Abstract
Purpose
Activating FGFR2 mutations have been identified in ~10% of endometrioid endometrial cancers (ECs). We have previously reported that mutations in FGFR2 are associated with shorter disease free survival (DFS) in stage I/II EC patients. Here we sought to validate the prognostic importance of FGFR2 mutations in a large, multi-institutional patient cohort.
Methods
Tumors were collected as part of the GOG 210 clinical trial “Molecular Staging of Endometrial Cancer” where samples underwent rigorous pathological review and had more than three years of detailed clinical follow-up. DNA was extracted and four exons encompassing the FGFR2 mutation hotspots were amplified and sequenced.
Results
Mutations were identified in 144 of the 973 endometrioid ECs, of which 125 were classified as known activating mutations and were included in the statistical analyses. Consistent with FGFR2 having an association with more aggressive disease, FGFR2 mutations were more common in patients initially diagnosed with stage III/IV EC (29/170;17%) versus stage I/II EC (96/803; 12%; p = 0.07, Chi-square test). Additionally, incidence of progression (progressed, recurred or died from disease) was significantly more prevalent (32/125, 26%) among patients with FGFR2 mutation versus wild type (120/848, 14%; p < 0.001, Chi-square test). Using Cox regression analysis adjusting for known prognostic factors, patients with FGFR2 mutation had significantly (p < 0.025) shorter progression-free survival (PFS; HR 1.903; 95% CI 1.177–3.076) and endometrial cancer specific survival (ECS; HR 2.013; 95% CI 1.096–3.696).
Conclusion
In summary, our findings suggest that clinical trials testing the efficacy of FGFR inhibitors in the adjuvant setting to prevent recurrence and death are warranted.
Keywords: Endometrial cancer, FGFR2, Mutation, Outcome, Prognosis
1. Introduction
Endometrial cancer (EC) is responsible for ~76,000 deaths worldwide and has a higher incidence in developed countries due to its association with obesity [1]. The majority of ECs are detected early (75%) and have a relatively good prognosis. However, if a patient presents with metastatic disease, or recurs after initial surgery, their prognosis is very poor, with a median survival of 7–12 months [2]. Although the endometrioid histological subtype is associated with good prognosis compared to other subtypes, due to its prevalence it is still responsible for ~50% of all EC deaths. For a woman diagnosed with early stage EC, a combination of clinicopathological features is currently used to guide decision making as to whether the patient should receive adjuvant therapy following initial surgery. These features include age, stage, histological subtype, tumor grade, depth of myometrial invasion, and tumor cell invasion of lymphatic vessels (lymphovascular space invasion: LVSI) [3]. However, these clinicopathological biomarkers fail to capture the heterogeneity of EC [4].
A recent review summarized promising prognostic and predictive biomarkers in EC [4], however they are not widely applied in the community. The development of tumor specific prognostic markers that could be used for risk stratification and to inform subsequent treatment options is clearly needed for early stage patients. This is especially true given that early stage patients include those patients that have not been surgically staged and for whom stage-specific prognosis is significantly worse. Identification of the most effective therapy for these higher risk patients (e.g. chemotherapy, radiation, or targeted therapy) is also a priority.
Fibroblast growth factor receptor 2 (FGFR2) has been shown to be activated in a number of cancers through a variety of mechanisms including gene amplification, translocations, and point mutations [5]. Our lab was the first to identify FGFR2 mutations, predominantly in the endometrioid histological subtype, which was subsequently confirmed by other groups [6–8]. Preclinical in vitro and in vivo studies in EC cell lines suggest that FGFR2 mutation status is predictive of response to anti-FGFR therapies [7,9,10]. An increasing number of FGFR inhibitors are entering clinical trials for breast, lung, and other cancers [5]. We previously reported that somatic activating FGFR2 mutations were associated with reduced disease free survival (DFS; hazard ratio [HR] = 3.24; 95% confidence interval, [CI] 1.35–7.77; p = 0.008) and overall survival (OS; HR = 2.00; 95% CI 1.09–3.65; p = 0.025) in early stage endometrioid EC (386 stage I and II cases) [6]. In the current study, we sought to validate the prognostic importance of FGFR2 mutations within the endometrioid subtype of EC in a large, multi-institutional cohort of patients with detailed clinical follow-up.
2. Materials and methods
2.1. Tumor samples and patient population
The GOG 210 clinical trial, “Molecular Staging of Endometrial Cancer,” was opened in 2003. In 2007 enrollment was limited to poor prognosis tumors and those occurring among non-obese and non-white patients. GOG 210 enrolled 6124 patients between 2003 and 2011. All participants provided written consent and specimens were prospectively collected at the time of surgery when all patients were comprehensively surgically staged (planned full pelvic and para-aortic lymph node dissection) based on the 1988 FIGO (International Federation of Gynecology and Obstetrics) staging system. Each case was reviewed for eligibility with respect to histological diagnosis and adequate surgical staging; 256 patients were deemed ineligible. Of the remaining 5869 eligible cases, 3713 (63.3%) enrolled during the unrestricted enrollment period. Of these, 2814 patients from 55 institutions had endometrioid histology. Patients in GOG-210 that had been previously analyzed as part of the WUSM cohort [6] were excluded from this study such that it comprises an independent cohort. The GOG Tissue Bank reviewed 1673 cases for tumor quality. All late stage cases (III/IV) and early stage (I/II) cases that recurred (n = 152) plus 841 random samples from early stage cases that did not recur and that had at least 3 years of follow-up were distributed for testing. Where available frozen specimens were used (n = 794). To ensure no bias was introduced by the inclusion of formalin fixed paraffin embedded (FFPE) samples, multiple age, grade, and stage matched samples that did not recur were included for every FFPE case that did recur. DNA extraction was successful from all samples; however, mutation analysis was unsuccessful in 20 samples. As such the patient cohort was comprised of 803 early stage patients (stage I, II) and 170 late stage (stage III/IV) patients. Institutional review boards at Washington University (St Louis, MO, USA), the Translational Genomics Research Institute (Phoenix, AZ, USA), and the Queensland University of Technology (Brisbane, Australia) approved this study.
