Abstract
Quadriceps and hamstrings weakness and co-activation present following total knee arthroplasty (TKA) and may impair functional performance. How surgery and post-operative rehabilitation influence muscle activation during walking early after surgery is unclear.
Purpose
Examine muscle strength and activation during walking before and one and 6-months post-TKA.
Methods
Ten patients (n=6 female; age:64.7±7.9years; body mass index[BMI]:29.2±2.5kg/m2) and 10 healthy adults (n=6 female; age:60.6±7.4years; BMI:25.5±4.0kg/m2) participated. The patients underwent bilateral quadriceps and hamstrings strength assessment and assessment of quadriceps/hamstrings co-activation and on/off timing using surface electromyography during a six-minute walk test (6MW). Groups, limbs, and changes with TKA surgery were compared.
Results
Patients reported greater 6MW knee pain pre- versus post-TKA and compared to controls (P<0.05). Patients had weaker surgical limb hamstrings (P<0.05) and bilateral quadriceps (P<0.05) than controls pre- and post-TKA. Before and 1-month post-TKA, patients had side-to-side differences in quadriceps and hamstrings strength (P<0.05). Controls walked farther than patients (P<0.01). Patients demonstrated greater surgical limb co-activation pre-operatively than controls (P<0.05). Co-activation was higher bilaterally one-month post-TKA compared to controls (P<0.05). Patients turned off their quadriceps later during stance than controls before and 1-month post-TKA (P<0.05).
Conclusions
Muscle strength, co-activation, and timing differed between patients and controls before and early after surgery. Rehabilitation to improve strength and muscle activation seems imperative to restore proper muscle firing patterns early after surgery.
Keywords: strength, walking, muscle activity, total knee replacement
1. INTRODUCTION
Total knee arthroplasty (TKA) is commonly performed to relieve pain and disability associated with knee osteoarthritis (OA). Despite pain relief and high patient satisfaction following surgery,[1] deficits in lower extremity muscle strength and functional performance persist post-operatively.[2] Previous research suggests that quadriceps and hamstrings weakness and impaired gait biomechanics may persist for years following surgery.[2,3]
Recovery of muscle strength is often difficult after TKA. In the first post-operative month, quadriceps strength declines by up to 60%.[4] While strength improves over time, quadriceps strength deficits of approximately 30% have been reported upwards of two years postoperatively compared to healthy adults.[5] Similarly, hamstrings strength is reduced up to 50% have been reported in the immediate post-operative period[6,7] compared to pre-operative levels. Hamstrings strength deficits of nearly 30% may persist years after surgery compared to healthy adults.[5]
Muscle weakness, particularly in the quadriceps, is related to impaired functional performance. Patients with quadriceps weakness following TKA walk slower and with aberrant biomechanics compared to healthy adults.[8,9] Patients frequently adopt a knee stiffening strategy during level walking following TKA, which is characterized by limited knee flexion range of motion and reduced demand placed on the quadriceps musculature.[10] Abnormal phasing of quadriceps and hamstrings muscle activity may also occur with a knee stiffening strategy during walking, possibly altering kinematics and joint loading, hastening disease progression.[10]
During daily activities, muscle groups surrounding the knee joint may fire individually to flex or extend the joint as necessary. Muscle co-activation is also common during daily activities, including walking, and occurs when multiple muscle groups are active at the same time. Increased levels of quadriceps/hamstrings co-activation have been reported frequently in patients with knee OA when compared with healthy adults, and may be related to dysfunction in these muscle groups.[11,12] In a comparison of patients measured two years following TKA to healthy adults, increased quadriceps/hamstrings co-activation occurred as a result of prolonged activity of the quadriceps during stance.[13] While this increased co-activation purportedly occurs to improve knee joint stability[12,14], it may also increase compressive forces through the joint and precipitate degeneration. Further, increased co-activation suggests that patients have difficulty isolating their quadriceps and hamstrings muscles during functional tasks, which may relate to muscle weakness after TKA.[15]
While the presence of co-activation has been reported in patients with knee OA and long term following TKA, quadriceps and hamstrings muscle activation patterns in the acute postoperative period remain unclear. Understanding activation patterns, including timing and co-activation, is necessary to successfully counter abnormal muscle activity during post-operative rehabilitation with targeted intervention strategies. This study, therefore, examined bilateral quadriceps and hamstrings strength and muscle activity during walking in patients before, one month after, and 6 months after TKA. Additionally, we compared the results to those obtained in healthy older adults. We hypothesized patients would demonstrate muscle weakness and higher levels of quadriceps/hamstrings co-activation at all time points compared to healthy adults.
