Abstract
Importance
Most cognitive functions decline with age. Prior studies suggest that testosterone treatment may improve these functions.
Objective
To determine if testosterone treatment compared with placebo is associated with improved verbal memory and other cognitive functions in older men with low testosterone and age-associated memory impairment (AAMI).
Design, Setting, and Participants
The Testosterone Trials (TTrials) were 7 trials to assess the efficacy of testosterone treatment in older men with low testosterone levels. The Cognitive Function Trial evaluated cognitive function in all TTrials participants. In 12 US academic medical centers, 788 men who were 65 years or older with a serum testosterone level less than 275 ng/mL and impaired sexual function, physical function, or vitality were allocated to testosterone treatment (n = 394) or placebo (n = 394). A subgroup of 493 men met criteria for AAMI based on baseline subjective memory complaints and objective memory performance. Enrollment in the TTrials began June 24, 2010; the final participant completed treatment and assessment in June 2014.
Interventions
Testosterone gel (adjusted to maintain the testosterone level within the normal range for young men) or placebo gel for 1 year.
Main Outcomes and Measures
The primary outcome was the mean change from baseline to 6 months and 12 months for delayed paragraph recall (score range, 0 to 50) among men with AAMI. Secondary outcomes were mean changes in visual memory (Benton Visual Retention Test; score range, 0 to −26), executive function (Trail-Making Test B minus A; range, −290 to 290), and spatial ability (Card Rotation Test; score range, −80 to 80) among men with AAMI. Tests were administered at baseline, 6 months, and 12 months.
Results
Among the 493 men with AAMI (mean age, 72.3 years [SD, 5.8]; mean baseline testosterone, 234 ng/dL [SD, 65.1]), 247 were assigned to receive testosterone and 246 to receive placebo. Of these groups, 247 men in the testosterone group and 245 men in the placebo completed the memory study. There was no significant mean change from baseline to 6 and 12 months in delayed paragraph recall score among men with AAMI in the testosterone and placebo groups (adjusted estimated difference, −0.07 [95% CI, −0.92 to 0.79]; P = .88). Mean scores for delayed paragraph recall were 14.0 at baseline, 16.0 at 6 months, and 16.2 at 12 months in the testosterone group and 14.4 at baseline, 16.0 at 6 months, and 16.5 at 12 months in the placebo group. Testosterone was also not associated with significant differences in visual memory (−0.28 [95% CI, −0.76 to 0.19]; P = .24), executive function (−5.51 [95% CI, −12.91 to 1.88]; P = .14), or spatial ability (−0.12 [95% CI, −1.89 to 1.65]; P = .89).
Conclusions and Relevance
Among older men with low testosterone and age-associated memory impairment, treatment with testosterone for 1 year compared with placebo was not associated with improved memory or other cognitive functions.
Aging is associated with declines in some cognitive functions, including verbal and visual memory, executive function, and spatial ability.1-3 Aging in men is also associated with a reduction in serum testosterone,4,5 raising the possibility that reduced circulating testosterone concentration may contribute to age-related cognitive decline. Support for this hypothesis comes from studies of clinical conditions that cause low testosterone levels,6,7 epidemiological investigations,8,9 and small randomized trials showing improved memory with testosterone supplementation.10 Together, these studies suggest that lower testosterone levels may be associated with poorer cognitive functioning in older men and that testosterone treatment may improve cognitive functioning, especially memory.
An Institute of Medicine panel11 recommended investigating the effects of testosterone treatment on conditions, including cognitive impairment, that might be caused by the decrease in testosterone. Men with age-associated memory impairment (AAMI) represent a clinically important group at risk for developing more severe memory impairment (eg, mild cognitive impairment and dementia)12 for whom testosterone intervention may be beneficial. AAMI is defined by subjective complaints of memory decline and scores at least 1 SD below the mean for young adults on objective memory testing.13 A large percentage of community-dwelling older adults meets these criteria.14
The Cognitive Function Trial determined the efficacy of testosterone treatment on cognitive outcomes among older men enrolled in the Testosterone Trials (TTrials) with low testosterone likely due to age.15 The primary hypothesis of the Cognitive Function Trial was that testosterone treatment for 1 year would improve or slow decline in verbal memory in the subgroup of men 65 years or older with an average testosterone level of less than 275 ng/dL and AAMI. Secondary aims were to determine if testosterone treatment affects other cognitive functions in these men with AAMI, and exploratory aims were to determine the effect of testosterone treatment on cognitive function in all men in the TTrials.
Methods
Study Design
The TTrials are a coordinated set of 7 double-blind, placebocontrolled trials conducted at 12 US academic medical centers.15 To qualify for the TTrials, participants had to qualify for the Sexual Function Trial, the Physical Function Trial, or the Vitality Trial.16 Cognitive tests were administered to all participants, but the Cognitive Function Trial's primary focus was the subgroup of men with AAMI.
The trial protocol (Supplement 1) and consent form were approved by the institutional review boards of the University of Pennsylvania and all trial sites. All participants provided written, informed consent. An unblinded data and safety monitoring board monitored accumulating safety data every 3 months.
Participants
Participants for the TTrials were recruited and screened as described.16 Respondents were screened first by telephone and then at 2 clinic visits. Inclusion criteria for the TTrials overall were 65 years or older and the mean of 2 morning serum testosterone concentrations less than 275 ng/dL (to convert to nmol/L, multiply by 0.0347). Additionally, inclusion in the Sexual Function Trial required self-reported decreased libido and sexual activity, inclusion in the Physical Function Trial required self-reported difficulty walking or climbing stairs and low gait speed, and inclusion in the Vitality Trial required self-reported fatigue and reduced vitality.16 Exclusion criteria included a recent history or evidence of increased risk of conditions that testosterone might exacerbate, cognitive impairment (Mini-Mental State Examination score <24), and severe depression (Patient Health Questionnaire-9 [PHQ-9] score ≥20) (eAppendix in Supplement 2).17
Men were classified as having AAMI if they had both subjective memory complaints and relative impairment on objective tests of memory performance. Subjective memory complaints were indicated by a score of 4 or 5 on at least 1 item of the Memory Assessment Clinics Questionnaire (MAC-Q).18 Objective memory impairment was defined by a score more than 1 SD below the performance for young men (aged 20-24 years) but not greater than 2 SD below the scores of age-matched men on tests of delayed paragraph recall or visual memory. Men were “normal for age” if they did not meet criteria for AAMI and had scores of 80 or more on the Modified Mini-Mental State Examination (3MSE) measure of global cognitive function. Demographic characteristics including self-reported race (white, African American, or other) and ethnicity (Hispanic or non-Hispanic) were collected because there are race differences in genetic risk factors for some conditions that affect cognitive function.
Treatment
In the TTrials overall, participants were allocated to treatment by minimization, with participants assigned to the optimally balanced treatment with 80% probability.19,20 Balancing variables included participation in each of the 3 main trials of the TTrials (Sexual Function Trial, Physical Function Trial, or Vitality Trial), trial site, screening testosterone concentration (≤200 ng/dL or >200 ng/dL), age (≤75 years or >75 years), and use of antidepressants and phosphodiesterase type 5 (PDE5) inhibitors. AAMI was not included as a balancing factor.
