in DXA-derived aBMD caused by osteophytes and aortic calcification,4,28 and allows assessment of trabecular bone, which testosterone treatment improves preferentially.18
These results are not surprising, however, in view of the effects on bone in men who are severely hypogonadal as a consequence of pituitary or testicular disease, who consistently show improvement in vBMD14 in response totestosterone treatment. The effect of testosterone on strength of trabecular bone in the spine, as estimated by FEA of QCT data in the men in the Bone Trial, is consistent with that of testosterone on trabecular bone in the distal tibia (a site also high in trabecular bone) of severely hypogonadal men, determined by FEA of magnetic resonance microimaging data.18
The effects of testosterone treatment on vBMD and estimated bone strength in these men seem to compare favorably with the effects of antiresorptive or anabolic agents meant for osteoporosis,32-35 although direct comparisons cannot be made because those studies were performed in postmenopausal (severely hypogonadal) women with osteoporosis, whereas men in this trial were generally moderately hypogonadal and not osteoporotic.
The mechanism by which testosterone treatment exerts these effects on bone cannot be discerned by this study design. Considerable evidence shows that much of the effect of testosterone on bone is mediated by conversion to estradiol.36-40 In these men, testosterone treatment was associated with a pronounced increase in both testosterone and estradiol concentrations. In the testosterone-treated men, the increases in vBMD of spine trabecular bone were significantly associated with the increases in testosterone and estradiol level.
The clinical significance of the effect of testosterone treatment on vBMD and estimated bone strength in these men will depend on whether testosterone treatment also reduces fracture risk. Some evidence suggests that it might. Bone strength, as estimated by FEA of QCT data, does correlate well with physical strength of human vertebrae29 and is associated with prevalent bone fractures41 and incident spine42 and hip30 fractures. Only a larger and longer trial, however, will determine whether testosterone treatment does reduce fracture risk in older men with low testosterone levels.
Limitations
The strengths of this trial include the unequivocally low testosterone concentrations of the participants, double-blind design, increase in serum testosterone to mid-normal for young men, and excellent participant retention. An important limitation of this trial is that because the participants were men with low serum testosterone levels, the results apply only to this population. In addition, because most men in this trial did not have osteoporosis by baseline T-scores, these results cannot be extrapolated to men who have osteoporosis but not low testosterone. An analytic limitation is the inflated probability of a false-positive finding due to multiple testing; however, the large number of significant findings is not likely due to chance alone, suggesting that testosterone treatment truly improves bone outcomes.
Conclusions
We conclude that testosterone treatment of older men with low testosterone levels significantly increased their vBMD and estimated bone strength, more so in the spine than hip and more so in trabecular bone than cortical-rich peripheral bone. These results should give impetus to a larger and longer trial to determine whether testosterone treatment of older men with low testosterone reduces fracture risk.
Supplementary Material
Key Points.
Question Will testosterone treatment of older men with low testosterone improve their bone density and strength?
Findings Testosterone treatment of older men with low testosterone increased volumetric trabecular bone mineral density of the lumbar spine and estimated bone strength significantly compared with placebo.
Meaning These results suggest that a larger and longer trial to determine whether testosterone treatment decreases fracture risk in this population is warranted.
Acknowledgments
Funding/Support: The Testosterone Trials were supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG030644). The Bone Trial was supported by a grant from the National Institute on Aging (R01 AG037679). AbbVie (formerly Solvay and Abbott Laboratories) provided funding, AndroGel, and placebo gel. Dr Matsumoto was supported by the Department of Veterans Affairs Puget Sound Health Care System. Dr Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging. The Yale Field Center was partially supported by the Claude D. Pepper Older Americans Independence Center (P30-AG021342) and UL1TR000142 from the National Center for Advancing Translational Science. Dr Basaria and the Boston Center were supported partly by the Boston Claude D. Pepper Older Americans Independence Center (6P30AG031679). Dr Lewis was supported by the National Institute for Diabetes, Digestive and Kidney Diseases, National Institutes of Health (DK079626) to the UAB Diabetes Research and Training Center. Dr Cauley was supported by the National Institute on Aging, National Institutes of Health (R01 AG37679).