2.2. Central pathology review
Pathologic diagnoses were made at participating GOG institutions and then reviewed centrally by the GOG Pathology Committee where there was at least two reviewers and structured adjudication of differences of opinion. Surgical stage was determined post-operatively and coded according to FIGO 1988 Staging criteria.
2.3. FGFR2 mutation analysis
Frozen tumor and matched normal tissues were reviewed to identify tumor specimens with high neoplastic cellularity (>60%) and normal myometrium (uninvolved by cancer). DNA was extracted from frozen samples (n = 794) as previously described [6]. For those cases for which FFPE tissues were used (n = 199), areas containing >60% tumor cellularity were manually macrodissected or microdissected (Arcturus PixCell II LCM instrument) prior to DNA extraction using the semi-automated Maxwell® 16 instrument (Promega). Matched normal tissues were similarly dissected and DNAs prepared using the Maxwell® 16 instrument (Promega).
PCR amplification of four exons (7, 10, 13, 15) of FGFR2 corresponding to the location of hotspot mutations was performed using M13 tailed primers. Exon 8 was also sequenced in 300 cases. Additional primers, which amplified smaller fragments, were used to amplify FGFR2 from the FFPE samples (primers available upon request). PCR fragments were then sent to Functional Biosystems (USA) at room temperature where they were cleaned up using an Exo/Sap protocol and sequenced in both directions using Sanger sequencing. Data was analyzed using Sequencher (v 4.0, Gene Codes). An independent PCR reaction was sequenced to validate each mutation. Confirmation of somatic status by sequencing the matched germline DNA was performed for all novel mutations and the majority of cases with hotspot mutations (~65%), and all mutations assessed were indeed somatic.
2.4. Statistical analyses
The relationship between gene mutation and covariates was assessed using Chi-square test, Fisher’s exact test, or Student’s t-test as appropriate. Endometrial cancer specific survival (ECS) was defined as the time from date of entry to death due to disease. Cause of death was based on confirmed death records and where necessary the site nurse and/or CRA queried for resolution on cause of death. All patients with documented relapse who died had confirmed cause of death due to endometrial cancer. Patients who did not die of disease were censored at the date of last contact. Progression-free survival (PFS) was defined as the time from surgery to time of first documented evidence of recurrence or progression. Based on the study protocol, recurrence was defined as discovery of disease not previously present by clinical, radiographic, and/or laboratory means. Progression was defined as 50% or greater increase in the product from any documented lesion, however, histologic confirmation of suspected progressive disease was left to the judgment of the attending physician. Kaplan-Meier product limit method was used to estimate PFS and ECS. Differences in PFS and ECS by mutation status were evaluated by using the log-rank test [11]. Univariate and multivariate Cox proportional hazard models were fitted to assess the effects of known covariates and mutation status on ECS and PFS. Clinically accepted prognostic factors that were significant on univariate analysis were included in the model including age, stage, and tumor grade. All analyses were two-sided and significance was set at a p-value of 0.05. Statistical analyses were performed using SAS 9.3.
3. Results
3.1. Characteristics of the GOG 210 patient cohort
The clinicopathologic characteristics of this GOG 210 patient cohort are consistent with the published literature for the general population. The majority of patients analyzed (83%) presented with early stage disease. The median age at diagnosis was 62 years (IQR: 55–69 years) with the majority of the women diagnosed between 50 and 70 (see Table 1). The distribution of patients across different age groups was consistent with the previously reported SEER data [12]. The patient cohort had a median follow up time of 68 months (IQR: 49–105 months). Thirteen percent of women had a BMI <25 (underweight and normal), 20% had a BMI between 25 and 30 (overweight) and 67% had a BMI above 30 (obese).
Table 1.
Relationship between FGFR2 mutation and clinic-pathological features.
| Characteristic | Value | FGFR2 mutation status
|
p valuea | |||
|---|---|---|---|---|---|---|
| Wild type
|
Mutant
|
|||||
| N | % | N | % | |||
| Age (years)b | <70 | 646 | 88.3 | 86 | 11.8 | 0.07 |
| ≥70 | 202 | 83.8 | 39 | 16.2 | ||
| BMIc | <25 | 109 | 83.9 | 21 | 16.2 | 0.22 |
| ≥25 | 735 | 87.7 | 103 | 12.3 | ||
| Race | Black | 51 | 96.2 | 2 | 5.5 | 0.10 |
| White | 760 | 86.5 | 119 | 13.5 | ||
| Other | 37 | 90.2 | 4 | 9.8 | ||
| Stage (FIGO 1988) | Early (I or II) | 707 | 88.0 | 96 | 12.0 | 0.07 |
| Late (III or IV) | 141 | 82.9 | 29 | 17.1 | ||
| Graded | 1 | 336 | 87.3 | 49 | 12.7 | 0.71 |
| 2 | 370 | 87.9 | 51 | 12.1 | ||
| 3 | 140 | 85.4 | 24 | 14.6 | ||
Chi-square test.
Age cut-off of 70 years is based on previous data showing age > 70 is associated with poorer outcomes [6].
BMI not available for 5 cases.
Tumor not graded for 3 cases.
3.2. Prevalence and spectrum of FGFR2 mutations
FGFR2 mutations were identified in 144/973 (15%) tumors investigated. Although the majority occurred at known mutational hotspots, the remaining mutations presumably include a proportion of “passenger” mutations attributable to the higher mutational load found in ECs with microsatellite instability (MSI) or carrying a somatic POLE mutation [8]. As such, the mutations have been characterized into those that are “known activating,” “putatively activating” and variants of unknown significance (VUS) where patients with the latter mutations were not included in the outcome analyses (Table 4).
Table 4.