2. METHODS
2.1 Participants
Ten patients and 10 healthy, older adults (controls) participated (Table 1). Patients were eligible if they were undergoing a unilateral, primary TKA for knee OA using a medial parapatellar approach.[7] Individuals were excluded from participation if they had: 1) current lower extremity orthopedic conditions besides knee OA in the patient cohort; 2) uncontrolled diabetes; 3) cardiovascular or neurological disease; 4) body mass index ≥35 kg/m2; or 5) any other medical conditions that severely limited function. Patients completed three testing sessions: pre-operative and one and 6 months post-operative. The control group completed only one testing session. Participants received rehabilitation as recommended by their surgeon, which typically consisted of 5–6 home physical therapy sessions over the first 1–2 weeks post-operatively, followed by 4–6 weeks of outpatient physical therapy (1–2 days/week). This study was approved by the university’s institutional review board. All participants provided written, informed consent prior to participation.
Table 1.
Participant baseline demographics. Data are mean±standard deviation unless otherwise noted.
| Patient (n=10) | Healthy (n=10) | |
|---|---|---|
| Sex (% female) | 60 | 60 |
| Age (years) | 64.70±7.90 | 60.60±7.44 |
| Body mass index (kg/m2)* | 29.15±2.50 | 25.47±4.00 |
indicates significant difference between groups (P<0.05)
2.2 Procedures
2.2.1 Pain Assessment
Participants completed a numerical rating of pain scale during testing. Pain was rated on a scale of 0–10 (0: no pain present; 10: worst pain possible) after participants completed the six-minute walk test.
2.2.2 Electromyography and Step Data
After cleaning the skin with isopropyl alcohol, surface electromyography (EMG) electrodes (DE2.3, Delsys Inc, Boston, MA, USA) were placed bilaterally over the vastus lateralis and biceps femoris.[16] A ground electrode was placed over the lateral humeral epicondyle. Data were amplified by 1000 and stored using the Myomonitor IV datalogger (Delsys Inc., Boston, MA). Heel strike timing for each step was determined by use of heel switches collecting synchronously with the EMG data.
2.2.3 Strength
Participants performed isometric quadriceps and hamstrings strength assessment using an electromechanical dynamometer (Humac Norm, CSMI Solutions, Stoughton, MA, USA). Strength data were collected in real-time using a computer running AcqKnowledge software (v3.8.2; Biopac, Inc., Goleta, CA, USA).[7,17] All strength assessments were performed bilaterally, beginning with the nonsurgical limb in patients and the left limb in the control group.
For all muscle strength testing, participants were seated with the hip and knee flexed to 85° and 60°, respectively.[7,17] Following two submaximal warm-up repetitions, participants performed a series of maximal voluntary isometric contractions (MVICs) until the torque values generated during two trials were within 5% of each other. A maximum of four trials were performed. Participants were given verbal and visual feedback during testing to elicit maximal effort. The highest torque value across all MVICs was normalized to participant body mass (Nm/kg) and used for analysis. The hamstrings were tested before the quadriceps.
2.2.4 Functional Performance
Physical function was assessed using the six-minute walk test (6MW). The 6MW assesses the distance a person can walk in six minutes.[18] To perform the 6MW, participants walked laps in a 30.5m hallway and the distance covered, in meters, was recorded.[19]
2.2.5 EMG Data Analysis
The EMG data were bandpass filtered using a fourth order, zero lag, Butterworth filter with 20–350Hz cutoff frequencies.[20] The Teager-Kaiser Energy Operator (TKEO) was applied to the filtered EMG signal. The TKEO combines the amplitude and instantaneous frequency[21] of the EMG signal and is recommended for use when determining timing in data with a low signal-to-noise ratio.[22] Linear envelopes were created with full-wave rectification and a fourth-order, zero phase lag, low-pass filter (6Hz cutoff). Linear envelope data were normalized by dividing by the mean amplitude of the signal during the 6MW.[23,24] EMG data were time normalized to 100% of the complete gait cycle (i.e., heel strike to heel strike). Ensemble averages were calculated from 10 strides of the 6MW test and used to calculate the dependent variables.