Role of the Funder/Sponsor: None of the funding agencies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: The authors acknowledge with gratitude the steadfast support of Evan Hadley, MD, and Sergei Romashkan, MD, PhD, of the National Institute on Aging throughout the trials. They received no compensation for their contributions.
Footnotes
Author Contributions: Dr Snyder had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Snyder, Kopperdahl, Ellenberg, Lee, Bhasin, Cunningham, Matsumoto.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Snyder, Cunningham, Matsumoto, Hou.
Critical revision of the manuscript for important intellectual content: Snyder, Kopperdahl, Stephens-Shields, Ellenberg, Cauley, Ensrud, Lewis, Barrett-Connor, Schwartz, Lee, Bhasin, Cunningham, Gill, Matsumoto, Swerdloff, Basaria, Diem, Wang, Cifelli, Dougar, Zeldow, Bauer, Keaveny.
Statistical analysis: Stephens-Shields, Ellenberg, Swerdloff, Hou, Zeldow.
Obtained funding: Snyder, Ellenberg, Cauley, Bhasin. Administrative, technical, or material support: Snyder, Kopperdahl, Cauley, Lewis, Barrett-Connor, Lee, Bhasin, Basaria, Cifelli, Dougar, Bauer, Keaveny. Supervision: Snyder, Stephens-Shields, Ellenberg, Ensrud, Barrett-Connor, Bhasin, Cunningham, Gill, Matsumoto, Basaria, Diem, Wang, Cifelli.
Conflict of Interest Disclosures: Dr Snyder reports grants from the National Institute on Aging (NIA), National Institutes of Health (NIH), grants and nonfinancial support from AbbVie (formerly Solvay and Abbott Laboratories), during the conduct of the study; personal fees from Watson Laboratories, outside the submitted work. Dr Kopperdahl is an employee of and has equity interest in O.N. Diagnostics. Dr Stephens-Shields reports grants from NIA and NIH, grants and other from AbbVie (formerly Solvay & Abbott Lab), during the conduct of the study. Dr Ellenberg reports grants from NIH and AbbVie, Inc, during the conduct of the study; grants from AbbVie, Inc, outside the submitted work. Dr Ensrud reports grants from NIA, during the conduct of the study. Dr Lewis reports grants from NIH and AbbVie, during the conduct of the study. Dr Schwartz reports grants from NIH, during the conduct of the study; personal fees from Amgen, Janssen Pharmaceutical, and Merck, and personal fees and nonfinancial support from Chugai Pharmaceutical, outside the submitted work. Dr Lee is an employee of and has equity interest in O.N. Diagnostics. Dr Bhasin reports grants from NIA, during the conduct of the study; grants and personal fees from Abbvie, Lilly, and Regeneron, and grants from Transition Therapeutics, outside the submitted work. In addition, Dr Bhasin has a patent free testosterone calculator pending and has equity interest in FPT, LLC. Dr Cunningham reports personal fees from AbbVie, Apricus, Besins, Clarus Therapeutics, Endo Pharma, Ferring, Lilly, Pfizer, and Repros Therapeutics, outside the submitted work. Dr Matsumoto reports personal fees from AbbVie, Endo, Lilly, Lipocine, and Clarus, outside the submitted work. Dr Swerdloff reports grants from the Bone Trial of the Testosterone Trials during the conduct of the study; grants and other from Clarus and Antares and grants from Lipesene, outside the submitted work. Dr Basaria reports other from Eli Lilly and Takeda Pharmaceuticals, outside the submitted work. Dr Diem reports grants from NIA, during the conduct of the study. Dr Wang reports grants from Besins Health International, other from Abbvie, during the conduct of the study; grants from Clarus Therapeutics, outside the submitted work. Dr Keaveny reports grants from NIA and NIH, grants and other from AbbVie (formerly Solvay & Abbott Lab), during the conduct of the study.
