Abstract
Purpose
The purpose of this study is to assess cone-mediated central retinal function in children with a history of preterm birth, including subjects with and without retinopathy of prematurity (ROP). The multifocal electroretinogram (mfERG) records activity of the postreceptor retinal circuitry.
Methods
mfERG responses were recorded to an array of 103 hexagonal elements that subtended 43° around a central fixation target. The amplitude and latency of the first negative (N1) and first positive (P1) response were evaluated in six concentric rings centered on the fovea. Responses were recorded from 40 subjects with a history of preterm birth (severe ROP, mild ROP, no ROP) and 19 term-born control subjects.
Results
The amplitude of N1 and P1 varied significantly with eccentricity and ROP severity. For all four groups, these amplitudes were largest in the center and decreased with eccentricity. At all eccentricities, N1 amplitude was significantly smaller in severe ROP and did not differ significantly among the other three groups (mild ROP, no ROP, term-born controls). P1 amplitude in all preterm groups was significantly smaller than in controls; P1 amplitude was similar in no ROP and mild ROP and significantly smaller in severe ROP.
Conclusions
These results provide evidence that premature birth alone affects cone-mediated central retinal function and that the magnitude of the effect varies with severity of the antecedent ROP. The lack of difference in mfERG amplitude between the mild and no ROP groups is evidence that the effect of ROP on the neurosensory retina may not depend solely on appearance of abnormal retinal vasculature.
Keywords: retinopathy of prematurity (ROP), multifocal electroretinogram (mfERG), retinal circuitry, prematurity
During normal development, cones in the fovea and central retina have a protracted course of maturation that continues well into childhood.1–4 Lingering immaturity makes the central retina, which includes the fovea, particularly vulnerable to the effects of retinopathy of prematurity (ROP).5 Both central retinal structure assessed using optical coherence tomography (OCT) and function assessed with the multifocal electroretinogram (mfERG) are abnormal in some adolescents with a history of ROP.6,7 Specifically, OCT studies have shown that the fovea is shallower and the central retina thicker in children with a history of ROP than in term-born children.7–10 In subjects with a history of ROP, mfERG amplitude is significantly reduced compared with that in term-born controls.6,8 mfERG responses are generated by postreceptor activity in a large number of discrete retinal regions centered on the fovea and provide a topographic assessment of postreceptor cell responses.11,12
To learn more about cone-mediated central retinal function, we examined mfERG responses in subjects with a history of preterm birth including those who never had ROP, those who had mild ROP that resolved without treatment, and those with severe ROP and compared their results to those in term-born control subjects.
Methods
Subjects
mfERG responses were recorded in 40 subjects with a history of preterm birth (Table). All subjects had serial fundus examinations in the newborn intensive care nursery in accordance with the American Academy of Pediatrics Section on Ophthalmology guidelines for ROP screening.13–15 ROP was classified using the International Classification of Retinopathy of Prematurity (ICROP) system in which location of the retinal vascular abnormalities is specified by zone, severity by stage, and extent by clock hours.16 ROP is an active disease at preterm ages and resolves by the early postterm weeks.17
Table.
Subject Characteristics
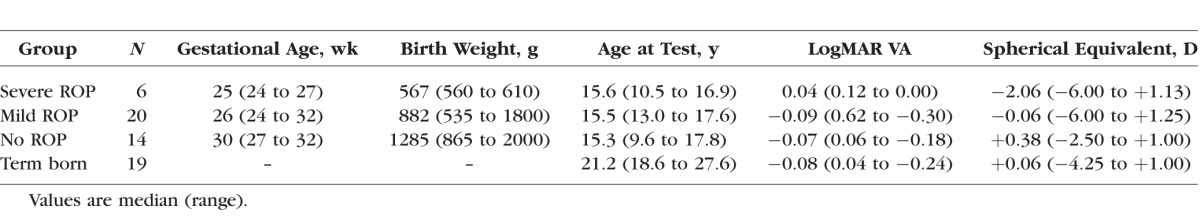
We categorized the subjects according to maximum severity of acute-phase ROP as severe ROP (n = 6), mild ROP (n = 20), or no ROP (n = 14). Those in the severe category were treated by laser ablation of avascular peripheral retina, which is eccentric to the retinal area tested by the mfERG. One of the six severe subjects had a localized retinal detachment (stage 4A) that resolved without intervention in early infancy; none of the other five had retinal detachment. In the mild ROP subjects, by clinical criteria, ROP resolved completely without treatment. All but one had stage 1 or 2 in zone II or III. One subject had 3 clock hours of stage 3 in zone II.16 For those in the no ROP category, serial examinations detected no ROP.