The quadriceps/hamstrings co-activation index (CAI) was calculated based on the integrated EMG signal.[25] Quadriceps and hamstrings on and off times (Qon, Qoff, Hon, Hoff) were calculated as the instances (in % gait cycle) when the linear envelope crossed 20% of the mean normalized linear envelope.
2.3 Statistical Analysis
The independent variables for analysis were group (patient and control), limb (surgical and nonsurgical), and time (pre-op, one month post-op, and six months post-op). Dependent variables were pain, quadriceps strength, hamstrings strength, 6MW distance, CAI, and quadriceps on/off times (Qon, Qoff), and hamstrings on/off times (Hon, Hoff). Independent samples t-tests compared the dependent variables between the control group and the patient group (at each time). Paired samples t-tests were used to compare dependent variables between limbs at each time. The a priori alpha level was 0.05. All statistical analyses were performed in IBM SPSS Statistics (version 19, Armonk, NY, USA).
3. RESULTS
3.1 Pain
Patients reported greater knee pain pre-operatively (5.5±2.7) than one (3.4±2.5; P=0.01) and six months (0.3±0.9; P<0.001) post-operatively. Patients had greater knee pain one than six months post-TKA (P=0.003).
The control group reported no 6MW knee pain. Compared to the control group, patients had greater knee pain before (P<0.001) and one month (P=0.002) following surgery. Knee pain did not differ between groups six months post-operatively (P=0.343).
3.2 Strength
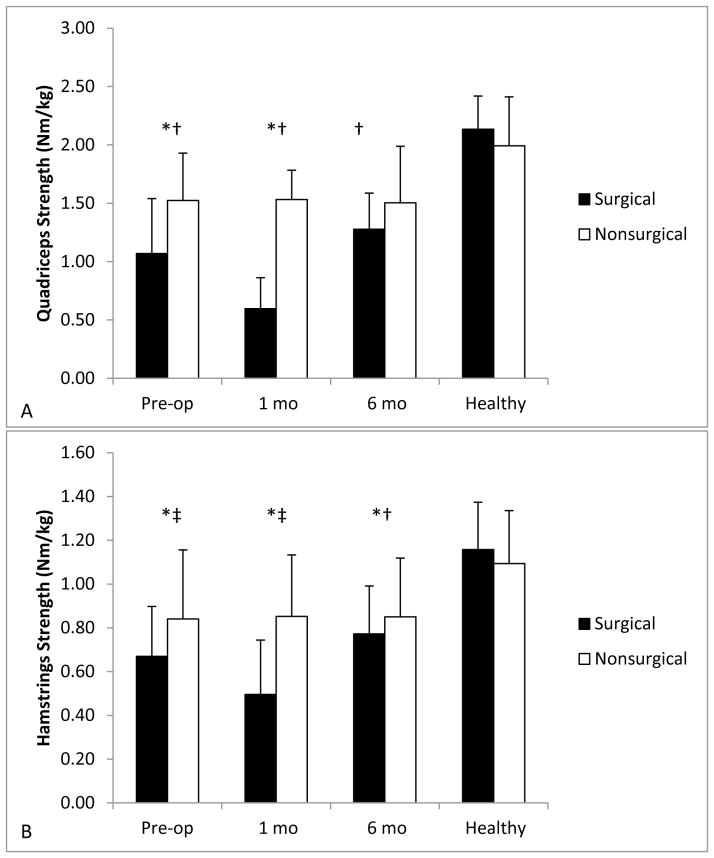
Quadriceps strength was greater in controls compared to patients at all time points (pre-op: surgical P<0.001, nonsurgical P=0.021; one month: surgical P<0.001, nonsurgical P=0.008; 6 months: surgical P<0.001, nonsurgical P=0.027; Figure 1A)). Hamstrings strength was greater in controls than in the patients’ surgical limb at all times (pre-op: P<0.001; 1 month: P<0.001; 6 months: P<0.001; Figure 1B). Hamstrings strength did not differ between the controls and patients’ nonsurgical limb pre-operatively (P=0.059) or one month post-operatively (P=0.054). However, hamstrings strength was greater in the control group compared to the patients’ nonsurgical limb six months post-operatively (P=0.047).