The testosterone gel was 1% concentration in a pump bottle (AndroGel, AbbVie). The initial dose was 5 g daily. The placebo gel was similar in appearance, smell, and consistency. Serum testosterone concentration was measured at month 1, 2, 3, 6, and 9 in a central laboratory (Quest Clinical Trials, Valencia, California). The dose of testosterone gel was adjusted by an unblinded staff person at the University of Pennsylvania data coordinating center following a prespecified algorithm after each measurement to keep the concentration within the mid-normal range for young men (500-800 ng/dL). To maintain blinding when the dose was adjusted in a participant taking testosterone, the dose was changed simultaneously in a participant taking placebo gel.
Hormone Assessment
Serum concentrations of testosterone, free testosterone, dihydrotestosterone, estradiol, and sex hormone–binding globulin were measured at the end of the trial in sera frozen at −80°C. Steroid assays were performed in the Brigham Research Assay Core Laboratory in Boston, Massachusetts, using liquid chromatography tandem mass spectroscopy and free testosterone was measured by equilibrium dialysis.16 All samples from each participant were measured in the same assay run.
Trial Outcomes
The primary outcome was mean change from baseline to 6 months and to 12 months in delayed paragraph recall score among men with AAMI. Secondary outcomes were change from baseline in visual memory, executive function, and spatial ability among men with AAMI. Exploratory outcomes included change from baseline on these measures among all men enrolled in the TTrials, as well as change in global cognition, subjective memory complaints, and immediate paragraph recall. We also performed additional exploratory analyses of the subgroup of men who were normal for age (normal for age subgroup) because recent trials in Alzheimer disease suggest that treatments may be less effective in the presence of cognitive impairment and irreversible neuronal damage that may be present among men with AAMI.21
Cognitive Assessment
The methods for cognitive assessments are detailed in Supplement 2. The cognitive battery was administered at baseline, 6 months, and 12 months and included measures of subjective memory complaints (MAC-Q); verbal memory by immediate paragraph recall (Wechsler Memory Scale-Revised Logical Memory I) and delayed paragraph recall (Wechsler Memory Scale-Revised Logical Memory II); visual memory (Benton Visual Retention Test [BVRT]; score range, 0 to −26)22; executive function (Trail-Making Test B minus A [TMT B − A]; range, −290 to 290); and spatial ability (Card Rotation Test; score range, −80 to 80). TMT B − A was used as an outcome because it provides a purer measure of executive function, adjusting for visuomotor speed and attention. Global cognitive function (3MSE) was assessed at baseline and 12 months. To minimize practice effects, 3 versions (A, B, and C) of Logical Memory, BVRT, and the Card Rotations Test were used in the test battery. All participants in the TTrials, regardless of AAMI status, were randomly allocated to 1 of 3 test battery orders for baseline, 6-month, and 12-month assessments (ABC, BCA, or CAB).
The Cognition Reading Center (CRC) at Wake Forest School of Medicine provided training, oversight, and quality control of cognitive testing following established procedures.23,24 CRC and National Institute on Aging investigators conducted a centralized in-person training session of testers from the 12 clinic sites. Subsequently, testers audio-taped a practice administration and submitted it for CRC certification. Recertification was required every 6 months for the first year and annually thereafter. During the trial, the CRC staff monitored the certified testers and assisted in training new testers. The CRC scored the cognitive measures and entered the data.
Statistical Analysis
Power Analysis
The statistical analytic plan is available in Supplement 1. Data from earlier studies of delayed paragraph recall showed testosterone-associated effect sizes ranging from 0.13 to 0.62.10,25 Based on our estimate of the proportion of men recruited who would meet the AAMI criteria, we determined that we would be able to detect an effect size of 0.3 (based on change from baseline to 12 months), corresponding to a 3-point difference between testosterone and placebo groups in change from baseline to 12 months in delayed paragraph recall. A 3-point difference is equivalent to a change in score from the 50th percentile performance for a man aged 70 to 74 years to that of a man aged 45 to 54 years.26
Data Analysis
Participants were analyzed in the treatment group to which they had been allocated, following the intention-to-treat principle. Primary analyses of outcomes from all time points were performed with mixed-effects models for longitudinal data. Models entered visit time (6 months or 12 months) as a categorical variable. Models initially included a main effect of treatment and treatment by visit interaction, but interaction terms were removed if they did not achieve statistical significance at the .05 level. Additional fixed effects were the baseline value for each outcome, balancing variables, and PHQ-9 depressive symptom score. Random intercepts were included for participants. After removing the treatment by visit interaction (nonsignificant for all cognitive outcomes), the estimated difference denotes the difference in the mean change from baseline to 6 months and to 12 months between treatment groups. All hypothesis tests were 2-sided and conducted at an α level of .05. We did not adjust analyses of the primary and secondary outcomes for multiple comparisons because the correlations among outcomes were expected to be high, making such adjustment excessively conservative. Mixed-effects models provide unbiased estimates of estimated differences under the assumption of data missing at random, meaning that missingness is associated at most with observed data and not unobserved responses. No additional sensitivity analyses were conducted to examine the influence of missing data on results given the small amount of missingness and nature of the results. We used the same statistical approach for analysis of outcomes for AAMI, all men, and the normal for age subgroup.
We also investigated whether the potential association between testosterone treatment and cognitive function was related to baseline testosterone levels (continuous variable) and to baseline global cognitive function (baseline 3MSE score groups: <90, 90-94, 95-100). All analyses were performed in SAS (SAS Institute), version 9.4.
Results
Participants
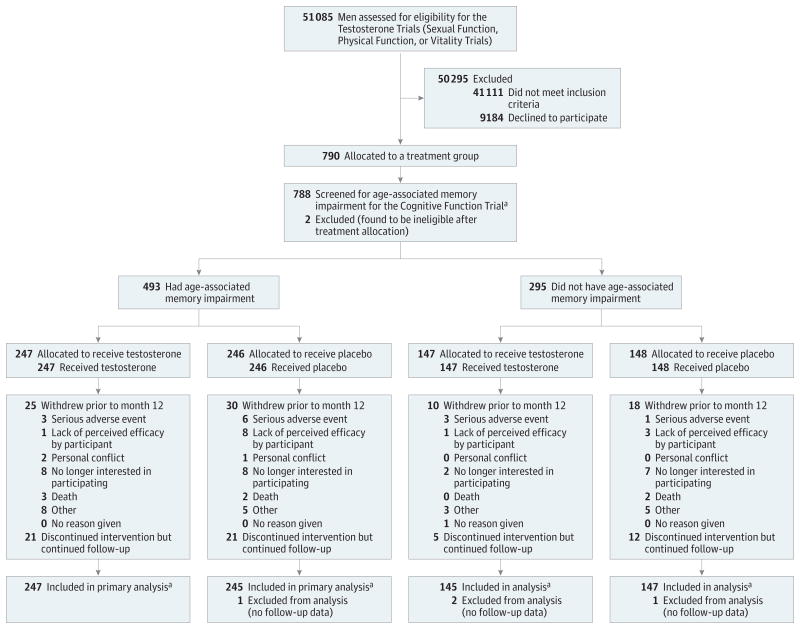
Recruitment began in June 2010, and planned accrual was completed in June 2013. Treatment was completed in June 2014. Of 788 men enrolled in the TTrials, 493 men (62.6%) met the criteria for AAMI, 247 in the testosterone group and 246 in the placebo group (Figure 1). Baseline characteristics by treatment group for men with AAMI and all men are presented in Table 1 and Table 2 and those for the normal for age subgroup are in eTable 2 in Supplement 2. In the AAMI group, placebo and testosterone treatment groups did not differ at baseline in most demographic and medical characteristics or in sex hormone levels or cognitive performance. Among all men enrolled in the TTrials, executive function performance at baseline was slightly better among those assigned to placebo than those assigned to testosterone.