Mutations Identified In Endometrial Cancer Patients.
| FGFR2 Mutation | # cases | Germline Syndrome | Functional data References | Species conservn | FGFR conservn | Mutation Assessor | PolyPhen2 | Reported as somatic in a cancer associated with FGFR dependence |
|---|---|---|---|---|---|---|---|---|
| Known Activating | ||||||||
| S252Wa | 64 | FGFR2 | [7,13–15] | Y | Y | Medium | Prob. Damaging | [6–8] |
| N550K b,c | 20 | FGFR2/3 | [7,16] | Y | Y | Neutral | Prob. Damaging | [6,7,17–20] |
| N550H | 4 | FGFR2/3 | [16] | Y | Y | Low | Prob. Damaging | [6,8] |
| N550T | 2 | [16] | Y | Y | Neutral | Prob. Damaging | ||
| N550D | 1 | Y | Y | Low | Prob. Damaging | [20] | ||
| C383R | 14 | FGFR1 | [7,21] | Y | Y | medium | Poss. Damaging | [6,7,18,19,22–25] |
| K660Ed | 7 | FGFR3 | [26] | Y | Y | low | Prob. Damaging | [6,8] |
| K660M | 1 | FGFR3 | [26] | Y | Y | low | Prob. Damaging | [7] |
| K660R | 1 | [26] | Y | Y | neutral | Prob. Damaging | ||
| Y376C | 6 | FGFR1/2/3 | [27,28] | Y | Y | medium | Prob. Damaging | [6,22,29] |
| P253R | 4 | FGFR2 | [7,13–15] | Y | Y | Low | Benign | [6–8] |
| G385R | 1 | FGFR2 | [30] | Y | Y | medium | Prob. Damaging | [30] |
| Putative Activating | ||||||||
| F276E | 1 | FGFR2 | Y | Y | High | Poss. Damaging | [22] | |
| A380S | 1 | Y | N | low | Poss. Damaging | |||
| A380T | 1 | Y | N | low | Poss. Damaging | |||
| Y382D | 1 | FGFR2 | [31] | Y | Y | Medium | Prob. Damaging | [32] |
| M392R | 2 | FGFR2 | [31] | Y | 1/2 | Medium | Poss. Damaging | [6] |
| V396D | 2 | N | 1/2/3 | Medium | Prob. Damaging | [6,18] | ||
| I548V | 1 | Y | Y | Neutral | Poss. Damaging | [6] | ||
| D651Y | 1 | Y | Y | low | Prob. Damaging | [33] | ||
| Variants of unknown Significance | ||||||||
| R251X | 1 | Y | Y | Truncation | Truncation | |||
| V274I | 1 | Y | Y | low | Prob. Damaging | |||
| V294M e | 1 | Y | N | low | Poss. Damaging | |||
| V311I e | 1 | Y | N | low | Benign | |||
| p.E378_C383delInsR | 1 | In/del | In/del | |||||
| p.I388_M391Idel | 1 | In/del | In/del | |||||
| D627Y | 1 | Y | Y | High | Prob. Damaging | |||
| A629E | 1 | Y | Y | High | Prob. Damaging | |||
| E637K | 1 | Y | Y | neutral | Prob. Damaging | |||
| L551I b | 1 | Y | Y | low | Prob. Damaging | |||
| L551F c | 1 | Y | Y | low | Prob. Damaging | |||
| V294La | 1 | Y | N | neutral | Benign | |||
| N653S d | 1 | Y | N | neutral | Benign | |||
One tumor carried a S252W and V294L mutation.
One tumor carried a N550K and L551I mutation.
One tumor carried a N550K and L551F mutation.
One tumor carried a K660E and N653S mutation.
MSI with MLH1 methylation.
Known activating mutations include all those mutations that occurred at codons previously identified as mutation “hotspots” [6,7] (Table 4). Many of these mutations have been functionally studied to determine how they result in receptor activation. The G385R mutation was included in this category of “known activating” mutations as this mutation has been reported in a patient with sporadic craniosynostosis [34], and the homologous mutation in FGFR3 has been identified in a multiple myeloma cell line where functional studies showed it was weakly activating [30]. The majority of sequence changes (125/144; 87%) occurred at one of these seven codons.
Another eight somatic mutations were defined as “putatively activating”. Although mutations were examined using mutation assessor and PolyPhen-2 (Table 4), it is difficult to determine with any certainty whether a particular missense change is likely to result in receptor activation, especially for those mutations in the transmembrane region where no structural data is available. As such we defined mutations as “putatively activating” if they had occurred in two independent cancer patients but there was either limited or no functional data. In some cases a mutation had been reported in another EC patient or in a patient with another cancer also characterized by FGFR2 activation (e.g. cholangiocarcinomas, ameloblastomas). We included two mutations where the homologous mutation in FGFR3 had been reported in bladder cancer, which is associated with FGFR3 activation. Also included in this category were two mutations associated with Bent bone dysplasia, which have been reported to show features associated with both loss and gain of receptor function [31] (Table 4).
Thirteen mutations were classified as VUS (Table 4). These included a nonsense mutation in the extracellular domain (R251*), a predicted loss of function missense mutation affecting the HRD consensus in the kinase domain (D627Y), two in-frame transmembrane deletions, and five other missense mutations for which no other data was available to support their role as potentially activating. Only two of these occurred in tumors with MLH1 methylation and MSI (Table 4). The remaining three mutations did not show a POLE mutation signature however they were designated VUS based on their low frequency and lack of functional data. This category included four additional novel mutations that were found in patients also carrying a known activating mutation (S252 W + V294 L, K660E + N653S, N550 K + L551I, and N550 K + L551F).
3.3. FGFR2 mutations are associated with poor outcomes
FGFR2 mutation showed a trend towards being more prevalent among advanced age (≥70 years) patients (16% vs. 12%, chi-square p value = 0.07). Consistent with FGFR2 having an association with more aggressive disease, FGFR2 mutations were more common in patients initially diagnosed with stage III/IV EC (29/170; 17%) versus stage I/II EC, although this did not reach statistical significance (96/803; 12%; p = 0.07, Chi-square test) (Table 1).