Figure 1.
A–B. Muscle strength pre-operatively and at 1 and 6 months post-operatively for Quadriceps (A) and Hamstrings (B). The surgical limb is represented by black bars and the nonsurgical limb by white bars. Data are mean+standard error. *Indicates significant side-to-side difference in muscle strength. †Indicates significant difference from the control group bilaterally. ‡Indicates significant difference from the controls compared to the operative limb only.
Patients demonstrated side-to-side differences in quadriceps strength pre-operatively (P=0.004) and one month post-operatively (P<0.001). Quadriceps strength did not differ between limbs six months post-TKA (P=0.135). Hamstrings strength was different side-to-side in patients before (P=0.009) and one month post-operatively (P=0.001). By six months post-operatively, hamstrings strength was not different between limbs (P=0.284). Controls did not demonstrate side-to-side differences in quadriceps (P=0.088) or hamstrings (P=0.321) strength.
3.3 Functional Performance
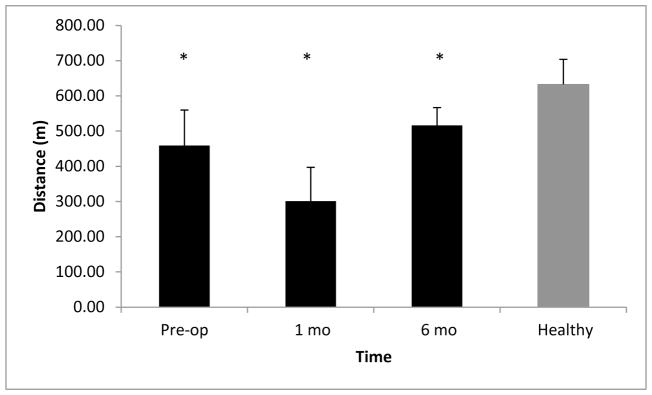
The 6MW distance differed between patients and controls at all time points (P<0.001; Figure 2). Healthy controls walked greater distances than patients.
Figure 2.
Six minute walk distance (m). Black bars indicate values for TKA patients pre-operatively and at 1 and 6 months post-operatively. Gray bars indicate values for healthy adults. Data are mean+standard error. *Indicates patients walked significantly less distance than healthy adults.
3.4 Electromyography
3.4.1 Co-activation Index
The CAI was greater in patients’ surgical limb compared to controls pre-operatively (P=0.029; Figure 3) and one month post-operatively (P=0.024), but these differences resolved by six months (P=0.402). The CAI was not different when the nonsurgical limb was compared to controls pre-operatively (P=0.167) or six months post-operatively (P=0.292). However, one month post-TKA, the nonsurgical limb demonstrated greater levels of muscle co-activation than in controls (P=0.049).
Figure 3.
Co-activation index for TKA patients (pre-operatively and 1 and 6 months postoperatively) and for healthy adults. The surgical limb is represented by black bars and the nonsurgical limb by white bars. Data are mean+standard error. *Indicates significantly greater co-activation in the surgical limb compared to healthy controls. †Indicates greater co-activation in the non-surgical limb compared to healthy controls.
Patients did not present with side-to-side differences in CAI at any time point (pre-op: P=0.098; 1 month: P=0.305; 6 months: P=0.568). Further, the healthy control group presented with no differences in CAI between limbs (P=0.266).
3.4.2 Quadriceps Off and On Times
The patients’ surgical limb quadriceps turned off later in the gait cycle compared to controls pre-operatively (P=0.001) and one month post-operatively (P=0.001; Table 2). These differences resolved six months after surgery (P=0.425). Surgical limb Qon time did not differ between groups (pre-op: P=0.659; one month: P=0.707; 6 months: P=0.792). In the nonsurgical limb, neither Qoff (pre-op: P=0.882; one month: P=0.556; 6 months: P=0.060) nor Qon (pre-op: P=0.667; 1 month: P=0.669; 6 months: P=0.916) times differed between groups.
Table 2.