Figure 1. Screening and Retention of Participants Throughout the Cognitive Function Trial.
AAMI indicates age-associated memory impairment. “Allocated to treatment incorrectly” indicates that the individuals were found to be ineligible after they received treatment allocation.
a The number analyzed is based on the primary outcome variable for the Cognitive Function Trial (delayed paragraph recall score).
Table 1. Baseline Characteristics of Men With AAMI in the Cognitive Function Trial and of All Men Enrolled in the Testosterone Trialsa.
| Men With AAMI in the Cognitive Function Trial | All Men Enrolled in the Testosterone Trials | |||
|---|---|---|---|---|
| Placebo (n = 246) | Testosterone (n = 247) | Placebo (n = 394) | Testosterone (n = 394) | |
| Demographics, No. (%) | ||||
| Age, mean (SD), y | 72.8 (6.1) | 72.3 (5.8) | 72.3 (5.8) | 72.1 (5.7) |
| Race | ||||
| White | 217 (88.2) | 213 (86.2) | 350 (88.8) | 348 (88.3) |
| African American | 13 (5.3) | 14 (5.7) | 20 (5.1) | 21 (5.3) |
| Other | 16 (6.5) | 20 (8.1) | 24 (6.1) | 25 (6.3) |
| Ethnicitya | ||||
| Hispanic | 7 (2.8) | 15 (6.1) | 10 (2.5) | 18 (4.6) |
| Non-Hispanic | 239 (97.2) | 232 (93.9) | 384 (97.5) | 375 (95.2) |
| College graduate | 112 (45.5) | 130 (52.6) | 197 (50.0) | 213 (54.1) |
| Married or living with partner | 189 (76.8) | 180 (72.9) | 303 (76.9) | 289 (73.4) |
| Concomitant Conditions, No. (%) | ||||
| BMI, mean (SD) | 30.7 (3.6) | 31.1 (3.5) | 31.0 (3.5) | 31.0 (3.5) |
| BMI >30 | 145 (58.9) | 164 (66.4) | 245 (62.2) | 251 (63.7) |
| Alcohol use (No. of drinks/wk), median (IQR) | 1.0 (0-6) | 1.0 (0-4) | 1.0 (0-5) | 1.0 (0-5) |
| Smoking | ||||
| Current | 24 (9.8) | 15 (6.1) | 34 (8.6) | 30 (7.6) |
| Ever | 165 (67.1) | 163 (66.0) | 268 (68.0) | 256 (65.0) |
| Diabetes | 88 (35.8) | 91 (36.8) | 144 (36.5) | 148 (37.6) |
| Hypertension | 172 (69.9) | 179 (72.5) | 279 (70.8) | 286 (72.6) |
| History of myocardial infarction | 44 (17.9) | 35 (14.2) | 63 (16.0) | 53 (13.5) |
| History of stroke | 12 (4.9) | 12 (4.9) | 17 (4.3) | 16 (4.1) |
| Sleep apnea | 40 (16.3) | 58 (23.5) | 76 (19.3) | 77 (19.5) |
| Hyperlipidemias | 186 (75.6) | 193 (78.1) | 295 (74.9) | 302 (76.6) |
| Coronary artery disease | 73 (29.7) | 67 (27.1) | 112 (28.4) | 101 (25.6) |
| Other Characteristics, No. (%) | ||||
| Medication use | ||||
| α-Blocking agents | 33 (13.4) | 38 (15.4) | 46 (11.7) | 55 (14.0) |
| 5α-Reductase inhibitors | 11 (4.5) | 9 (3.6) | 16 (4.1) | 14 (3.6) |
| Phosphodiesterase inhibitors | 20 (8.1) | 20 (8.1) | 36 (9.1) | 30 (7.6) |
| Antihypertensives | 169 (68.7) | 168 (68.0) | 270 (68.5) | 272 (69.0) |
| Antidepressants | 36 (14.6) | 36 (14.6) | 54 (13.7) | 54 (13.7) |
| Sex hormones, mean (SD) | ||||
| Testosterone, ng/dL | 234.0 (67.4) | 234.4 (65.1) | 236.1 (66.7) | 231.8 (63.1) |
| Free testosterone, pg/mL | 64.5 (23.1) | 63.0 (22.4) | 64.9 (23.4) | 62.0 (21.4) |
| Dihydrotestosterone, ng/dL | 21.0 (12.3) | 21.2 (10.5) | 20.8 (13.0) | 21.2 (11.6) |
| Estradiol, pg/mL | 20.3 (6.4) | 20.3 (6.4) | 20.4 (6.3) | 20.3 (6.7) |
| Sex hormone–binding globulin, nM | 29.5 (13.6) | 30.9 (14.5) | 29.5 (14.7) | 31.3 (15.2) |
Abbreviations: AAMI, age-associated memory impairment; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
SI conversion factors: To convert dihydrotestosterone to nmol/L, multiply by 0.0344; estradiol to pmol/L, multiply by 3.671; free testosterone to pmol/L, multiply by 3.47; testosterone to nmol/L, multiply by 0.0347.
Ethnicity data were missing for 1 participant in the testosterone group for all men in the Testosterone Trials.