Gestational age at birth ranged from 24 to 32 weeks (median, 27 weeks), and birth weight was from 535 to 2000 g (median, 913 g). Former preterm subjects with a history of ROP were born earlier and had lower birth weight than those who never had ROP. Although among the groups there was considerable overlap in gestational age and weight at birth, ANOVA indicated that both varied significantly with group. Post hoc tests showed there were no significant differences in either gestational age or weight at birth between those in the mild and severe groups, but both of these groups differed significantly from the no ROP group (Table).
At the time of mfERG testing, the subjects ranged in age from 9 to 17 years (median, 15 years). Nineteen term-born control subjects age 18 to 27 years (median, 21 years) also participated. Gerth et al.18 found that amplitude and implicit time of the mfERG do not change significantly over these ages in normal subjects. Molnar et al.19 found no significant relationship between age and amplitude in 5- to 15-year-old term-born children; implicit time increased slightly.
Before mfERG testing, visual acuity was measured using an Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, and refraction was assessed by autorefraction while the subject fixated a distant target (WR-5100K; Grand Seiko, Hiroshima, Japan). ANOVA showed no significant difference in visual acuity among the four groups, but spherical equivalent in the severe and mild ROP groups was significantly more myopic than in the no ROP and term-born groups (Table).
The study conformed to the tenets of the Declaration of Helsinki and was approved by the Boston Children's Hospital Committee on Clinical Investigation. After explanation of the nature and possible consequences of the study, written informed consent was obtained from all control subjects and from the parents of prematurely born subjects, and assent was obtained from the children before each session.
Procedure
Before testing, the subject's pupil was dilated with tropicamide 1%. Then, after instillation of proparacaine 0.5%, a bipolar Burian-Allen electrode (Hansen Ophthalmic Development Lab, Coralville, IA, USA) was placed on the cornea of one eye, chosen at random. A ground electrode was placed on the skin over the ipsilateral mastoid. Responses were differentially amplified (bandpass, 0.3 to 100 Hz; gain, 100,000), digitized, and displayed using the VERIS multifocal system (EDI, Redwood City, CA, USA). The input signal from the electrode was monitored, and segments contaminated by noise were rejected and recorded again.
Responses were recorded to an array of 103 hexagons scaled by eccentricity and centered on a fixation cross that subtended 1°. The total horizontal extent of the array was 45°. The centers of the hexagons on the horizontal meridian were at approximately 0°, ±2.5°, ±5.9°, ±10.0°, ±14.8°, and ±19.9°. The average luminance of the stimulus was ∼100 cd/m2, and the contrast between the white and black hexagons was >90%. Each hexagon alternated between white and black using a random m sequence with exponent 14. Thus, each hexagon in the pattern changed 214−1 times during a 4-minute recording period that was divided into 12 segments. Fifty-one subjects were tested using a high-resolution stimulator with a fixation monitoring system (EDI) that uses a liquid crystal on silicon (LCOS) display. Eight subjects were tested with stimuli presented on a high-resolution Visual Graphics Array (VGA) monitor (Nortech Imaging Technologies, Mount Prospect, IL, USA). Stimulus parameters (luminance, contrast, and spatial extent) were identical in the two devices. We compared responses from 17 control subjects tested with the FMS stimulator to responses from 14 control subjects tested with the VGA monitor and found no systematic differences in P1 amplitude or latency between the two groups. Therefore, data obtained using the two stimulators have been combined.
Analyses
Responses to the stimuli were processed using the VERIS software (version 6.4.4; EDI) with one iteration of artifact removal and spatial averaging with one-sixth of the surrounding responses. For each subject, responses were combined in six concentric rings. Ring 1 is at the center and includes the response from the fovea. Ring 6 is the most eccentric.20 The amplitude of the first-order kernel11,12 was measured from the baseline to the first negative trough (N1) and from the baseline to the first positive peak (P1) of the waveform. Latency was measured from the start of the trace to the trough of N1 and to the peak of P1. Amplitude and latency of N1 and P1 were evaluated as a function of group (severe ROP, mild ROP, no ROP, term born) and eccentricity (ring 1, 2, 3, 4, 5, 6) using a two-factor repeated-measures ANOVA. Post hoc comparisons were made using the Scheffé test. For all statistical tests, the level of significance was P ≤ 0.05.