Muscle On/Off Times as a Percentage of the Gait Cycle. Data are mean±standard deviation.
| Patient | Healthy | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Op | 1 Month | 6 Months | ||||||
| Surgical | Nonsurgical | Surgical | Nonsurgical | Surgical | Nonsurgical | Right | Left | |
| Qon | 32.91±13.00 | 19.87±11.38 | 30.44±14.29† | 24.51±12.09 | 15.36±5.56 | 14.29±1.91 | 15.58±1.89 | 20.62±10.42 |
| Qoff | 91.47±5.04*† | 91.07±3.30 | 91.38±7.83* | 89.18±8.35 | 90.96±2.09 | 90.82±3.21 | 90.54±3.94 | 90.46±2.72 |
| Hon | 24.80±9.62 | 23.84±28.14 | 34.42±24.34 | 13.80±8.26 | 26.89±28.14 | 23.27±28.70 | 24.34±28.11 | 24.28±36.78 |
| Hoff | 78.51±7.57 | 78.36±6.55 | 81.38±7.49 | 81.24±5.61 | 81.76±7.52 | 79.67±9.40 | 74.50±23.79 | 76.62±7.44 |
Indicates significant difference from control group (P<0.05)
Indicates significant difference between limbs (P<0.05)
The surgical limb quadriceps turned off later in the gait cycle than the nonsurgical limb in patients pre-operatively (P=0.05; Table 2). The Qoff time did not differ between limbs at one (P=0.354) or 6 (P=0.782) months post-operatively. There were no side-to-side differences in Qon time pre-operatively (P=0.849) or six months (P=0.812) post-operatively; however, the surgical limb quadriceps turned on later than the nonsurgical limb quadriceps one month postoperatively (P=0.013). Neither Qoff (P=0.923) or Qon (P=0.179) differed between limbs in healthy controls.
3.4.3 Hamstrings Off and On Times
The Hoff times were not different between groups for the surgical limb (pre-op: P=0.963; one month: P=0.817; 6 months: P=0.988) or nonsurgical limb (pre-op: P=0.977; one month: P=0.336; 6 months: P=0.874; Table 2). Similarly, neither surgical limb (pre-op: P=0.635; one month: P=0.412; 6 months: P=0.447) nor nonsurgical limb (pre-op: P=0.598; 1 month: P=0.112; 6 months: P=0.391; Table 2) Hon times differed in patients compared to healthy controls.
Patients demonstrated similar side-to-side Hoff times pre-operatively (P=0.932) and six months post-operatively (P=0.864). However, one month after surgery the surgical limb hamstrings turned off later during stance compared to the nonsurgical limb hamstrings (P=0.020). The Hon time did not differ between limbs at any time point in patients (pre-op: P=0.925; one month: P=0.981; 6 months: P=0.262; Table 2). Hoff (P=0.793) and Hon (P=0.997) did not differ between limbs in controls.
4. DISCUSSION
This study examined muscle strength, co-activation, and timing during walking in patients before and after TKA. Our results suggest that quadriceps/hamstrings co-activation is elevated bilaterally in patients one month after TKA compared to healthy adults. These findings highlight the need for clinicians to emphasize bilateral strength and muscle activation retraining during early, post-operative rehabilitation. This retraining may minimize secondary pathology due to compensatory movement strategies.
In accordance with our hypothesis, patients demonstrated both quadriceps and hamstrings weakness in their surgical limb pre-operatively compared to the healthy control group. These strength deficits were exacerbated one month post-operatively and still present six months after surgery compared to healthy controls. Previous investigators have demonstrated similar patterns of muscle strength before and after TKA.[4,26]
Pre-operatively, patients demonstrated greater surgical limb quadriceps/hamstrings co-activation than healthy adults. By one month after surgery, muscle co-activation was further elevated bilaterally in patients compared to healthy adults. Changes in muscle co-activation in response to muscle weakness are not unique to the TKA population. Increased co-activation has been reported frequently in response to muscle weakness, particularly in patients following stroke[27] and even in healthy, older adults as a result of age-related strength declines.[28] Elevated levels of co-activation in the muscles surrounding the knee joint were demonstrated in individuals with knee instability, such as those with osteoarthritis[11] or anterior cruciate ligament injury.[29] These patients often present with a “stiff knee” gait. These aberrant neuromechanical patterns purportedly represent an adaptive strategy to overcome muscle weakness, particularly in the quadriceps, and increase joint stability during walking. Given the presence of muscle weakness pre-operatively and its exacerbation immediately after surgery, it seems that the patients in the present study utilized muscle co-activation to accommodate for the strength loss they experienced. However, we did not assess differences in gait kinematics or joint stability, and cannot be certain how these influenced the levels of muscle co-activation present in our participants.