Table 2. Baseline Cognitive Function Characteristics of Men With AAMI in the Cognitive Function Trial and in All Men Enrolled in the Testosterone Trials.
| Men With AAMI in the Cognitive Function Trial | All Men in the Testosterone Trials | |||
|---|---|---|---|---|
| Placebo (n = 246) | Testosterone (n = 247) | Placebo (n = 394) | Testosterone (n = 394) | |
| Performance, Mean (SD) | ||||
| Mini-Mental State Examination | 28.3 (1.8) | 28.3 (1.7) | 28.4 (1.7) | 28.4 (1.7) |
| Gait speed, m/s | 1.1 (0.2) | 1.1 (0.2) | 1.1 (0.2) | 1.1 (0.2) |
| Derogatis Interview for Sexual Functioninga | 13.3 (7.7) | 13.5 (7.8) | 14.0 (7.8) | 13.9 (7.7) |
| FACIT scaleb | 36.1 (8.8) | 36.6 (8.8) | 36.8 (8.8) | 37.0 (8.6) |
| PANASc | ||||
| Positive affect | 15.8 (3.4) | 15.9 (3.4) | 16.0 (3.6) | 16.2 (3.6) |
| Negative affect | 7.2 (2.5) | 7.2 (2.5) | 7.2 (2.7) | 7.1 (2.5) |
| PHQ-9 (score range, 0 to 21) | 5.5 (3.9) | 5.7 (4.1) | 5.3 (4.0) | 5.4 (3.9) |
| Cognitive Outcomes, Mean (SD) | ||||
| Delayed paragraph recall (score range, 0 to 50)d | 14.4 (6.4) | 14.0 (6.6) | 15.7 (6.6) | 15.3 (6.8) |
| Benton Visual Retention Test Errors (score range, 0 to −26)e | −8.2 (3.1) | −8.2 (3.2) | −7.3 (3.8) | −7.5 (3.9) |
| Card Rotation Test (score range, −80 to 80) | 30.0 (14.8) | 28.7 (14.1) | 31.4 (15.1) | 29.7 (15.0) |
| Trail-Making Test, s | ||||
| A (maximum score, 300 seconds) | 42.3 (16.8) | 42.2 (18.2) | 41.7 (16.7) | 42.1 (20.4) |
| B (maximum score, 300 seconds) | 119.0 (60.1) | 128.4 (72.0) | 114.9 (59.6) | 125.0 (71.0) |
| B − A (range, −290 to 290)f | 76.7 (54.2) | 86.4 (64.8) | 73.3 (52.2) | 83.1 (63.2) |
| 3MSE (score range, 0 to 100) |
93.0 (4.9) | 92.3 (6.6) | 93.4 (4.9) | 92.8 (6.5) |
| MAC-Q (score range, 6 to 30) |
24.9 (3.8) | 25.3 (3.9) | 23.5 (5.1) | 23.3 (5.3) |
| Immediate paragraph recall (score range, 0 to 50)g | 18.6 (6.2) | 18.3 (6.5) | 19.7 (6.3) | 19.5 (6.6) |
Abbreviations. 3MSE, Modified Mini-Mental State Examination; AAMI, Age-associated memory impairment; FACIT, Functional Assessment of Chronic Illness Therapy Scale; MAC-Q, Memory Assessment Clinics Questionnaire; PANAS, Positive and Negative Affect Schedule; PHQ-9, Patient Health Questionnaire-9.
The Derogatis Interview for Sexual Functioning measures sexual desire.
The FACIT scale measures fatigue.
PANAS is a 20-item, self-reported measure of mood by 2 scales (positive and negative affect).
Delayed paragraph recall was measured with the Wechsler Memory Scale-Revised Logical Memory II test.
BVRT error scores were inverted so that higher scores reflect better performance for all cognitive outcomes, except Trail-Making Test A, B, and B − A wherein higher time in seconds reflects poorer performance.
The only significant difference between testosterone and placebo groups was Trail-Making Test B and Trail-Making Test B − A, wherein scores were better in the placebo than testosterone group for all men (P < .05).
Immediate paragraph recall was measured with the Wechsler Memory Scale-Revised Logical Memory I test.
Adherence and Hormone Levels
Adherence to treatment in the TTrials was assessed by weighing gel bottles at each visit and was judged as excellent (mean adherence >92% at each site). Testosterone treatment increased the serum concentrations of total and free testosterone and estradiol in men with AAMI (eFigure 1 in Supplement 2) and in all men16 to levels in the mid-normal range for healthy young men aged 19 to 40 years. These levels were unchanged in men receiving placebo.
Primary Outcome Analysis
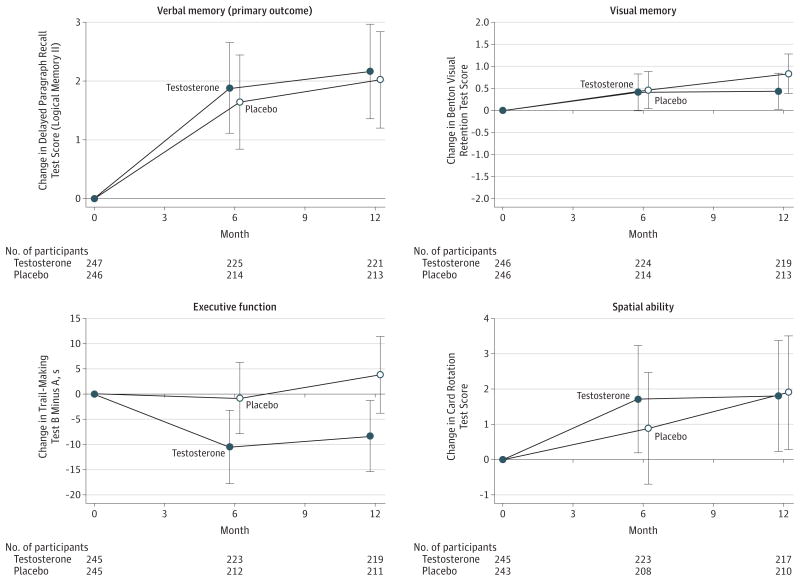
Among men with AAMI, testosterone treatment compared with placebo was not associated with significant differences in the mean change from baseline to month 6 and to month 12 in delayed paragraph recall (adjusted estimated difference, −0.07 [95% CI, −0.92 to 0.79]; P = .88) (Table 3 and Figure 2). Mean delayed paragraph recall scores (score range, 0 to 50) were 14.0 at baseline, 16.0 at month 6, and 16.2 at month 12 in the testosterone group and 14.4 at baseline, 16.2 at month 6, and 16.5 at month 12 in the placebo group. The difference in mean change between treatment groups did not vary significantly by visit month. Analyses evaluating whether the association of testosterone treatment with memory and cognitive function varied by baseline testosterone level and by baseline global cognition yielded similar results.
Table 3.
