Abstract
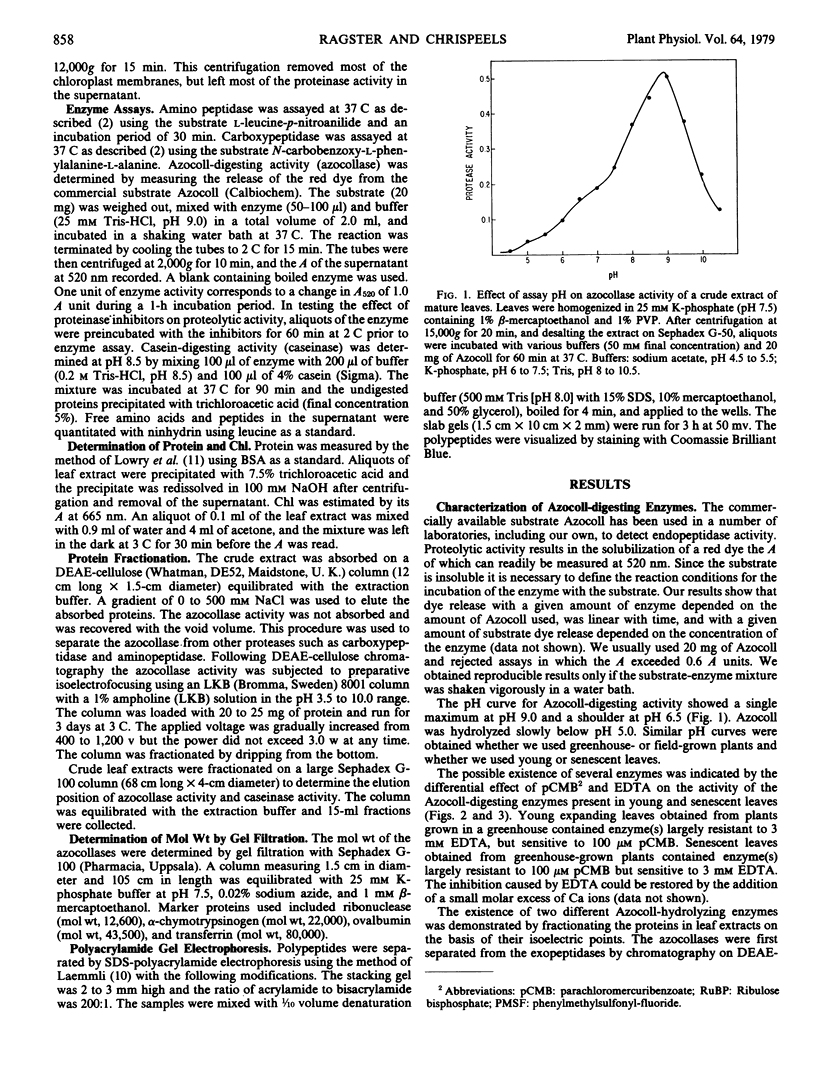
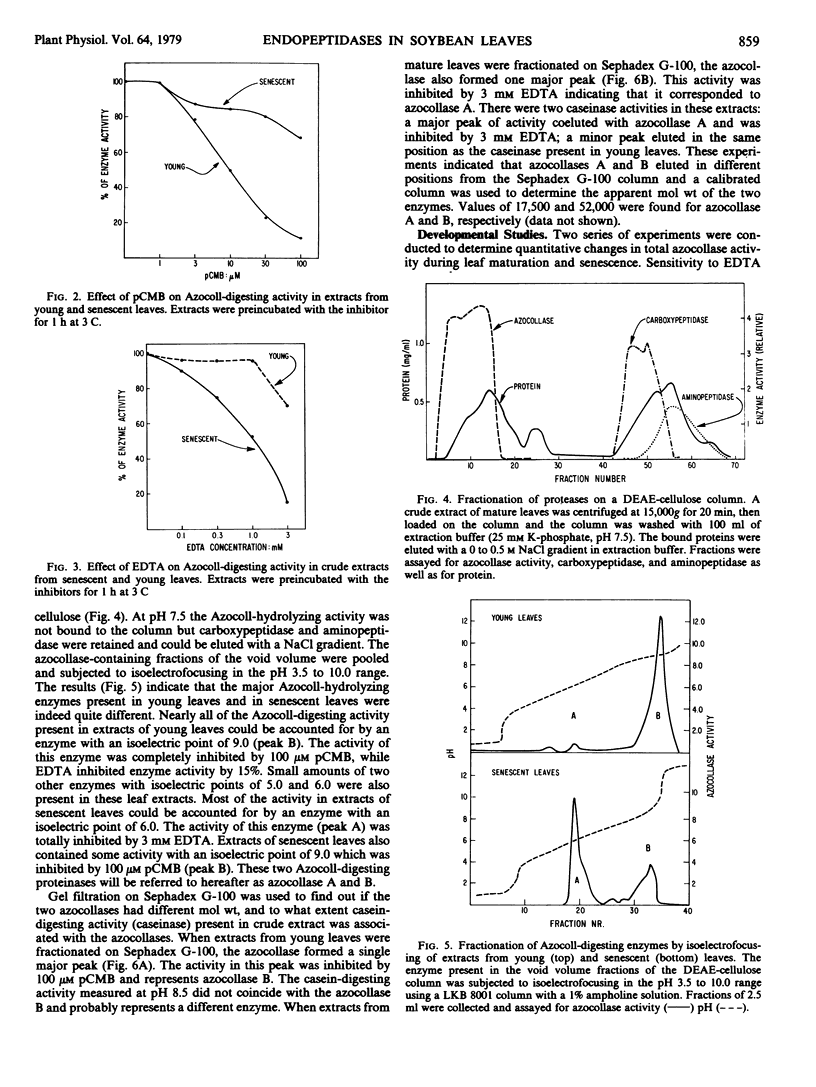
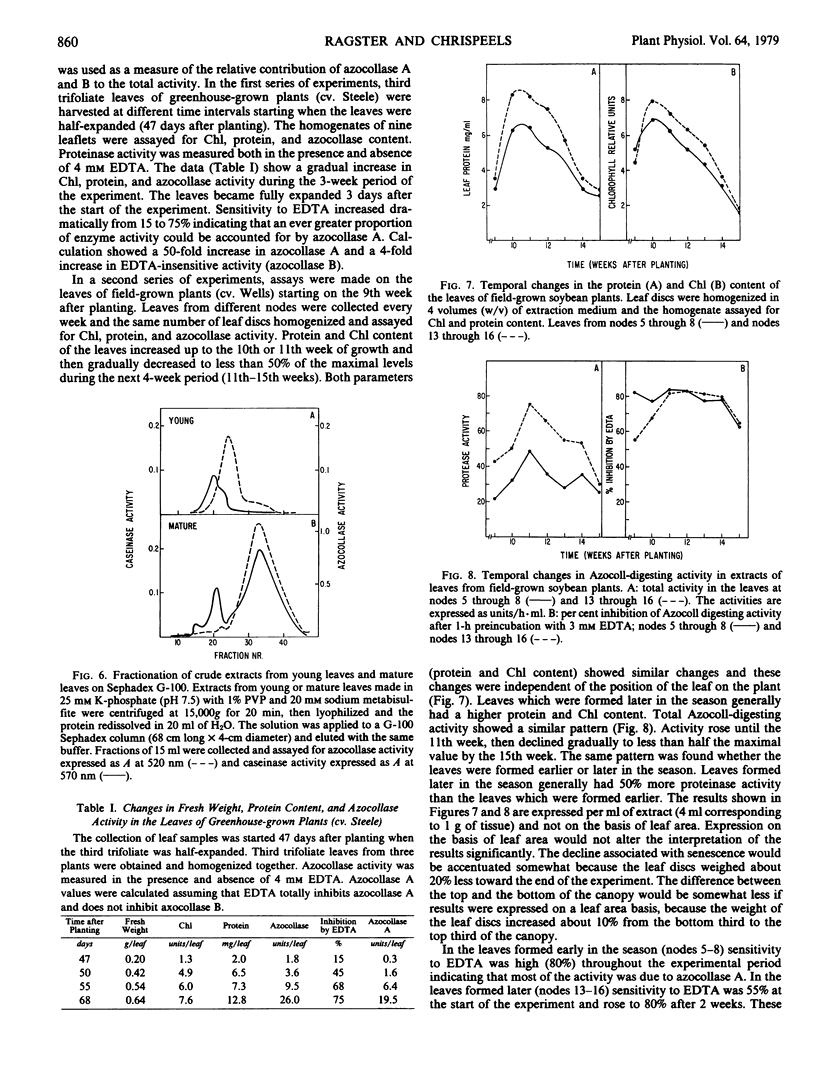
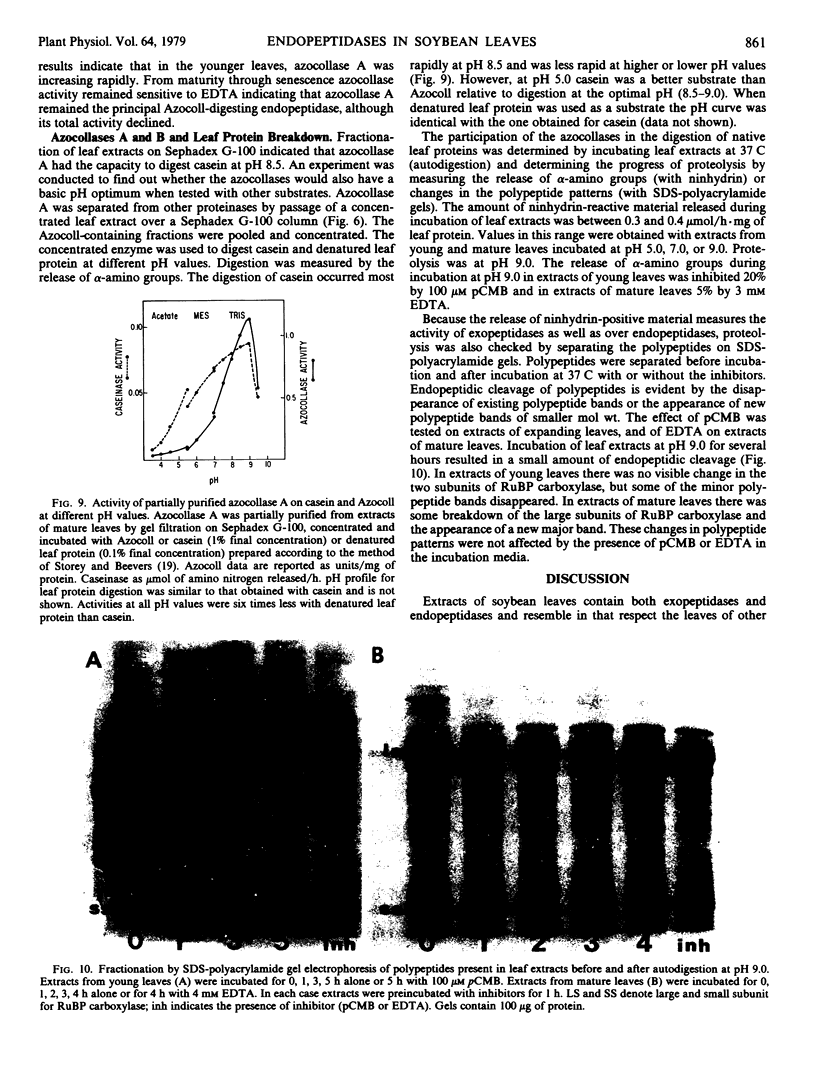
Two different endopeptidases which digest the chromogenic substrate Azocoll were found in soybean leaves. Azocollase A has a molecular weight of 17,500 and a pI of 6.0. Azocollase B has a molecular weight of 52,000 and a pI of 9.0. Both digest Azocoll optimally at pH 9.0. Azocollase A is inhibited by 3 millimolar ethylenediamine tetraacetate (EDTA) and azocollase B by 100 micromolar parachloromercuribenzoate. Studies on whole plants grown in the greenhouse and in the field show that total azocollase activity gradually increased during leaf maturation when leaf protein and chlorophyll increased, and then declined again during leaf senescence. Young leaves which are still expanding contain mostly azocollase B and little azocollase