Results
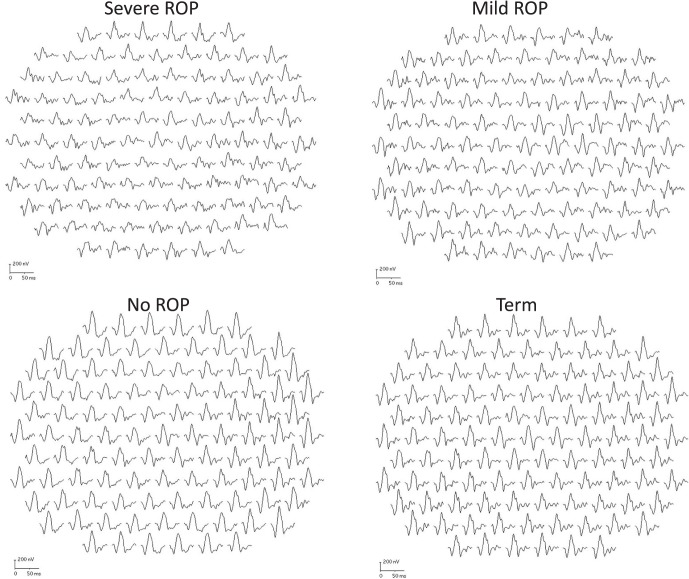
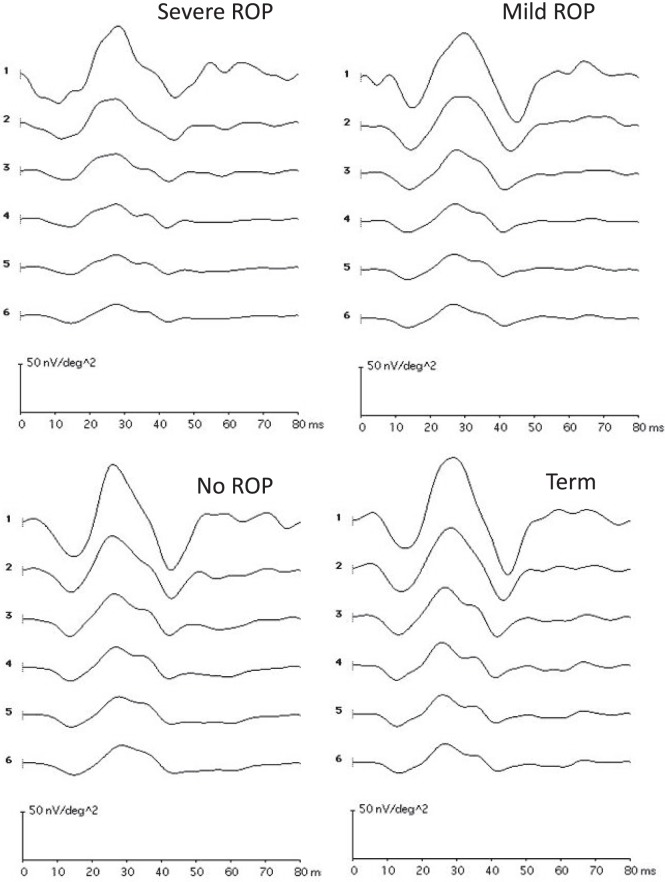
Responses from a representative subject from each group are shown in Figure 1; their ring averages are shown in Figure 2. These subjects had P1 amplitude near the median for their group.
Figure 1.
Sample mfERG records for a representative subject from each group. The 103 first-order responses are shown. Each of these subjects had P1 amplitudes near the median for their group. Calibration bars are the same in all panels.
Figure 2.
The response to the central hexagon (ring 1) and the average of responses to all hexagons in each concentric ring (2 to 6) plotted for the subjects illustrated in Figure 1. Calibration bars are the same in all panels.