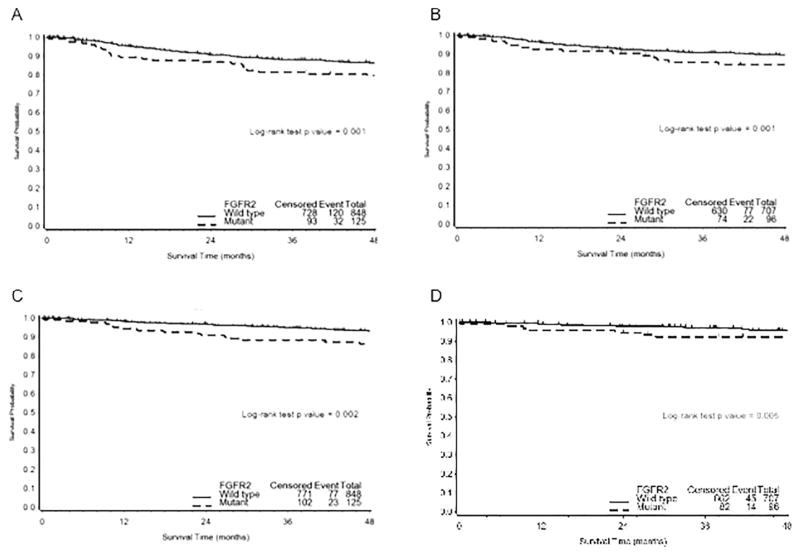
Additionally, incidence of progression (progressed, recurred or died from disease) was significantly more prevalent (32/125, 26%) among patients with FGFR2 mutations versus wild type (120/848, 14%), (Chi-square test p < 0.001). Consistent with previous studies [35] Cox proportional hazard regression analysis identified increasing age, later stage (III, IV), and higher tumor grade as unfavorable prognostic factors relative to PFS, as well as ECS (Table 2). In addition, univariate analysis demonstrated, activating mutations in FGFR2 to be independently prognostic for worse outcome, that is shorter PFS (HR 1.867; 95% CI 1.264–2.758; p = 0.002) and ECS (HR 2.075; 95% CI 1.303–3.307; p = 0.002). Similar analyses were carried out including the additional 10 cases defined as putatively activating with similar results, albeit with a slight reduction in significance (Table 2). In multivariate analysis, adjusting for known prognostic factors age, stage, and grade, the relative risk of failure was significantly greater among patients with FGFR2 mutation. Specifically, patients with FGFR2 mutation had significantly (p < 0.025) shorter PFS (HR 1.584; 95% CI 1.063–2.361) and ECS (HR 1.665; 95% CI 1.032–2.687) (Table 2). The Kaplan Meier survival plot for ECS survival according to activating FGFR2 mutation status is presented in Fig. 1. FGFR2 mutations were significantly associated with shorter PFS (log rank test, p = 0.001) (data not shown) and decreased ECS (log rank test, p = 0.004) in the total cohort of 973 patients. In those patients with grade 3 disease recurrence/progression was seen in 54/140 (38%) patients with wildtype FGFR2 and 11/24 (46%) patients with mutant FGFR2.
Table 2.
Univariate and multivariate outcome analysis of all EC patients (n = 973).
| Univariate analysis | Disease-free survival (DFS)
|
EC survival (ECS)
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | Pa | HR | 95% CI | pa | |
| Age (years.) | 1.030 | 1.014–1.045 | <0.001 | 1.031 | 1.012–1.050 | 0.001 |
| Race (ref = white) | ||||||
| Black | 1.435 | 0.776–2.653 | 0.250 | 1.369 | 0.634–2.953 | 0.424 |
| Other | 0.972 | 0.429–2.203 | 0.947 | 1.005 | 0.369–2.738 | 0.992 |
| Stage (ref = 1A/1B) | ||||||
| IC or II | 1.632 | 1.088–2.447 | 0.018 | 1.820 | 1.082–3.059 | 0.024 |
| III or IV | 3.343 | 2.313–4.832 | <0.001 | 4.492 | 2.860–7.053 | <0.001 |
| Grade (ref = 1 Well) | ||||||
| 2 Moderate | 1.623 | 1.077–2.444 | 0.021 | 1.791 | 1.033–3.107 | 0.038 |
| 3 Poor | 3.736 | 2.434–5.736 | <0.001 | 5.528 | 3.208–9.527 | <0.001 |
| FGFR2 (ref = Wild type) | ||||||
| “known activating” | 1.867 | 1.264–2.758 | 0.002 | 2.075 | 1.303–3.307 | 0.002 |
| FGFR2 (ref = WT) | ||||||
| “known activating + putative” | 1.682 | 1.139–2.484 | 0.009 | 1.878 | 1.179–2.993 | 0.008 |
| Multivariable analysis | ||||||
| Age (years) | 1.030 | 1.014–1.046 | <0.001 | 1.032 | 1.012–1.052 | 0.002 |
| Stage (ref = IA/IB) | ||||||
| IC/II | 1.333 | 0.883–2.011 | 0.171 | 1.443 | 0.852–2.442 | 0.172 |
| III/IV | 2.504 | 1.698–3.693 | <0.001 | 3.125 | 1.940–5.034 | <0.001 |
| Grade (ref = 1 Well) | ||||||
| 2 Moderate | 1.487 | 0.986–2.243 | 0.059 | 1.606 | 0.925–2.790 | 0.093 |
| 3 Poor | 2.936 | 1.888–4.565 | <0.001 | 4.061 | 2.319–7.112 | <0.001 |
| FGFR2b (ref = WT) | ||||||
| ”known activating” | 1.584 | 1.063–2.361 | 0.024 | 1.665 | 1.032–2.687 | 0.0368 |
WT = wild type.