While muscle co-activation is inherent in daily activity, the elevated levels of quadriceps/hamstrings co-activation one month after surgery in our patients is concerning. Elevated muscle co-activation may compress the knee joint and accelerate the progression of knee OA. The same response may be observed after TKA, whereby increased muscle co-activation in the nonsurgical limb may hasten degeneration in that limb. Increases in muscle co-activation likely arise due to weakness and altered muscle activation timing in the quadriceps muscle group. Specifically, delayed Qoff during stance seems to drive increases in co-activation. At the pre-operative and one month post-operative time points, the surgical limb quadriceps in the patients turned off later in the gait cycle than those in the healthy adult group, resulting in periods of quadriceps activity that were approximately twice as long as healthy adults. Benedetti et al.[13] suggested that the on/off timing of the quadriceps and hamstrings is impaired following TKA, and noted periods of prolonged quadriceps muscle activity compared to healthy adults. Altered on/off timing is not only the result of a compensatory strategy employed by the patient, but it also results from impaired proprioception arising before or during surgery.[30–32] Considering this, full restoration of normal muscle firing may require a comprehensive rehabilitation plan including exercises to improve strength and neuromuscular control as well as the integration of these during functional tasks.
Elevated levels of muscle co-activation may increase joint stiffness in response to pain in individuals with knee OA.[33] This idea is confirmed by the present data, considering that knee pain levels in patients were greater than controls at the same time points (pre-operatively and one month post-operatively) as co-activation. It seems that reducing pain may be essential to restoring levels of muscle co-activation following TKA.
An important finding in the present investigation is the increase in muscle co-activation bilaterally one month following TKA. These data suggest a potentially hazardous compensatory strategy in the non-surgical limb. While increases in muscle co-activation around the knee joint may improve stability[12,14], this same strategy may also increase joint contact forces and precipitate degeneration.[34] These findings highlight the need for clinicians to emphasize bilateral strength and muscle activation retraining during early, post-operative rehabilitation. While it is well documented that patients after TKA benefit from early rehabilitation and neuromuscular electrical stimulation use to improve quadriceps strength[35–38], little evidence exists to suggest the optimal mode of exercise to improve muscle firing patterns post-operatively. Well-designed, randomized controlled trials should examine methods by which to isolate the quadriceps muscle group during functional activities, rather than relying on quadriceps/hamstrings co-activation as this may help to optimize outcomes. Additional investigations should seek to develop a comprehensive rehabilitation approach that also includes EMG or visual biofeedback during functional activities and/or gait retraining to optimize muscle firing.
Six months post-operatively, the magnitude of muscle co-activation and the timing of muscle onset/offset did not differ between patients and healthy adults. This finding is in contrast to previous reports by Benedetti and colleagues[13] that the quadriceps and hamstrings muscles demonstrated prolonged firing during gait in patients six months after surgery.[13] These authors noted the presence of prolonged muscle firing up to two years following TKA and attribute its presence to an adaptive, stabilization strategy.[13] The reasons why patients in the previous investigation demonstrated abnormal muscle firing six months after surgery and our patients did not is unclear, though differences in rehabilitation strategy may contribute. However, our patients demonstrated muscle firing patterns similar to healthy adults six months post-operatively, which suggests that this apparent adaptive strategy can be reversed. Early restoration of muscle firing patterns may minimize secondary pathology due to compensatory movement strategies, such as increased nonsurgical limb loading precipitating joint degeneration in that limb.
The quadriceps and hamstrings muscles do not act as a simple agonist/antagonist pair of muscles that cross the knee. Rather, the rectus femoris and hamstrings are biarticular, also acting on the hip. Because we did not record muscle activity from the rectus femoris, we are confident that the quadriceps EMG activity reported represents knee extension muscle activity. However, some of the changes in hamstrings activity may have been the result of these muscles acting as hip extensors. Without gait kinetic and kinematic data or EMG recordings from other hip extensors, the degree to which these factors may have influenced changes in hamstrings EMG recordings in the present study cannot be determined.