References
- 1.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 2.Wu FC, Tajar A, Pye SR, et al. European Male Aging Study Group Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 3.Orwoll ES, Oviatt SK, McClung MR, Deftos LJ, Sexton G. The rate of bone mineral loss in normal men and the effects of calcium and cholecalciferol supplementation. Ann Intern Med. 1990;112(1):29–34. doi: 10.7326/0003-4819-112-1-29. [DOI] [PubMed] [Google Scholar]
- 4.Zmuda JM, Cauley JA, Glynn NW, Finkelstein JS. Posterior-anterior and lateral dual-energy x-ray absorptiometry for the assessment of vertebral osteoporosis and bone loss among older men. J Bone Miner Res. 2000;15(7):1417–1424. doi: 10.1359/jbmr.2000.15.7.1417. [DOI] [PubMed] [Google Scholar]
- 5.Riggs BL, Melton LJ, Robb RA, et al. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res. 2008;23(2):205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samelson EJ, Christiansen BA, Demissie S, et al. QCT measures of bone strength at the thoracic and lumbar spine: the Framingham Study. J Bone Miner Res. 2012;27(3):654–663. doi: 10.1002/jbmr.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyeri A, Kaptoge S, Luben RN, et al. Estimation of absolute fracture risk among middle-aged and older men and women: the EPIC-Norfolk population cohort study. Eur J Epidemiol. 2009;24(5):259–266. doi: 10.1007/s10654-009-9337-8. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JS, Klibanski A, Neer RM, Greenspan SL, Rosenthal DI, Crowley WF., Jr Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med. 1987;106(3):354–361. doi: 10.7326/0003-4819-106-3-. [DOI] [PubMed] [Google Scholar]
- 9.Greenspan SL, Neer RM, Ridgway EC, Klibanski A. Osteoporosis in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1986;104(6):777–782. doi: 10.7326/0003-4819-104-6-777. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90(12):6410–6417. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 11.Wadhwa VK, Weston R, Mistry R, Parr NJ. Long-term changes in bone mineral density and predicted fracture risk in patients receiving androgen-deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU Int. 2009;104(6):800–805. doi: 10.1111/j.1464-410X.2009.08483.x. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 13.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23(31):7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 14.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81(12):4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 15.Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 16.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82(8):2386–2390. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 17.Benito M, Vasilic B, Wehrli FW, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20(10):1785–1791. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- 18.Al Mukaddam M, Rajapakse CS, Bhagat YA, et al. Effects of testosterone and growth hormone on the structural and mechanical properties of bone by micro-MRI in the distal tibia of men with hypopituitarism. J Clin Endocrinol Metab. 2014;99(4):1236–1244. doi: 10.1210/jc.2013-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(6):1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- 20.Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56(5):M266–M272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- 21.Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89(2):503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 22.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 23.Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11(3):362–375. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder PJ, Bhasin S, Cunningham GR, et al. Testosterone Trials Investigators Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cauley JA, Fluharty L, Ellenberg SS, et al. Recruitment and screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015;70(9):1105–1111. doi: 10.1093/gerona/glv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 27.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 28.Orwoll ES, Oviatt SK, Mann T. The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J Clin Endocrinol Metab. 1990;70(4):1202–1207. doi: 10.1210/jcem-70-4-1202. [DOI] [PubMed] [Google Scholar]
- 29.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744–750. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 30.Orwoll ES, Marshall LM, Nielson CM, et al. Osteoporotic Fractures in Men Study Group Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res. 2009;24(3):475–483. doi: 10.1359/JBMR.081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopperdahl DL, Morgan EF, Keaveny TM. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone. J Orthop Res. 2002;20(4):801–805. doi: 10.1016/S0736-0266(01)00185-1. [DOI] [PubMed] [Google Scholar]
- 32.Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580. doi: 10.1210/jc.2012-2972. [DOI] [PubMed] [Google Scholar]
- 33.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22(1):149–157. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- 34.Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50(1):165–170. doi: 10.1016/j.bone.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Keaveny TM, McClung MR, Genant HK, et al. Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res. 2014;29(1):158–165. doi: 10.1002/jbmr.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 37.Carani C, Qin K, Simoni M, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337(2):91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 38.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106(12):1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88(1):204–210. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein JS, Lee H, Leder BZ, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126(3):1114–1125. doi: 10.1172/JCI84137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melton LJ, III, Riggs BL, Keaveny TM, et al. Relation of vertebral deformities to bone density, structure, and strength. J Bone Miner Res. 2010;25(9):1922–1930. doi: 10.1002/jbmr.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Sanyal A, Cawthon PM, et al. Osteoporotic Fractures in Men (MrOS) Research Group Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27(4):808–816. doi: 10.1002/jbmr.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.