Wald test.
Multivariate model adjusting for age, stage and tumor grade.
Fig. 1. Kaplan-Meier curves for survival by FGFR2 mutation status.
A–B. Progression-free survival (PFS) and endometrial specific survival (ECS) by FGFR2 status (known activating mutation versus wild type) in the cohort of 973 endometrioid patients. C–D. Progression-free survival (PFS) and endometrial specific survival (ECS) by FGFR2 status (known activating mutation versus wild type) in the cohort of 803 early stage endometrioid patients. Vertical bars represent censored cases.
The utility of FGFR2 mutation in early stage disease, where prognostic biomarkers are needed most, was evaluated (Table 3). Among patients with stage I/II disease, activating mutations were shown to be independently associated with shorter PFS (HR 2.141; 95% CI 1.333–3.3439; p = 0.002) and ECS (HR 2.302; 95% CI 1.263–4.194; p = 0.007). Similar results were obtained when patients carrying “known + putative” activating mutations were analyzed (Table 3). The association between known activating mutations and poorer outcomes remained when multivariate analysis was performed, revealing that activating mutations were associated with shorter PFS (HR 1.903; 95% CI 1.177–3.076; p = 0.009) and ECS (HR 2.013; CI 95% 1.096–3.696; p = 0.024).
Table 3.
Univariate and multivariate analysis for stage I/II EC patients (n = 803).
| Univariate analysis | Progression-free survival (DFS)
|
Endometrial Cancer survival (ECS)
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | pa | HR | 95% CI | pa | |
| Age (years) | 1.035 | 1.016–1.054 | <0.001 | 1.036 | 1.011–1.061 | 0.004 |
| Race (ref = white) | ||||||
| Black | 1.787 | 0.900–3.549 | 0.097 | 1.963 | 0.844–4.567 | 0.117 |
| Other | 0.528 | 0.130–2.146 | 0.372 | 0.130 | – | – |
| Stage (ref = IA/IB) | ||||||
| IC or II | 1.634 | 1.090–2.451 | 0.018 | 1.823 | 1.084–3.064 | 0.024 |
| Grade (ref = 1 Well) | ||||||
| 2 Moderate | 1.302 | 0.834–2.033 | 0.245 | 1.136 | 0.618–2.085 | 0.682 |
| 3 Poor | 2.028 | 1.167–3.524 | 0.012 | 2.973 | 1.545–5.724 | 0.001 |
| FGFR2 (ref = WT) | ||||||
| ”known activating” | 2.141 | 1.333–3.439 | 0.002 | 2.302 | 1.263–4.194 | 0.007 |
| FGFR2 (ref = WT) | ||||||
| “known activating + putative” | 1.927 | 1.200–3.095 | 0.007 | 2.077 | 1.140–3.785 | 0.017 |
| Multivariable analyses | ||||||
| Age (years) | 1.032 | 1.013–1.051 | 0.001 | 1.032 | 1.008–1.057 | 0.001 |
| Stage (ref = IA/IB) | ||||||
| IC/II | 1.359 | 0.898–2.057 | 0.147 | 1.465 | 0.861–2.492 | 0.159 |
| Grade (ref = 1 Well) | ||||||
| 2 Moderate | 1.257 | 0.805–1.964 | 0.314 | 1.068 | 0.581–1.963 | 0.832 |
| 3 Poor | 1.882 | 1.078–3.285 | 0.026 | 2.755 | 1.422–5.338 | 0.003 |
| FGFR2b (ref = WT) | ||||||
| “known activating” | 1.903 | 1.177–3.076 | 0.009 | 2.013 | 1.096–3.696 | 0.024 |
Wald test.
Multivariate model adjusting for age (>70), stage (IC/II) and tumor grade (grade 3).
4. Discussion
Current risk stratification of EC patients is not ideal, with recurrences estimated to occur in ~15% of patients with grade 1/2 tumors who are often not offered adjuvant therapy, as well as significant morbidity in EC patients with grade 3 tumors who receive adjuvant therapy but carry a low risk of their tumor recurring. Molecular profiling of endome-trial cancer by TCGA has revealed that there are 3 subtypes within the endometrioid histological subtype of EC that differ in their somatic mutational load and have corresponding differences in their prognosis [8].
We have analyzed FGFR2 from a large series of patients enrolled in the GOG 210 multi-institutional clinical trial focused on specimen banking for future molecular analyses. In addition to the large number of cases, several other characteristics of the study are notable including 1) samples were prospectively collected, 2) each sample underwent rigorous pathological review within the GOG Tissue Bank to confirm diagnosis and ensure it had sufficient tumor cellularity prior to DNA extraction, and 3) detailed follow-up for at least 3 years was available for all cases.
The most significant difference between the current analyses and that previously reported is that in the GOG 210 patient cohort, FGFR2 mutations were found at a similar frequency across all three grades whereas in the WUSM cohort, they were significantly less common in grade 3 endometrioid endometrial cancers [6]. Although both patient cohorts were graded based on the 1988 FIGO grading system, it is well accepted that quantification of the percentage of solid growth is open to inter-observer variability near the diagnostic cut-points between grades, as is the qualitative scoring of nuclear atypia [36]. We suspect that this new finding of FGFR2 mutations in 15% of poorly differentiated ECs is due to differences in grading by the pathologists involved and we believe the multi-institutional cohort data is more reliable. The frequency of FGFR2 hotspot mutations detected in the TCGA cohort with whole exome sequencing (22/248; 9%) was lower than we identified in this study, however this is likely due to differences in the patient population. The TCGA cohort was primarily composed of prospectively collected early stage patients whereas this cohort of GOG-210 patients had been enriched to include all late stage cases and those early stage cases that recurred.