A potential limitation of the present study is that the small sample size may limit generalizability of these findings. Further, we recruited patients from multiple orthopedic surgeons using a variety of prostheses, post-operative pain management, and rehabilitation strategies. However, this variety may enhance the generalizability of our findings. Additionally, the inclusion criteria utilized may have yielded a high functioning patient population, which may have contributed to differences in walking speed between the two groups and possibly influenced EMG activity and muscle co-activation. Despite these limitations, our findings are consistent with those reported previously. Finally, we did not assess gait biomechanics during the 6MW test. As such, it remains unknown how the reported changes in muscle strength and co-activation may influence lower extremity biomechanics during walking and, therefore, the development/progression of non-surgical limb degeneration. Inclusion of gait kinetics and kinematics in future studies may lend further insight into the relation between muscle weakness, EMG, and aberrant biomechanics.
CONCLUSION
Patients demonstrated muscle weakness at all time points as well as greater quadriceps/hamstrings co-activation before and one month after surgery compared to healthy adults. Muscle co-activation was not different between limbs or when patients were compared to healthy adults six months after TKA. These findings suggest that to minimize compensatory movement strategies post-operatively and optimize muscle-firing patterns, clinicians should emphasize bilateral strength and muscle activation retraining in the immediate post-operative period.
HIGHLIGHTS.
Muscle co-activation is elevated bilaterally in patients one month after TKA.
Muscle co-activation is greater in patients than healthy adults.
Quadriceps activity is prolonged during stance before and early after TKA.
Rehabilitation should integrate strength and neuromuscular control retraining.
Acknowledgments
This study was supported by the NIH (K23AG029978) and, in part, by NIH/NCATS Colorado CTSI (UL1TR000154). Contents are the author’s sole responsibility and do not necessarily represent official NIH views. None of the sponsors had any influence on the study design, implementation, or data analysis and interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loughead JM, Malhan K, Mitchell SY, et al. Outcome following knee arthroplasty beyond 15 years. Knee. 2008 Mar;15(2):85–90. doi: 10.1016/j.knee.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005 Feb;(431):157–165. doi: 10.1097/01.blo.0000150130.03519.fb. [DOI] [PubMed] [Google Scholar]
- 3.Huang CH, Cheng CK, Lee YT, Lee KS. Muscle strength after successful total knee replacement: a 6- to 13-year followup. Clin Orthop Relat Res. 1996 Jul;(328):147–154. doi: 10.1097/00003086-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005 May;87(5):1047–1053. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silva M, Shepherd EF, Jackson WO, Pratt JA, McClung CD, Schmalzried TP. Knee strength after total knee arthroplasty. J Arthroplasty. 2003 Aug;18(5):605–611. doi: 10.1016/s0883-5403(03)00191-8. [DOI] [PubMed] [Google Scholar]
- 6.Judd DL, Eckhoff DG, Stevens-Lapsley JE. Muscle strength loss in the lower limb after total knee arthroplasty. Am J Phys Med Rehabil. Mar;91(3):220–226. doi: 10.1097/PHM.0b013e3182411e49. quiz 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens-Lapsley JE, Balter JE, Kohrt WM, Eckhoff DG. Quadriceps and Hamstrings Muscle Dysfunction after Total Knee Arthroplasty. Clin Orthop Relat Res. Jan 20; doi: 10.1007/s11999-009-1219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995 Nov;50(Spec No):55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 9.Walsh M, Woodhouse LJ, Thomas SG, Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther. 1998 Mar;78(3):248–258. doi: 10.1093/ptj/78.3.248. [DOI] [PubMed] [Google Scholar]