Figure 3 shows mean N1 and P1 amplitude as a function of ring number, that is eccentricity, for the four groups. For all groups, amplitude was largest at the center (ring 1) and decreased with eccentricity (ring number). ANOVA showed that N1 amplitude (Fig. 3, upper panel) differed significantly between groups (F = 11.6; df = 3,55; P < 0.001) and varied significantly with eccentricity (F = 134.7; df = 5,275; P < 0.001). The post hoc Scheffé tests showed that N1 amplitude in severe ROP subjects was significantly smaller than in the other groups, and that the other three groups (mild ROP, no ROP, and term) did not differ from each other.
Figure 3.
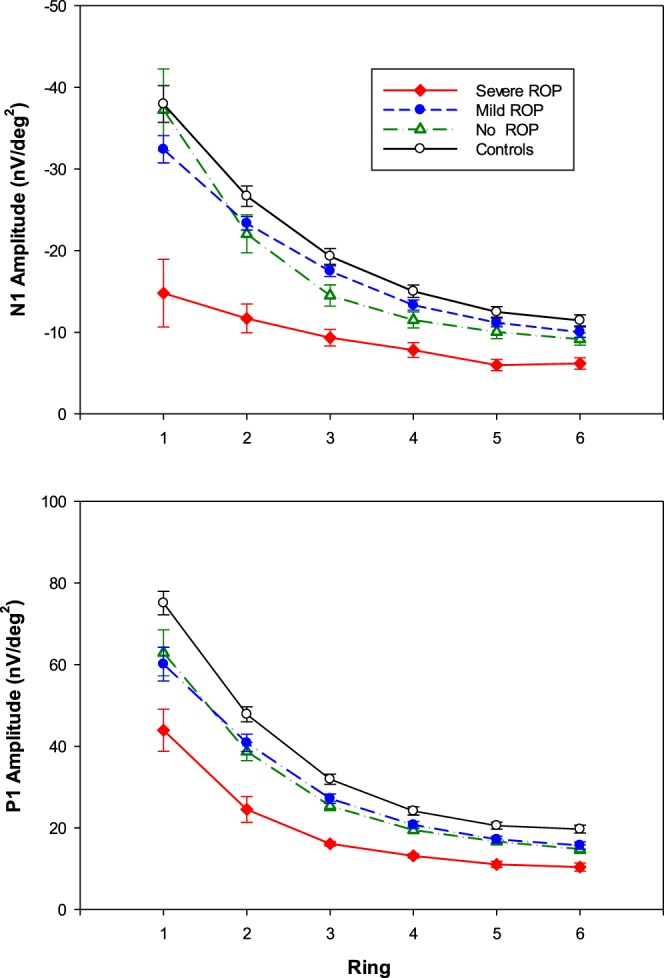
Mean amplitude (±SEM) for each group plotted as a function of eccentricity (ring number) for N1 (upper) and P1 (lower).
P1 amplitude was largest in ring 1 and decreased with eccentricity (Fig. 3, lower panel). ANOVA showed that P1 amplitude varied significantly with group (F = 12.4; df = 3,55; P < 0.001) and eccentricity (F = 346.8; df = 5,275; P < 0.001) and that there was a significant interaction of group and eccentricity (F = 3.5; df = 15,275; P < 0.001). The Scheffé test showed that amplitude in severe ROP subjects was significantly smaller than in any other group. At each of the six eccentricities, responses in no ROP and mild ROP subjects did not differ significantly. P1 amplitude in all three preterm groups (severe ROP, mild ROP, and no ROP) was significantly smaller than that in the term-born control group (Fig. 3, lower panel). Neither N1 nor P1 latency varied significantly with group or eccentricity.
Discussion
The results show that, compared with term-born controls, the amplitude of mfERG responses in prematurely born subjects was significantly reduced even if they never had ROP. This is evidence that the late maturing central retina, which includes the fovea and mediates the mfERG response, is vulnerable to the effects of prematurity. Other evidence of the central retina's vulnerability to prematurity, even in the absence of a history of ROP, has been demonstrated by OCT studies of the macula.8,9 These studies showed thickening of the postreceptor laminae, which is interpretable as a failure of centrifugal migration of postreceptor cells that occurs in normal development.7
The mfERG responses in severe ROP were significantly smaller than in any other group. The attenuated mfERG response in severe ROP cannot be a direct effect of the antecedent laser treatment; there is no physical overlap of the laser spots and the retinal region tested by the mfERG stimulus (radius, 22.5°). The combined impacts of laser, ROP, and prematurity on the mfERG cannot be specified at this time.