One of the main advantages of the GOG 210 cohort is that detailed clinical follow-up is available which allows us to test for an association between FGFR2 mutation status and ECS. Indeed, the current study found that patients carrying an activating FGFR2 mutation were twice as likely to die from their disease compared to patients with wildtype FGFR2 (HR 2.075; 95% CI 1.303–3.307; p = 0.002), which remained significant when other poor prognosis features were taken into account in multivariate analysis (HR 1.67; 95% CI 1.03–2.69; p = 0.037). In the PORTEC 1/2 molecular risk stratification study where MSI and mutation status in 14 genes was assessed, FGFR2 mutations were found almost exclusively in the MSI (9%) and copy number low/NSMP (no specific molecular profile) subtypes (12%) [37]. In the latter study, FGFR2 mutation was not associated with recurrence or overall survival, whereas the presence of TP53 mutations (characteristically associated with the serous histological subtype) did predict recurrence and reduced overall survival, confirming the molecular subgroups proposed by TCGA. The GOG-210 cohort differs from the PORTEC 1/2 cohort in that tumors with mixed or serous histology were excluded and stage III/IV tumors were included. Given the finding within the PORTEC cohort that FGFR2 mutations occur almost exclusively in the MSI and NSMP subtypes, the data presented herein suggests that FGFR2 mutation status could possibly further stratify patients with poor prognoses within these latter subtypes. The findings described here are clinically relevant as EC patients often present with other comorbidities and it shows that patients diagnosed with FGFR2 mutation positive EC are indeed dying due to their disease rather than from other comorbidities. This provides important clinical data supporting the testing of more specific FGFR inhibitors in this patient population.
A wide variety of in vitro and in vivo data support a role for FGFR2 signaling in driving cell migration. FGFR2 has been shown to be essential for keratinocyte migration both in vivo using conditional knockout mice as well as in vitro using keratinocytes derived from these mice [38]. FGF7 and FGF10, which only bind to FGFR2, have also been shown to drive migration and/or invasion in a variety of tissue types and our lab has also shown that FGF10 stimulation of FGFR2 in the Ishikawa endometrial cancer cell line drives migration and invasion (unpublished data). We therefore hypothesize that FGFR2 mutation-positive EC have an increased ability to form micro-metastases outside the uterus, which results in the significantly shorter PFS seen in these patients following their initial surgical treatment.
Molecular biomarkers can either be diagnostic or prognostic and/or predictive of response to a certain therapy. There have been several studies showing that EC cell lines with FGFR2 mutations are more sensitive to FGFR inhibition [7,9,10]. Dovitinib, a “first generation” multi-kinase inhibitor with anti-FGFR activity has been assessed in a Phase II trial in endometrial cancer patients with and without somatic FGFR2 mutations [39]. Similar activity was seen in both arms suggesting the anti-angiogenic activity of dovitinib was responsible for these responses. Although longer lasting responses were seen in patients with FGFR2 mutant tumors (~20 months) versus the non-mutant group (~9 months) this might reflect the different histological subtypes included within the two arms, rather than the FGFR activity of dovitinib, as more serous and clear cell EC were included in the non-mutant arm. This would be consistent with the lack of “on target” side effects including hyperphosphatemia and tissue calcification seen in the dovitinib trial. These side effects are characteristically seen with the “second generation” more specific FGFR inhibitors currently being evaluated in Phase I/II trials in multiple other FGFR-dependent malignancies [5]. Perhaps early signals of efficacy may come from “basket trials” open to patients with any solid malignancy with aberrations in FGFR1, FGFR2 or FGFR3, and where EC patients whose tumors carry FGFR2 activating mutations may enroll.
Following the identification of the most effective and best tolerated FGFR inhibitor in patients with metastatic disease, we propose that administration of an FGFR inhibitor in the adjuvant setting following initial surgery might show a significant benefit with respect to ECS, reminiscent of trastuzumab in breast cancer [40]. Treating patients with FGFR2 mutation positive EC with anti-FGFR agents at this earlier stage is expected to be more effective due to the reduced burden of tumor cells in the patient and less tumor heterogeneity, resulting in a decrease in the emergence of acquired resistance. We propose the following approach to assess the efficacy of FGFR inhibition to reduce the risk of recurrence of high-risk EC: 1) identify an effective FGFR inhibitor in the metastatic setting (perhaps, even in other FGFR dependent cancers); 2) combine FGFR inhibition with contemporary radiation protocols and compare to radiation therapy alone; 3) stratify by MSI and copy number low/NSMP molecular subtypes. Given the frequency of these cases, this is likely to require an international multi-site clinical trial in order to facilitate recruitment.
HIGHLIGHTS.
FGFR2 mutations are more common in patients with late stage endometrioid EC.
FGFR2 mutations are more common in patients with recurrent endometrioid EC.
FGFR2 mutations are associated with shorter PFS and EC specific death.
Acknowledgments
This study was supported by National Cancer Institute grants to PJG and PMP (R21 CA133295, P50 CA1342540) as well as to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), the GOG Tissue Bank (U10 CA27469, U24 CA114793, and U10 CA180868), and the NRG Oncology Group (U10 CA180822). PMP has been supported by an NHMRC career development fellowship. In addition, this research was supported in part by funds provided by the intramural research program of the National Cancer Institute, National Institutes of Health.
The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, Northwestern University, University of Mississippi, University of Colorado-Anschutz Cancer Pavilion, University of California at Los Angeles, Fred Hutchinson Cancer Research Center, Penn State Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center, Indiana University Medical Center, Wake Forest University Health Sciences, University of California Medical Center at Irvine – Orange Campus, Magee Women’s Hospital – University of Pittsburgh Medical Center, University of New Mexico, Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council/Ohio State University, University of Massachusetts Memorial Health Care, Fox Chase Cancer Center, Women’s Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago, Mayo Clinic, Case Western Reserve University, Moffitt Cancer Center and Research Institute, Yale University, University of Wisconsin Hospital, Women and Infants’ Hospital of Rhode Island, The Hospital of Central Connecticut at New Britain General, GYN Oncology of West Michigan, PLLC and Community Clinical Oncology Program.