Effect of Testosterone on Cognitive Function Outcomes Among Men With AAMI inthe Cognitive Function Trial
| No. of Participants | Mean (95% CI) | Diffrence (95% CI)c,d | P Valued | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda | Adjusted Change From Baseline Valuesb | ||||||||
| Baseline | Month 6 | Month 12 | Month 6 | Month 12 | |||||
| Primary Outcome | |||||||||
| Delayed paragraph recall (Logical Memory II) | Testosterone | 247 | 14.0 (13.2 to 14.8) |
16.0 (15.1 to 16.9) |
16.2 (15.3 to 17.1) |
1.1 (−0.1 to 2.3) |
1.3 (0.1 to 2.5) |
−0.07 (−0.92 to 0.79) | .88 |
| Placebo | 246 | 14.4 (13.6 to 15.2) |
16.2 (15.3 to 17.2) |
16.5 (15.6 to 17.5) |
1.1 (−0.1 to 2.3) |
1.4 (0.3 to 2.6) |
|||
| Secondary Outcomes | |||||||||
| Visual memory (Benton Visual Retention Test) | Testosterone | 246 | −8.2 (−8.6 to −7.8) |
−7.7 (−8.2 to −7.2) |
−7.7 (−8.2 to −7.2) |
0.2 (−0.4 to 0.9) |
0.3 (−0.4 to 0.9) |
−0.28 (−0.76 to 0.19) |
.24 |
| Placebo | 246 | −8.2 (−8.6 to −7.8) |
−7.7 (−8.1 to −7.2) |
−7.3 (−7.9 to −6.8) |
0.3 (−0.3 to 1.0) |
0.7 (0.0 to 1.4) |
|||
| Spatial ability (Card Rotation Test) | Testosterone | 245 | 28.7 (26.9 to 30.5) |
30.8 (28.8 to 32.8) |
31.1 (29.0 to 33.2) |
0.6 (−1.9 to 3.0) |
0.6 (−1.8 to 3.1) |
−0.12 (−1.89 to 1.65) |
.89 |
| Placebo | 243 | 30.0 (28.1 to 31.8) |
31.6 (29.5 to 33.8) |
32.4 (30.2 to 34.7) |
0.2 (−2.3 to 2.7) |
1.2 (-1.3 to 3.7) |
|||
| Executive function (Trail-Making Test B − A), s | Testosterone | 245 | 86.4 (78.3 to 94.6) |
74.5 (67.5 to 81.4) |
76.0 (68.5 to 83.6) |
−2.1 (−12.4 to 8.2) |
−0.0 (−10.3 to 10.3) |
−5.51 (−12.91 to 1.88) |
.14 |
| Placebo | 245 | 76.7 (69.9 to 83.5) |
74.3 (66.6 to 82.0) |
78.5 (70.4 to 86.6) |
1.8 (−8.6 to 12.2) |
7.1 (−3.3 to 17.5) |
|||
| Exploratory Outcomes | |||||||||
| Subjective memory complaints (MAC-Q) | Testosterone | 247 | 25.3 (24.8 to 25.8) |
23.9 (23.3 to 24.5) |
24.2 (23.7 to 24.8) |
−1.7 (−2.6 to −0.9) |
−1.5 (−2.3 to 0.6) |
−0.05 (−0.67 to 0.57) |
.87 |
| Placebo | 246 | 24.9 (24.4 to 25.8) |
23.8 (23.3 to 24.5) |
24.3 (23.6 to 24.9) |
−1.8 (−2.6 to −0.9) |
−1.3 (−2.2 to 0.5) |
|||
| Global cognitive function (3MSE) | Testosterone | 244 | 92.3 (91.5 to 93.1) |
93.0 (92.2 to 93.7) |
−0.4 (−1.4 to 0.5) |
−0.36 (−1.08 to 0.36) |
.33 | ||
| Placebo | 242 | 93.0 (92.4 to 93.6) |
93.5 (92.8 to 94.2) |
−0.1 (−1.0 to 0.9) |
|||||
| Immediate paragraph recall (Logical Memory I) | Testosterone | 247 | 18.3 (17.5 to 19.1) |
19.8 (18.9 to 20.7) |
20.3 (19.4 to 21.2) |
0.8 (−0.4 to 2.0) |
1.3 (0.1 to 2.5) |
−0.01 (−0.89 to 0.86) |
.98 |
| Placebo | 246 | 18.6 (17.9 to 19.4) |
20.2 (19.3 to 21.1) |
20.5 (19.6 to 21.3) |
1.0 (−0.3 to 2.2) |
1.2 (−0.0 to 2.4) |
|||
Abbreviation: 3MSE, Modified Mini-Mental State Examination; MAC-Q, Memory Assessment Clinics Questionnaire.
BVRT error scores were inverted so that higher scores reflect better cognitive function except for Trail-Making Test B minusAand MAC-Q scores wherein lower scores indicate better cognitive function.
Positive values indicate greater increase or less decrease for participants allocated to testosterone vs placebo. Variables included for adjustment are listed in footnote c.
The difference is the mean difference in the change from baseline to 6 months and 12 months in participants allocated to testosterone vs placebo adjusted for balancing factors: baseline testosterone level (≤200ng/dL), age(≤75), site, participation in main trials, use of antidepressants, and use of phosphodiesterase type 5 inhibitors. Analyses were also adjusted for education level. A positive estimated difference indicates greater increases, smaller decreases, or both for the testosterone group compared with the placebo group.
The estimated difference and P value were determined by a linear mixed-model with a random effect for participants using outcomes at month 6 and month 12.
Figure 2. Adjusted Mean Change From Baseline to 6 Months and 12 Months for Men With AAMI by Treatment Group (Testosterone vs Placebo) for Verbal Memory (Delayed Paragraph Recall), Visual Memory, Executive Function, and Spatial Ability.
AAMI indicates age-associated memory impairment. Error bars indicate 95% CIs. The score range for the delayed paragraph recall test (Wechsler Memory Scale-Revised Logical Memory II) was 0 to 50. Benton Visual Retention Test scores could range from 0 to 26 but were inverted to −26 to 0 so that higher scores indicated better performance. Possible scores for the Trail-Making Test B minus A range from −290 to 290. The range of possible scores for the Card Rotation Test was −80 to 80. Some participants completed the Delayed Paragraph Recall Test at baseline but not the secondary assessments.
Secondary Outcome Analyses
Among men with AAMI, there was no significant association between testosterone treatment and mean change from baseline to month 6 and month 12 in visual memory (adjusted estimated difference, −0.28 [95% CI, −0.76 to 0.19]; P = .24), executive function (adjusted estimated difference, −5.51 [95% CI, −12.91 to 1.88]; P = .14), or spatial ability (adjusted estimated difference, −0.12 [95% CI, −1.89 to 1.65]; P = .89) (Table 3 and Figure 2). Mean scores for visual memory were −8.2 at baseline, −7.7 at 6 months, and −7.7 at 12 months in the testosterone group and −8.2 at baseline, −7.7 at 6 months, and −7.3 at 12 months in the placebo group. Mean scores for spatial ability were 28.7 at baseline, 30.8 at 6 months, and 31.1 at 12 months in the testosterone group and 30.0 at baseline, 31.6 at 6 months, and 32.4 at 12 months in the placebo group. Mean scores for executive function were 86.4 at baseline, 74.5 at 6 months, 76.0 at 12 months in the testosterone group and 76.7 at baseline, 74.3 at 6 months, and 78.5 at 12 months in the placebo group.
Exploratory Outcome Analyses
Among all men in the TTrials (with and without AAMI), there were also no significant associations of testosterone treatment with mean change from baseline to month 6 and month 12 in delayed paragraph recall (adjusted estimated difference, 0.09 [95% CI, −0.57 to 0.75]; P = .80), visual memory (adjusted estimated difference, −0.34 [95% CI, −0.70 to 0.01]; P = .06), or spatial ability (adjusted estimated difference, −0.08 [95% CI, −1.44 to 1.28]; P = .91) (Table 4). Mean scores among all men for delayed paragraph recall were 15.3 at baseline, 17.3 at 6 months, and 17.6 at 12 months in the testosterone group and 15.7 at baseline, 17.3 at 6 months, and 17.5 at 12 months in the placebo group. Mean scores among all men for visual memory were −7.5 at baseline, −7.3 at 6 months, and −7.1 at 12 months in the testosterone group and −7.3 at baseline, −7.1 at 6 months, and −6.6 at 12 months in the placebo group. Mean scores among all men for spatial ability were 29.7 at baseline, 31.8 at 6 months, and 32.7 at 12 months in the testosterone group and 31.4 at baseline, 33.0 at 6 months, and 34.0 at 12 months in the placebo group. In all men, executive function showed a small improvement in the testosterone group compared with the placebo group (adjusted estimated difference, −5.68 [95% CI, −11.18 to −0.17]; P = .04). Mean scores among all men for executive function were 83.1 at baseline, 69.2 at 6 months, and 70.5 at 12 months in the testosterone group and 73.3 at baseline, 70.6 at 6 months, and 72.9 at 12 months in the placebo group.