We are aware of three prior studies of mfERG responses in subjects with a history of preterm birth.6,8,21 All three studies conclude that mfERG amplitude in preterms is smaller than in term-born controls, but assessment of the effect of ROP severity on the mfERG was not done. The present study was organized to make that comparison. Our 2005 study showed that mfERG amplitude was reduced in mild ROP subjects compared with term-born controls.6 Michalczuk et al.21 found that P1 differed significantly between severe ROP and no ROP subjects only in the most central ring, ring 1. Akerblom et al.8 found that amplitude did not differ between those who had ROP (mild and severe groups combined) and those in the no ROP category. Thus, although it is clear that responses in preterms were smaller than in term born subjects in all studies, it was not possible to evaluate the effect of ROP severity from the previously published data.
The mfERG response is a complex waveform that combines contributions from the cone photoreceptors and potentials from cone ON and OFF bipolar cells of the postreceptor retina.22,23 Photoreceptors are thought to contribute to N1 in the central 6° that includes rings 1 and 2. In this sample, N1 was smallest in severe ROP subjects (Fig. 3). Thus, low N1 amplitude may be a consequence of low cone photoreceptor sensitivity in severe ROP as previously shown by full-field electroretinography.24
The mfERG P1 component is formed by interaction of recovery of the photoreceptor response and potentials associated with depolarization and recovery of cone ON and OFF bipolar cells.22,23 P1 amplitude was lower in the former preterm subjects than in the controls (Fig. 3, lower panel). One possibility is that the reduced P1 amplitude may be a consequence of damage to the cones.11 Recovery of the cone photoreceptor response may be slower than normal in ROP, as it is for rod deactivation.25 We had previously shown by adaptive optics imaging of extrafoveal cones that the optical properties of cones in severe ROP were altered.26 Perhaps the cones are dysmorphic, as were the rods in a rat model of ROP.27 The relative balance of cone driven ON and OFF bipolar activity may be altered by ROP, but evidence of an ON–OFF imbalance was not found by full-field electroretinography.24 Possibly, the imbalance could be elucidated by using other mfERG stimulus sequences.28,29
Our results provide evidence that premature birth alone has an effect on central retinal function and that, among those diagnosed with ROP in infancy, the magnitude of the effect varies with severity of the antecedent ROP. The lack of difference in mfERG amplitude between the mild and no ROP groups is evidence that the effect on the neurosensory retina does not depend solely on categorization of ROP, which is based on appearance of the retinal vasculature at the time of examination in the nursery with no regard to assessment of the neurosensory retina. Investigation of visual threshold in the regions of altered mfERG function may help further define the effects of prematurity on the neurosensory retina. Use of adaptive optics imaging methods7 have identified subclinical changes in the microvasculature in mild ROP subjects. These may well be present subjects without ROP but, to our knowledge, have not been reported.
Acknowledgments
The authors thank Jennifer Bush and Rotem Kimia for assistance.
Supported by National Eye Institute Grant R01 EY-010597 and the Massachusetts Lions Eye Research Fund.
