Abstract
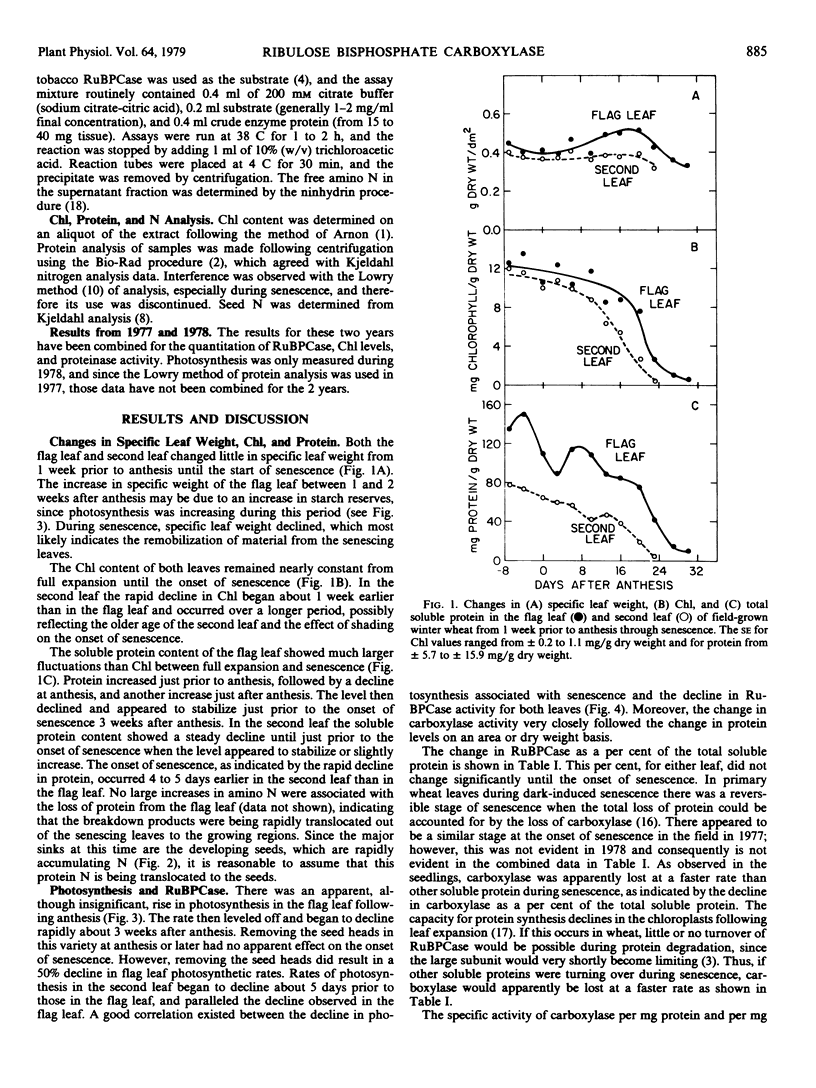
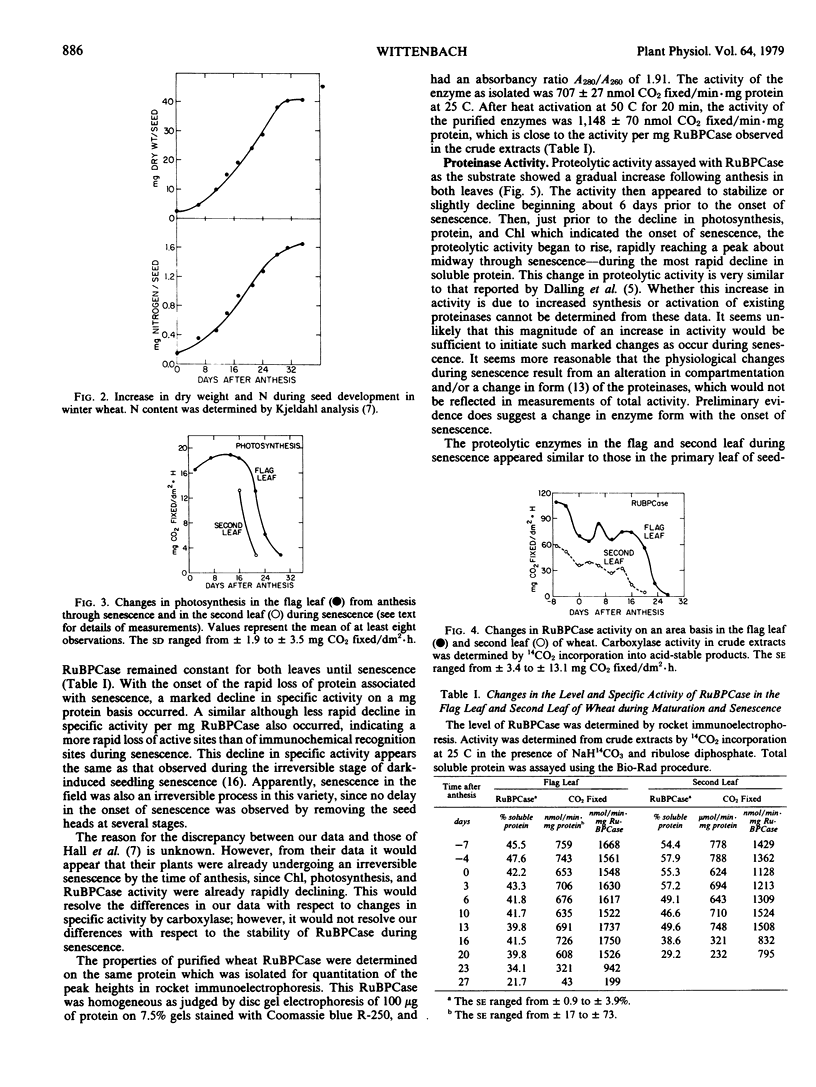
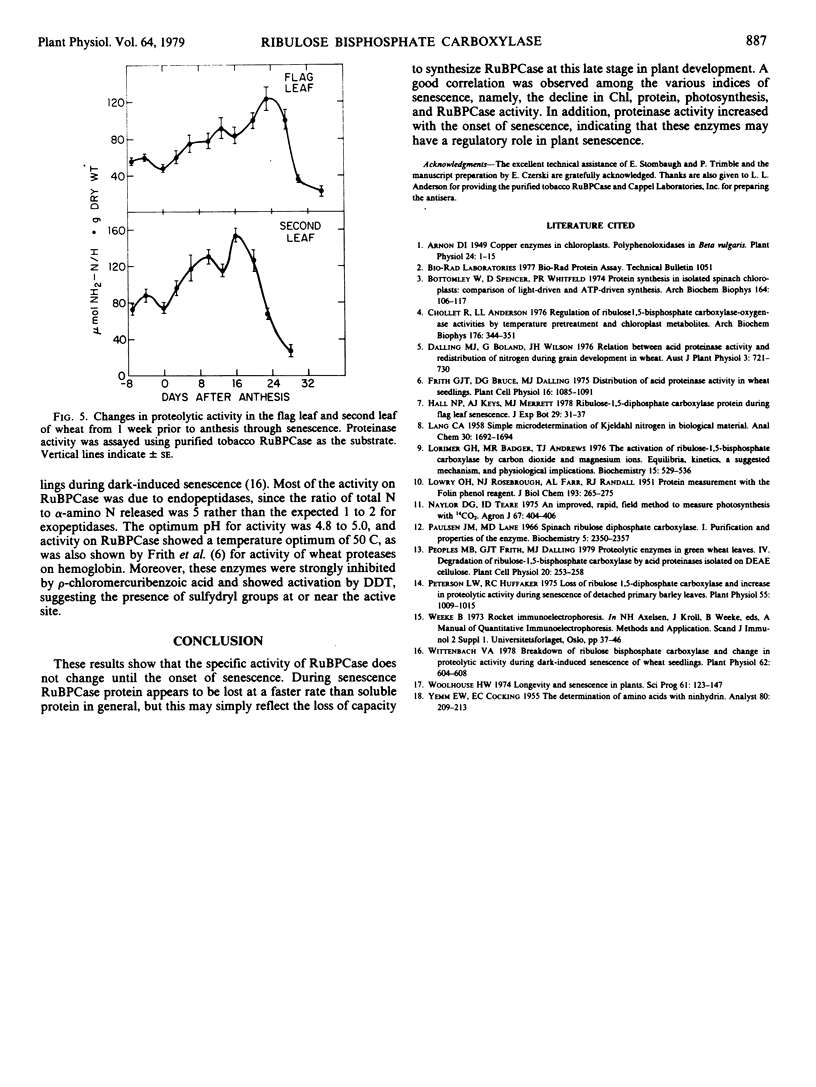
Changes in ribulose bisphosphate carboxylase (RuBPCase) and proteolytic activity were followed in the flag leaf and second leaf of field-grown winter wheat (cv. Arthur). These changes were followed in relation to changes in leaf chlorophyll, protein, and photosynthesis, and seed development. Levels of RuBPCase were determined by rocket immunoelectrophoresis as described previously (Wittenbach 1978 Plant Physiol 62: 604-608). RuBPCase constituted 40 to 45% of the total soluble protein in the flag leaf and an even higher percentage of the soluble protein in the second leaf. This ratio remained unchanged until senescence when RuBPCase protein was apparently lost at a faster rate than total soluble protein. No change in the specific activity of RuBPCase on either a milligram protein or RuBPCase basis was observed until senescence. A close correlation existed among the various indices of senescence in the field, namely, the decline in chlorophyll, protein, photosynthesis, and RuBPCase activity. In addition, proteinase activity increased with the onset of senescence. These enzymes readily degraded RuBPCase, exhibiting a pH optimum of 4.8 to 5.0 and a temperature optimum of 50 C. Proteinase activity was modified by sulfydryl reagents suggesting the presence of sulfydryl groups at or near the active sites.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Chollet R., Anderson L. L. Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch Biochem Biophys. 1976 Sep;176(1):344–351. doi: 10.1016/0003-9861(76)90173-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]