Table 4. Effect of Testosterone on Cognitive Function Outcomes Among All Men in the Testosterone Trials.
| No. of Participants | Mean (95% CI) | Difference (95% CI)c,d | P Valued | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusteda | Change From Baseline Values, Adjustedb | ||||||||
| Baseline | Treatment Period Values | ||||||||
| Month 6 | Month 12 | Month 6 | Month 12 | ||||||
| Primary Outcome | |||||||||
| Delayed paragraph recall (Logical Memory II) | Testosterone | 392 | 15.3 (14.6 to 16.0) |
17.3 (16.5 to 18.0) |
17.6 (16.9 to 18.4) |
0.5 (−0.4 to 1.4) |
0.8 (−0.1 to 1.7) |
0.09 (−0.57 to 0.75) |
.80 |
| Placebo | 393 | 15.7 (15.0 to 16.3) |
17.3 (16.5 to 18.0) |
17.5 (16.8 to 18.3) |
0.4 (−0.5 to 1.3) |
0.7 (−0.2 to 1.6) |
|||
| Secondary Outcome | |||||||||
| Visual memory (Benton Visual Retention Test) | Testosterone | 391 | −7.5 (−7.8 to −7.1) |
−7.3 (−7.8 to −6.9) |
−7.1 (−7.5 to −6.7) |
−0.2 (−0.7 to 0.3) |
−0.0 (−0.5 to 0.5) |
−0.34 (−0.70 to 0.01) |
.06 |
| Placebo | 393 | −7.3 (−7.6 to −6.9) |
−7.1 (−7.5 to −6.7) |
−6.6 (−7.0 to −6.2) |
−0.0 (−0.5 to 0.5) |
0.4 (−0.1 to 0.9) |
|||
| Spatial ability (Card Rotation Test) | Testosterone | 387 | 29.7 (28.2 to 31.2) |
31.8 (130.2 to 33.2) |
32.7 (31.0 to 34.5) |
0.9 (−1.1 to 2.8) |
1.4 (−0.5 to 3.4) |
−0.08 (−1.44 to 1.28) |
.91 |
| Placebo | 388 | 31.4 (29.9 to 32.9) |
33.0 (31.3 to 34.6) |
34.0 (32.3 to 35.8) |
0.8 (−1.1 to 2.7) |
1.6 (−0.3 to 3.6) |
|||
| Executive function (Trail-Making Test B − A), s | Testosterone | 389 | 83.1 (76.8 to 89.4) |
69.2 (63.5 to 74.8) |
70.5 (64.9 to 76.1) |
−4.5 (−12.4 to 3.3) |
−1.8 (−9.7 to 6.1) |
−5.68 (−11.18 to −0.17) |
|
| Placebo | 392 | 73.3 (68.1 to 78.5) |
70.6 (64.7 to 76.5) |
72.9 (66.9 to 79.0) |
0.7 (−7.0 to 8.4) |
4.3 (−3.4 to 12.0) |
|||
| Exploratory Outcomes | |||||||||
| Subjective memory complaints (MAC-Q) | Testosterone | 392 | 23.3 (22.7 to 23.8) |
22.6 (22.0 to 23.1) |
22.9 (22.3 to 23.4) |
−0.8 (−1.5 to −0.2) |
−0.6 (−1.3 to 0.1) |
−0.24 (−0.72 to 0.23) |
.32 |
| Placebo | 393 | 23.5 (23.0 to 24.0) |
23.0 (22.5 to 23.5) |
23.2 (22.7 to 23.8) |
−0.6 (−1.2 to 0.1) |
−0.4 (−1.1 to 0.3) |
|||
| Global cognitive function (3MSE) | Testosterone | 388 | 92.8 (92.2 to 93.4) |
93.7 (93.1 to 94.3) |
−0.5 (−1.2 to 0.2) |
−0.22 (−0.76 to 0.31) |
.41 | ||
| Placebo | 386 | 93.4 (92.9 to 93.9) |
94.0 (93.4 to 94.5) |
−0.3 (−1.0 to 0.4) |
|||||
| Immediate paragraph recall (Logical Memory I) | Testosterone | 392 | 19.5 (18.8 to 20.1) |
20.7 (20.0 to 21.4) |
21.5 (20.7 to 22.2) |
0.1 (−0.9 to 1.0) |
0.7 (−0.2 to 1.7) |
−0.05 (−0.72 to 0.61) |
.87 |
| Placebo | 393 | 19.7 (19.1 to 20.3) |
20.9 (20.2 to 21.6) |
21.3 (20.6 to 21.9) |
0.3 (−0.6 to 1.2) |
0.6 (−0.4 to 1.5) |
|||
Abbreviation: 3MSE, Modified Mini-Mental State Examination; MAC-Q, Memory Assessment Clinics Questionnaire.
Higher scores reflect better cognitive function except for Trail-Making Test B minus A and MAC-Q scores wherein lower scores indicate better cognitive function.
Positive values indicate greater increase or less decrease for participants allocated to testosterone vs placebo. Variables included for adjustment are listed in footnote c.
The difference is the mean difference in the change from baseline to 6 months and 12 months in participants allocated to testosterone vs placebo adjusted for balancing factors: baseline testosterone level (≤200ng/dL), age(≤75), site, participation in main trials, use of antidepressants, and use of phosphodiesterase type 5 inhibitors. Analyses were also adjusted for education level. A positive estimated difference indicates greater increases, smaller decreases, or both for the testosterone group compared with the placebo group.
The estimated difference and P value were determined by a linear mixed-model with a random effect for participants using outcomes at month 6 and month 12.
Testosterone and placebo groups did not differ significantly in any of the exploratory outcomes of immediate paragraph recall, subjective memory complaints, and global cognitive function (AAMI shown in Table 2, all men shown in Table 3). There was no significant association of testosterone treatment with any cognitive outcomes for the normal for age subgroup (eTable 3 and eFigure 2 in Supplement 2).