Disclosure: P. Altschwager, None; A. Moskowitz, None; A.B. Fulton, None; R.M. Hansen, None
References
- 1. Hendrickson A,, Possin D,, Vajzovic L,, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012; 154: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hendrickson AE. Primate foveal development: a microcosm of current questions in neurobiology. Invest Ophthalmol Vis Sci. 1994; 35: 3129–3133. [PubMed] [Google Scholar]
- 3. Provis JM,, Diaz CM,, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998; 54: 549–580. [DOI] [PubMed] [Google Scholar]
- 4. Vajzovic L,, Hendrickson AE,, O'Connell RV,, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012; 154: 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Isenberg SJ. Macular development in the premature infant. Am J Ophthalmol. 1986; 101: 74–80. [DOI] [PubMed] [Google Scholar]
- 6. Fulton AB,, Hansen RM,, Moskowitz A,, Barnaby AM. Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol. 2005; 111: 7–13. [DOI] [PubMed] [Google Scholar]
- 7. Hammer DX,, Iftimia NV,, Ferguson RD,, et al. Foveal fine structure in retinopathy of prematurity: an adaptive optics Fourier domain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2008; 49: 2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akerblom H,, Andreasson S,, Holmstrom G. Macular function in preterm children at school age. Doc Ophthalmol. 2016; 133: 151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowl W,, Stieger K,, Bokun M,, et al. OCT-based macular structure–function correlation in dependence on birth weight and gestational age—the Giessen Long-Term ROP Study. Invest Ophthalmol Vis Sci. 2016; 57: OCT235–OCT241. [DOI] [PubMed] [Google Scholar]
- 10. Ecsedy M,, Szamosi A,, Karko C,, et al. A comparison of macular structure imaged by optical coherence tomography in preterm and full-term children. Invest Ophthalmol Vis Sci. 2007; 48: 5207–5211. [DOI] [PubMed] [Google Scholar]
- 11. Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000; 19: 607–646. [DOI] [PubMed] [Google Scholar]
- 12. Sutter EE,, Tran D. The field topography of ERG components in man. I. The photopic luminance response. Vision Res. 1992; 32: 433–446. [DOI] [PubMed] [Google Scholar]
- 13. Screening examination of premature infants for retinopathy of prematurity. A joint statement of the American Academy of Pediatric, the American Association for Pediatric Ophthalmology and Strabismus, and the American Academy of Ophthalmology. Ophthalmology. 1997; 104: 888–889. [PubMed] [Google Scholar]
- 14. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2001; 108: 809–811. [DOI] [PubMed] [Google Scholar]
- 15. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006; 117: 572–576. [DOI] [PubMed] [Google Scholar]
- 16. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123: 991–999. [DOI] [PubMed] [Google Scholar]
- 17. Repka MX, Palmer EA, Tung B; for the Cryotherapy for Retinopathy of Prematurity Cooperative Group. . Involution of retinopathy of prematurity. Arch Ophthalmol. 2000; 118: 645–649. [DOI] [PubMed] [Google Scholar]
- 18. Gerth C,, Sutter EE, Werner JS. mfERG response dynamics of the aging retina. Invest Ophthalmol Vis Sci. 2003; 44: 4443–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molnar AE,, Andreasson SO,, Larsson EK,, Akerblom HM,, Holmstrom GE. Macular function measured by binocular mfERG and compared with macular structure in healthy children. Doc Ophthalmol. 2015; 131: 169–176. [DOI] [PubMed] [Google Scholar]
- 20. Bearse MA, Jr,, Adams AJ,, Han Y,, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006; 25: 425–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Michalczuk M,, Urban B,, Chrzanowska-Grenda B,, Ozieblo-Kupczyk M,, Bakunowicz-Lazarczyk A,, Kretowska M. The assessment of multifocal ERG responses in school-age children with history of prematurity. Doc Ophthalmol. 2016; 132: 47–55. [DOI] [PubMed] [Google Scholar]
- 22. Hare WA,, Ton H. Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Effects of APB, PDA, and TTX on monkey ERG responses. Doc Ophthalmol. 2002; 105: 189–222. [DOI] [PubMed] [Google Scholar]
- 23. Hood DC,, Frishman LJ,, Saszik S,, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002; 43: 1673–1685. [PubMed] [Google Scholar]
- 24. Fulton AB,, Hansen RM,, Moskowitz A. The cone electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008; 49: 814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hansen RM,, Fulton AB. Recovery of the rod photoresponse in infants. Invest Ophthalmol Vis Sci. 2005; 46: 764–768. [DOI] [PubMed] [Google Scholar]
- 26. Ramamirtham R,, Akula JD,, Soni G,, et al. Extrafoveal cone packing in eyes with a history of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2016; 57: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fulton AB,, Reynaud X,, Hansen RM,, Lemere CA,, Parker C,, Williams TP. Rod photoreceptors in infant rats with a history of oxygen exposure. Invest Ophthalmol Vis Sci. 1999; 40: 168–174. [PubMed] [Google Scholar]
- 28. Kondo M,, Miyake Y. Assessment of local cone on- and off-pathway function using multifocal ERG technique. Doc Ophthalmol. 2000; 100: 139–154. [DOI] [PubMed] [Google Scholar]
- 29. Lung JC,, Swann PG,, Chan HH. The multifocal on- and off-responses in the human diabetic retina. PLoS One. 2016; 11: e0155071. [DOI] [PMC free article] [PubMed] [Google Scholar]