- 10.Andriacchi TP. Functional analysis of pre and post-knee surgery: total knee arthroplasty and ACL reconstruction. J Biomech Eng. 1993 Nov;115(4B):575–581. doi: 10.1115/1.2895543. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph KS, Schmitt LC, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007 Nov;87(11):1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res. 2008 Sep;26(9):1180–1185. doi: 10.1002/jor.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech (Bristol, Avon) 2003 Nov;18(9):871–876. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa S, Solomonow M, Luo Z, Lu Y, D’Ambrosia R. Muscular co-contraction and control of knee stability. J Electromyogr Kinesiol. 1991 Sep;1(3):199–208. doi: 10.1016/1050-6411(91)90035-4. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida Y, Mizner RL, Snyder-Mackler L. Association between long-term quadriceps weakness and early walking muscle co-contraction after total knee arthroplasty. Knee. 2013 Jan 23; doi: 10.1016/j.knee.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermens HJ, Freriks B, Merletti R, et al. European Recommendations for Surface ElectroMyoGraphy: Results of the SENIAM Project. Roessingh Research and Development; 1999. [Google Scholar]
- 17.Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther. Feb;92(2):210–226. doi: 10.2522/ptj.20110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002 Feb;82(2):128–137. doi: 10.1093/ptj/82.2.128. [DOI] [PubMed] [Google Scholar]
- 19.Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil. 2002 Jan;83(1):70–80. doi: 10.1053/apmr.2002.27337. [DOI] [PubMed] [Google Scholar]
- 20.Walker S, Peltonen H, Avela J, Hakkinen K. Kinetic and electromyographic analysis of single repetition constant and variable resistance leg press actions. J Electromyogr Kinesiol. Apr;21(2):262–269. doi: 10.1016/j.jelekin.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Hug F. Can muscle coordination be precisely studied by surface electromyography? J Electromyogr Kinesiol. Feb;21(1):1–12. doi: 10.1016/j.jelekin.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Aruin A. Muscle activity onset time detection using teager-kaiser energy operator. Conf Proc IEEE Eng Med Biol Soc. 2005;7:7549–7552. doi: 10.1109/IEMBS.2005.1616259. [DOI] [PubMed] [Google Scholar]
- 23.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J Electromyogr Kinesiol. 2010 Dec;20(6):1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984 Sep;65(9):517–521. [PubMed] [Google Scholar]
- 25.Winter DA, MacKinnon CD, Ruder GK, Wieman C. An integrated EMG/biomechanical model of upper body balance and posture during human gait. Prog Brain Res. 1993;97:359–367. doi: 10.1016/s0079-6123(08)62295-5. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech (Bristol, Avon) 2008 Mar;23(3):320–328. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamontagne A, Richards CL, Malouin F. Coactivation during gait as an adaptive behavior after stroke. J Electromyogr Kinesiol. 2000 Dec;10(6):407–415. doi: 10.1016/s1050-6411(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 28.Woollacott MH, Shumway-Cook A. Changes in posture control across the life span--a systems approach. Phys Ther. 1990 Dec;70(12):799–807. doi: 10.1093/ptj/70.12.799. [DOI] [PubMed] [Google Scholar]
- 29.Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004 Sep;22(5):925–930. doi: 10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991 Jan;73(1):53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- 31.Cash RM, Gonzalez MH, Garst J, Barmada R, Stern SH. Proprioception after arthroplasty: role of the posterior cruciate ligament. Clin Orthop Relat Res. 1996 Oct;(331):172–178. [PubMed] [Google Scholar]
- 32.Sharma L, Pai YC. Impaired proprioception and osteoarthritis. Curr Opin Rheumatol. 1997 May;9(3):253–258. doi: 10.1097/00002281-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Fisher NM, Pendergast DR. Reduced muscle function in patients with osteoarthritis. Scandinavian journal of rehabilitation medicine. 1997 Dec;29(4):213–221. [PubMed] [Google Scholar]
- 34.Liu W, Maitland ME. The effect of hamstring muscle compensation for anterior laxity in the ACL-deficient knee during gait. J Biomech. 2000 Jul;33(7):871–879. doi: 10.1016/s0021-9290(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 35.Lewek M, Stevens J, Snyder-Mackler L. The use of electrical stimulation to increase quadriceps femoris muscle force in an elderly patient following a total knee arthroplasty. Phys Ther. 2001 Sep;81(9):1565–1571. doi: 10.1093/ptj/81.9.1565. [DOI] [PubMed] [Google Scholar]
- 36.Stevens JE, Mizner RL, Snyder-Mackler L. Neuromuscular electrical stimulation for quadriceps muscle strengthening after bilateral total knee arthroplasty: a case series. J Orthop Sports Phys Ther. 2004 Jan;34(1):21–29. doi: 10.2519/jospt.2004.34.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005 Jul;35(7):424–436. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 38.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: A single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004 Apr;85(4):546–556. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]