Adverse Events
Among all TTrials participants, men treated with testosterone compared with placebo were more likely to experience erythrocytosis (hemoglobin level, ≥17.5 g/dL), but the 2 groups did not differ in other adverse events, as previously reported.16
Discussion
Among older men with symptomatic hypogonadism and low baseline testosterone, testosterone treatment compared with placebo for 1 year was not associated with significant improvement in memory and other cognitive functions. The lack of association between testosterone treatment and cognition was apparent across all cognitive domains assessed among men with AAMI, in spite of an increase in circulating total and free testosterone concentrations in the testosterone group to levels typical of men aged 19 to 40 years.16
Verbal memory by delayed paragraph recall performance among men with AAMI was selected a priori as the primary outcome based on prior findings in small clinical trials and its clinical importance. Verbal memory declines with age,1-3 and the decline is accelerated in the years preceding clinical dementia, including Alzheimer disease.26-28 Delayed paragraph recall performance requires integrity of the hippocampus, which contains both androgen and estrogen receptors, providing a biological basis for the actions of testosterone or its active metabolite, estradiol.29-31 In addition, verbal memory has been associated with circulating testosterone levels in epidemiologic studies of aging men,8,32,33 may be impaired with androgen deprivation,6,7,34 and has shown improvement after testosterone treatment in some previous short-term trials among older men.10,35
Cognitive assessments were performed among all men in the TTrials, although the principal analyses focused on the subgroup of men who met criteria for AAMI, which defined a sample of men with subjective symptoms of memory decline and objective reductions in memory performance. Testosterone-treated men did show a small increase in executive function with the increased statistical power in all men combined but no other outcomes; it is difficult to interpret this difference in a single exploratory outcome given the multiple outcomes assessed. We found no significant association between testosterone treatment and memory or other cognitive function in the normal for age subgroup.
In spite of previously reported associations between testosterone and verbal memory, the results of this Cognitive Function Trial offers no support for a benefit to memory and little or no support for a benefit to other cognitive functions in older hypogonadal men. It is possible that the mode of treatment and participant characteristics might have contributed to differences between the current findings and those reported previously. Some prior studies used doses of injectable testosterone preparations that cause supraphysiological peak and higher average testosterone levels over time than in our study of testosterone gels. Testosterone gels provide more stable physiological levels. Effects of injectable testosterone may reflect acutely changing testosterone levels, depending on timing of cognitive testing relative to testosterone peaks and troughs. However, our findings are consistent with the lack of significant cognitive effects in a smaller, but longer-term (36 months), placebo-controlled trial of biweekly intramuscular testosterone injections in older men.36 Another difference between men enrolled in the TTrials and those studied in many prior trials was the selection of men with subjective memory complaints and unequivocally low baseline testosterone levels.
The Cognitive Function Trial of the TTrials is, to our knowledge, the largest placebo-controlled study conducted to date of testosterone effects on cognition in older men with low testosterone levels. The trial addresses limitations of prior studies including small sample sizes, variability in baseline testosterone levels and cognitive function of participants, variability in dose and duration of testosterone treatment, and sensitivity of the cognitive outcome measures to hormone action and practice effects. The Cognitive Function Trial had more than 90% power to detect a clinically meaningful increase in verbal memory performance, although it was not powered to detect very small effects, and was conducted in older men who had unequivocally low testosterone and well-defined cognitive function at baseline. The testosterone treatment increased circulating total and free testosterone and estradiol levels to within the normal ranges for young men. The Cognitive Function Trial included assessment of a range of cognitive functions, including verbal and visual memory, spatial ability, and executive function, with alternate forms and randomized test orders to minimize practice effects with repeated use of the same test. The cognitive domains and specific measures were selected based on prior studies showing associations with circulating testosterone in observational studies8,9,32,33 as well as beneficial effects of testosterone on memory function in small randomized trials of younger and older men.10,35,37 The procedures for cognitive assessment and scoring were rigorous and followed established procedures for centralized training, certification, and scoring.23,24 One year of testosterone treatment had no or little effect on cognition.
This study had several limitations. First, AAMI status was not a balancing factor in treatment allocation, although the 2 treatment groups did not differ with respect to baseline demographic characteristics, clinical characteristics, or cognitive performance. Second, the participants in this study were older men with symptomatic hypogonadism and unequivocally low baseline testosterone with no known cause other than aging, so the findings may not generalize to other populations, such as men with normal testosterone or older or younger men, or men with more severe androgen deficiency due to testicular or pituitary disease. Third, it is possible that a longer treatment duration could yield a different result.
Conclusions
Among older men with low testosterone and AAMI, testosterone treatment for 1 year was not associated with improved memory or other cognitive functions.
Supplementary Material
Key Points.
Question Is treatment with testosterone for 1 year associated with improved memory in older men with low testosterone and age-associated memory impairment?
Findings In this placebo-controlled study of 788 older men with symptomatic hypogonadism, 493 had age-associated memory impairment, defined by subjective memory complaints and impaired performance on tests of verbal and visual memory. Compared with placebo, 1 year of testosterone treatment was not significantly associated with improved memory or other cognitive functions in these men.
Meaning This study does not support the use of testosterone for the treatment of age-associated memory decline in older men with symptomatic hypogonadism.
Acknowledgments
Funding/Support: The Testosterone Trials were supported by grant U01 AG030644 from the NIA/NIH, supplemented by funds from the National Heart, Lung, and Blood Institute; National Institute of Neurological Disorders and Stroke; and National Institute of Child Health and Human Development. AbbVie provided funding, AndroGel, and placebo gel. The Boston site was partially supported by grant P30-AG013679 from the Claude D. Pepper Older Americans Independence Center (OAIC). The Yale Field Center was partially supported by grant P30AG021342 from the Claude D. Pepper OAIC and grant UL1TR000142 from the Yale Clinical and Translational Science Award. Additional support was from the Department of Veterans Affairs Puget Sound Health Care System (Dr Matsumoto); the Academic Leadership Award (K07AG043587) from the NIA (Dr Gill); the Intramural Research Program of NIA/NIH (Dr Resnick); grant DK079626 from the National Institute for Diabetes and Digestive and Kidney Diseases of NIH to the University of Alabama at Birmingham Diabetes Research and Training Center (Dr Lewis); grant R01 AG37679 from NIA/NIH (Dr Cauley); and grant 5P30AG031679 from the Boston Claude D. Pepper OAIC (Dr Bhasin).
Role of the Funder/Sponsors: The NIA gave advice on the design and conduct of the study. None of the sponsoring organizations participated in collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Additional Contributions: We thank Evan Hadley, MD, and Sergei Romashkan, MD, PhD (both from NIA), for their support throughout the trials. We also thank Monique Cherrier, PhD, and Suzanne Craft, PhD (both from University of Washington); Jeri Janowsky, PhD (Oregon Health Sciences University); and Scott Moffat, PhD (Wayne State University), for their contributions during the planning phase of the Cognitive Function Trial. There was no compensation provided for their contributions.
Footnotes
Author Contributions: Dr Resnick had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Resnick and Matsumoto contributed equally to this work.
Concept and design: Resnick, Matsumoto, Ellenberg, Bhasin, Cunningham, Farrar, Cifelli, Snyder.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Resnick, Matsumoto, Pleasants, Hou.
Critical revision of the manuscript for important intellectual content: Matsumoto, Stephens-Shields, Ellenberg, Gill, Shumaker, Barrett-Connor, Bhasin, Cauley, Cella, Crandall, Cunningham, Ensrud, Farrar, Lewis, Molitch, Pahor, Swerdloff, Cifelli, Anton, Basaria, Diem, Wang, Snyder.