A. Leaf maturation was associated with a dramatic increase in azocollase A (40- to 50-fold), while azocollase B activity increased more slowly. This increase in azocollase A occurred in the 2- to 3-week period following leaf expansion. Azocollase A, separated from other proteinases by gel filtration on Sephadex G-100, digested denatured leaf protein and casein, resulting in the release of free α-amino groups. Break-down of leaf proteins by autodigestion of extracts at pH 9.0 resulted in the release of free α-amino groups and endopeptidic cleavage of polypeptides. However, polypeptide cleavage was not inhibited by parachloromercuribenzoate or EDTA indicating that the azocollases do not play a major role in the hydrolysis of leaf proteins in crude extracts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of Senescing Oat Leaves: II. Reaction to Substrates and Inhibitors. Plant Physiol. 1978 Apr;61(4):501–505. doi: 10.1104/pp.61.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller U. K., Soong T. S., Hageman R. H. Leaf Proteolytic Activities and Senescence during Grain Development of Field-grown Corn (Zea mays L.). Plant Physiol. 1977 Feb;59(2):290–294. doi: 10.1104/pp.59.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. The Hydrolysis of Endosperm Protein in Zea mays. Plant Physiol. 1974 Mar;53(3):453–457. doi: 10.1104/pp.53.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J. V., Varner J. E. Gibberellic Acid-induced synthesis of protease by isolated aleurone layers of barley. Plant Physiol. 1967 Nov;42(11):1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavreinová S., Turková J. SH-proteinase from bean Phaseolus vulgaris var. Perlicka. Biochim Biophys Acta. 1975 Oct 22;403(2):506–513. doi: 10.1016/0005-2744(75)90078-9. [DOI] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]