Footnotes
Conflicts of interest
Drs. Pollock and Goodfellow are listed as inventors of two patents involving the detection of FGFR2 mutations for diagnostic or prognostic purposes in endometrial cancer. Drs. Pollock and Powell have received compensation as a member of a scientific advisory board for Novartis and Eisai respectively. The remaining authors declare no conflicts of interests.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet] International Agency for Research on Cancer; Lyon, France: 2015. [Google Scholar]
- 2.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, Gotlieb WH, Hoskins PJ, Ghatage P, Tonkin KS, Mackay HJ, Mazurka J, Sederias J, Ivy P, Dancey JE, Eisenhauer EA. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC clinical trials group. J Clin Oncol. 2011;29:3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Werner HM, Salvesen HB. Current status of molecular biomarkers in endometrial cancer. Curr Oncol Rep. 2014;16:403. doi: 10.1007/s11912-014-0403-3. [DOI] [PubMed] [Google Scholar]
- 5.Hierro C, Rodon J, Tabernero J. Fibroblast growth factor (FGF) receptor/FGF inhibitors: novel targets and strategies for optimization of response of solid tumors. Semin Oncol. 2015;42:801–819. doi: 10.1053/j.seminoncol.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Byron SA, Gartside M, Powell MA, Wellens CL, Gao F, Mutch DG, Goodfellow PJ, Pollock PM. FGFR2 point mutations in 466 endometrioid endometrial tumors: relationship with MSI, KRAS, PIK3CA, CTNNB1 mutations and clinicopathological features. PLoS One. 2012;7:e30801. doi: 10.1371/journal.pone.0030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, Nicoletti R, Winckler W, Grewal R, Hanna M, Wyhs N, Ziaugra L, Richter DJ, Trovik J, Engelsen IB, Stefansson IM, Fennell T, Cibulskis K, Zody MC, Akslen LA, Gabriel S, Wong KK, Sellers WR, Meyerson M, Greulich H. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105:8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byron SA, Gartside MG, Wellens CL, Mallon MA, Keenan JB, Powell MA, Goodfellow PJ, Pollock PM. Inhibition of activated fibroblast growth factor receptor 2 in endometrial cancer cells induces cell death despite PTEN abrogation. Cancer Res. 2008;68:6902–6907. doi: 10.1158/0008-5472.CAN-08-0770. [DOI] [PubMed] [Google Scholar]
- 10.Konecny GE, Kolarova T, O’Brien NA, Winterhoff B, Yang G, Qi J, Qi Z, Venkatesan N, Ayala R, Luo T, Finn RS, Kristof J, Galderisi C, Porta DG, Anderson L, Shi MM, Yovine A, Slamon DJ. Activity of the fibroblast growth factor receptor inhibitors dovitinib (TKI258) and NVP-BGJ398 in human endometrial cancer cells. Mol Cancer Ther. 2013;12:632–642. doi: 10.1158/1535-7163.MCT-12-0999. [DOI] [PubMed] [Google Scholar]
- 11.Bland J, Altman D. The Log Rank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin K. SEER Cancer Statistics Review, 1975–2011. N.C. Institute; Bethesda, MD: 2014. http://seer.cancer.gov/csr/1975_2011/ (based on November 2013 SEER data submission, posted to the SEER web site, April 2014) [Google Scholar]
- 13.Ibrahimi OA, Eliseenkova AV, Plotnikov AN, Yu K, Ornitz DM, Mohammadi M. Structural basis for fibroblast growth factor receptor 2 activation in Apert syndrome. Proc Natl Acad Sci U S A. 2001;98:7182–7187. doi: 10.1073/pnas.121183798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahimi OA, Chiu ES, McCarthy JG, Mohammadi M. Understanding the molecular basis of Apert syndrome. Plast Reconstr Surg. 2005;115:264–270. [PubMed] [Google Scholar]
- 15.Yu K, Herr AB, Waksman G, Ornitz DM. Loss of fibroblast growth factor receptor 2 ligand-binding specificity in Apert syndrome. Proc Natl Acad Sci U S A. 2000;97:14536–14541. doi: 10.1073/pnas.97.26.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Ma J, Li W, Eliseenkova AV, Xu C, Neubert TA, Miller WT, Mohammadi M. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol Cell. 2007;27:717–730. doi: 10.1016/j.molcel.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gatius S, Velasco A, Azueta A, Santacana M, Pallares J, Valls J, Dolcet X, Prat J, Matias-Guiu X. FGFR2 alterations in endometrial carcinoma. Mod Pathol. 2011;24:1500–1510. doi: 10.1038/modpathol.2011.110. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney RT, McClary AC, Myers BR, Biscocho J, Neahring L, Kwei KA, Qu K, Gong X, Ng T, Jones CD, Varma S, Odegaard JI, Sugiyama T, Koyota S, Rubin BP, Troxell ML, Pelham RJ, Zehnder JL, Beachy PA, Pollack JR, West RB. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014;46:722–725. doi: 10.1038/ng.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell MA, Sill MW, Goodfellow PJ, Benbrook DM, Lankes HA, Leslie KK, Jeske Y, Mannel RS, Spillman MA, Lee PS, Hoffman JS, McMeekin DS, Pollock PM. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2014;135:38–43. doi: 10.1016/j.ygyno.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, Brown C, Pugh TJ, Stojanov P, Cho J, Lawrence MS, Getz G, Bragelmann J, DeBoer R, Weichselbaum RR, Langerman A, Portugal L, Blair E, Stenson K, Lingen MW, Cohen EE, Vokes EE, White KP, Hammerman PS. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Mangasarian K, Mansukhani A, Basilico C. Activation of FGF receptors by mutations in the transmembrane domain. Oncogene. 1997;14:1397–1406. doi: 10.1038/sj.onc.1200983. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJ, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470–1473. doi: 10.1038/ng.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS, Elenitoba-Johnson KS, Betz BL. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517–5526. doi: 10.1158/1078-0432.CCR-14-1069. [DOI] [PubMed] [Google Scholar]