Statistical analysis: Stephens-Shields, Ellenberg, Cella, Swerdloff, Hou.
Obtained funding: Matsumoto, Ellenberg, Bhasin, Snyder.
Administrative, technical, or material support: Shumaker, Cauley, Lewis, Cifelli, Basaria, Snyder. No additional contributions: Farrar, Anton.
Other: Snyder.
Other - liaison to cognition reading center, including training and monitoring: Resnick.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Resnick reports receiving grants from the National Institute on Aging (NIA), National Institutes of Health (NIH), and AbbVie (formerly Solvay & Abbott Lab) and being an intramural employee of NIA/NIH. Dr Matsumoto reports receiving personal fees from AbbVie, Endo, Lilly, Lipocine, and Clarus Therapeutics. Dr Stephens-Shields reports receiving grants from NIA, NIH, and AbbVie. Dr Ellenberg reports receiving grants from NIH and AbbVie. Dr Gill reports receiving grants from NIH. Dr Bhasin reports receiving grants from NIA, AbbVie, Lilly, and Transition Therapeutics; personal fees from AbbVie, Lilly, and Regeneron; a patent pending for a free testosterone calculator; and holding equity interest in FPC, LLC. Dr Cunningham reports receiving personal fees from AbbVie, Apricus Biosciences, Besins Healthcare, Clarus Therapeutics, Endo, Ferring Pharmaceuticals, Lilly, Pfizer, Repros Therapeutics. Dr Ensrud reports receiving grants from NIA. Dr Farrar reports receiving grants from NIA, NIH, and AbbVie. Dr Lewis reports receiving grants from NIH and AbbVie. Dr Molitch reports receiving grants from NIH and Abbott Laboratories and personal fees from AbbVie, Lilly, and Pfizer. Dr Swerdloff reports receiving grant funding from the Bone Trial of the Testosterone Trial, Clarus Therapeutics, Lipocine, and Antares Pharma and personal fees from Clarus Therapeutics and Antares Pharma. Dr Basaria reports receiving personal fees from Lilly and Takeda. Dr Diem reports receiving grants from NIA. Dr Wang reports receiving grants from Besins Health International, Clarus Therapeutics, and Lipocine and personal fees from Antares, TesoRx, and Besins Health International. Dr Snyder reports receiving grants from NIA, NIH, and AbbVie and personal fees from Watson Laboratories. No other disclosures were reported.
References
- 1.Schaie KW, Willis SL. Age difference patterns of psychometric intelligence in adulthood: generalizability within and across ability domains. Psychol Aging. 1993;8(1):44–55. doi: 10.1037//0882-7974.8.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- 3.Price L, Said K, Haaland KY. Age-associated memory impairment of logical memory and visual reproduction. J Clin Exp Neuropsychol. 2004;26(4):531–538. doi: 10.1080/13803390490496678. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 6.Almeida OP, Waterreus A, Spry N, Flicker L, Martins RN. One year follow-up study of the association between chemical castration, sex hormones, β-amyloid, memory and depression in men. Psychoneuroendocrinology. 2004;29(8):1071–1081. doi: 10.1016/j.psyneuen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Bussiere JR, Beer TM, Neiss MB, Janowsky JS. Androgen deprivation impairs memory in older men. Behav Neurosci. 2005;119(6):1429–1437. doi: 10.1037/0735-7044.119.6.1429. [DOI] [PubMed] [Google Scholar]
- 8.Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87(11):5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50(4):707–712. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]
- 10.Cherrier MM, Asthana S, Plymate S, et al. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57(1):80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- 11.Liverman CT, Blazer DG, editors. Testosterone and Aging: Clinical Research Directions. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 12.Goldman WP, Morris JC. Evidence that age-associated memory impairment is not a normal variant of aging. Alzheimer Dis Assoc Disord. 2001;15(2):72–79. doi: 10.1097/00002093-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Crook T, Bartus RT, Ferris SH, Whitehouse P, Cohen GD, Gershon S. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change—report of a National Institute of Mental Health Work Group. Dev Neuropsychol. 1986;2(4):261–276. [Google Scholar]
- 14.Smith G, Ivnik RJ, Petersen RC, Malec JF, Kokmen E, Tangalos E. Age-associated memory impairment diagnoses: problems of reliability and concerns for terminology. Psychol Aging. 1991;6(4):551–558. doi: 10.1037//0882-7974.6.4.551. [DOI] [PubMed] [Google Scholar]
- 15.Snyder PJ, Ellenberg SS, Cunningham GR, et al. The Testosterone Trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11(3):362–375. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder PJ, Bhasin S, Cunningham GR, et al. Testosterone Trials Investigators Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. doi: 10.1056/NEJMoa1506119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the Patient Health Questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Crook TH, III, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr. 1992;4(2):165–176. doi: 10.1017/s1041610292000991. [DOI] [PubMed] [Google Scholar]
- 19.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15(5):443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 20.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 21.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228) doi: 10.1126/scitranslmed.3007941. 228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benton A. Revised Visual Retention Test. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 23.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19(6):604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 24.Resnick SM, Coker LH, Maki PM, Rapp SR, Espeland MA, Shumaker SA. The Women's Health Initiative Study of Cognitive Aging (WHISCA): a randomized clinical trial of the effects of hormone therapy on age-associated cognitive decline. Clin Trials. 2004;1(5):440–450. doi: 10.1191/1740774504cn040oa. [DOI] [PubMed] [Google Scholar]
- 25.Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003;24(4):568–576. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Memory Scale—Revised. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- 27.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 28.Bilgel M, An Y, Lang A, et al. Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimers Dement. 2014;10(6):735–742.e4. doi: 10.1016/j.jalz.2014.04.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tohgi H, Utsugisawa K, Yamagata M, Yoshimura M. Effects of age on messenger RNA expression of glucocorticoid, thyroid hormone, androgen, and estrogen receptors in postmortem human hippocampus. Brain Res. 1995;700(1-2):245–253. doi: 10.1016/0006-8993(95)00971-r. [DOI] [PubMed] [Google Scholar]
- 30.Cherrier MM, Matsumoto AM, Amory JK, et al. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64(2):290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- 31.Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138(3):1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84(10):3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- 33.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31(5):565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Green HJ, Pakenham KI, Headley BC, et al. Altered cognitive function in men treated for prostate cancer with luteinizing hormone-releasing hormone analogues and cyproterone acetate: a randomized controlled trial. BJU Int. 2002;90(4):427–432. doi: 10.1046/j.1464-410x.2002.02917.x. [DOI] [PubMed] [Google Scholar]
- 35.Cherrier MM, Matsumoto AM, Amory JK, et al. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32(1):72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28(6):875–882. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- 37.Cherrier MM, Anawalt BD, Herbst KL, et al. Cognitive effects of short-term manipulation of serum sex steroids in healthy young men. J Clin Endocrinol Metab. 2002;87(7):3090–3096. doi: 10.1210/jcem.87.7.8570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.