- 24.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, McKenna A, Carter SL, Cibulskis K, Sivachenko A, Saksena G, Voet D, Ramos AH, Auclair D, Thompson K, Sougnez C, Onofrio RC, Guiducci C, Beroukhim R, Zhou Z, Lin L, Lin J, Reddy R, Chang A, Landrenau R, Pennathur A, Ogino S, Luketich JD, Golub TR, Gabriel SB, Lander ES, Beer DG, Godfrey TE, Getz G, Bass AJ. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet. 2013;45:478–486. doi: 10.1038/ng.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, Leary A, Edmondson RJ, Powell ME, Crosbie EJ, Kitchener HC, Mileshkin L, Pollock PM, Smit VT, Creutzberg CL. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–844. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Huang Z, Dutta K, Blais S, Neubert TA, Li X, Cowburn D, Traaseth NJ, Mohammadi M. Cracking the molecular origin of intrinsic tyrosine kinase activity through analysis of pathogenic gain-of-function mutations. Cell Rep. 2013;4:376–384. doi: 10.1016/j.celrep.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byron SA, Gartside MG, Wellens CL, Goodfellow PJ, Birrer MJ, Campbell IG, Pollock PM. FGFR2 mutations are rare across histologic subtypes of ovarian cancer. Gynecol Oncol. 2010;117:125–129. doi: 10.1016/j.ygyno.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Robertson SC, Meyer AN, Hart KC, Galvin BD, Webster MK, Donoghue DJ. Activating mutations in the extracellular domain of the fibroblast growth factor receptor 2 function by disruption of the disulfide bond in the third immunoglobulin-like domain. Proc Natl Acad Sci U S A. 1998;95:4567–4572. doi: 10.1073/pnas.95.8.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephens PJ, Davies HR, Mitani Y, Van Loo P, Shlien A, Tarpey PS, Papaemmanuil E, Cheverton A, Bignell GR, Butler AP, Gamble J, Gamble S, Hardy C, Hinton J, Jia M, Jayakumar A, Jones D, Latimer C, McLaren S, McBride DJ, Menzies A, Mudie L, Maddison M, Raine K, Nik-Zainal S, O’Meara S, Teague JW, Varela I, Wedge DC, Whitmore I, Lippman SM, McDermott U, Stratton MR, Campbell PJ, El-Naggar AK, Futreal PA. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. 2013;123:2965–2968. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ronchetti D, Greco A, Compasso S, Colombo G, Dell’Era P, Otsuki T, Lombardi L, Neri A. Deregulated FGFR3 mutants in multiple myeloma cell lines with t(4;14): comparative analysis of Y373C, K650E and the novel G384D mutations. Oncogene. 2001;20:3553–3562. doi: 10.1038/sj.onc.1204465. [DOI] [PubMed] [Google Scholar]
- 31.Merrill AE, Sarukhanov A, Krejci P, Idoni B, Camacho N, Estrada KD, Lyons KM, Deixler H, Robinson H, Chitayat D, Curry CJ, Lachman RS, Wilcox WR, Krakow D. Bent bone dysplasia-FGFR2 type, a distinct skeletal disorder, has deficient canonical FGF signaling. Am J Hum Genet. 2012;90:550–557. doi: 10.1016/j.ajhg.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Knowles E, Hernandez S, Malats N, Kogevinas M, Lloreta J, Carrato A, Tardon A, Serra C, Real FX. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66:7401–7404. doi: 10.1158/0008-5472.CAN-06-1182. [DOI] [PubMed] [Google Scholar]
- 33.Lott S, Wang MS, Zhang SB, MacLennan GT, Lopez-Beltran A, Montironi R, Sung MT, Tan PH, Cheng L. FGFR3 and TP53 mutation analysis in inverted urothelial papilloma: incidence and etiological considerations. Mod Pathol. 2009;22:627–632. doi: 10.1038/modpathol.2009.28. [DOI] [PubMed] [Google Scholar]
- 34.Pulleyn LJ, Reardon W, Wilkes D, Rutland P, Jones BM, Hayward R, Hall CM, Brueton L, Chun N, Lammer E, Malcolm S, Winter RM. Spectrum of craniosynostosis phenotypes associated with novel mutations at the fibroblast growth factor receptor 2 locus. Eur J Hum Genet. 1996;4:283–291. doi: 10.1159/000472215. [DOI] [PubMed] [Google Scholar]
- 35.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a gynecologic oncology group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 36.Clarke BA, Gilks CB. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63:410–415. doi: 10.1136/jcp.2009.071225. [DOI] [PubMed] [Google Scholar]
- 37.Stelloo E, Nout RA, Osse EM, Jurgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Nijman HW, Putter H, Bosse T, Creutzberg CL, Smit VT. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016;22:4215–4224. doi: 10.1158/1078-0432.CCR-15-2878. [DOI] [PubMed] [Google Scholar]
- 38.Meyer M, Muller AK, Yang J, Moik D, Ponzio G, Ornitz DM, Grose R, Werner S. FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin. J Cell Sci. 2012;125:5690–5701. doi: 10.1242/jcs.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konecny GE, Finkler N, Garcia AA, Lorusso D, Lee PS, Rocconi RP, Fong PC, Squires M, Mishra K, Upalawanna A, Wang Y, Kristeleit R. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2-non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol. 2015;16:686–694. doi: 10.1016/S1470-2045(15)70159-2. [DOI] [PubMed] [Google Scholar]
- 40.Recondo G, Diaz Canton E, de la Vega M, Greco M, Recondo G, Valsecchi ME. Therapeutic options for HER-2 positive breast cancer: perspectives and future directions. World J Clin Oncol. 2014;5:440–454. doi: 10.5306/wjco.v5.i3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]