HMGB1 in the late phase of sepsis plays a specific role in the development of post-sepsis immunosuppression, and specifically neutrophil dependent antibacterial defense mechanisms.
Keywords: NADPH oxidase, sepsis, HMGB1, neutrophils, immunosuppression
Abstract
Sepsis is accompanied by the initial activation of proinflammatory pathways and long-lasting immunosuppression that appears to contribute to late-occurring mortality. Although high-mobility group box 1 (HMGB1) is involved in many aspects of inflammation, its role in sepsis-induced immune suppression remains unclear. In this study, we examined HMGB1’s contribution to neutrophil NADPH oxidase activity dysfunction and associated neutrophil-dependent bacterial clearance in mice subjected to sepsis and in patients who survive septic shock. Using a murine model of polymicrobial septic peritonitis, we demonstrated that treatment with anti-HMGB1 Ab significantly diminished sepsis-induced dysfunction of neutrophil NADPH oxidase activity. In a subsequent set of experiments, we found that blocking HMGB1 preserved the ability of neutrophils from patients recovering from septic shock to activate NADPH oxidase. Taken together, our data suggest that HMGB1 accumulation in the late phase of sepsis plays a specific role in the development of postsepsis immunosuppression and specifically affects neutrophil-dependent antibacterial defense mechanisms. Thus, blocking HMGB1 may be a promising therapeutic intervention to diminish the adverse effects of sepsis-induced immunosuppression.
Introduction
Sepsis is one of the most frequent causes of morbidity and mortality in the ICU and affects more than 1 million critically ill patients each year in the United States alone [1]. Although activation of the innate immune system is an essential step in bacterial clearance, the initial proinflammatory response that accompanies sepsis subsequently evolves into immunoparalysis, also known as immunosuppression [2–4]. In particular, critically ill patients who have chronic critical illness (>2 wk) often progress to persistent immunosuppression that is characterized by enhanced apoptosis of lymphocytes and immune cell dysfunction [4–6]. It has been suggested that preservation of immune function in septic patients, as well as recovery from immunosuppression, will result in improved outcomes [2, 7]. Despite advances in clinical and translational research, therapeutic interventions for sepsis are limited to use of antibiotics and fluid resuscitation, as specific pharmacological treatment is not available for this detrimental condition. Similarly, there are no effective pharmacologic approaches to accelerate the recovery of dysfunctional neutrophils, monocytes, dendritic cells, and lymphocytes from sepsis-induced immunosuppression [1, 8–11]. Of note, loss of phagocytic function and diminished microbial killing during severe infection are associated with deficient production of ROS, which has adverse effects on dissemination of existing infection and increased susceptibility to nosocomial infections and viral reactivation [12–14].
Neutrophils and macrophages are essential cell populations responsible for bacterial eradication, primarily through production of anti-bacterial peptides, cytokines, and ROS/RNS [13, 15]. Among antimicrobial mediators, activation of NADPH oxidase is a key antimicrobial mechanism linked to high superoxide output, known as the respiratory burst [16, 17]. NADPH oxidase consists of several subunits that form an active membrane-bound or soluble complex, assembled in response to specific signals triggered by microbial or fungal products [18]. Dysfunction in NADPH oxidase has serious adverse effects on neutrophil-dependent bacterial clearance in experimental models of intra-abdominal polymicrobial sepsis and in patients with chronic granulomatous disease [16, 19, 20]. It is important to note that, although NADPH oxidase is activated during the initial phase of polymicrobial sepsis, this event is followed by impairment of PMN-dependent ROS production at later time points [12]. The exact mechanism responsible for development of such neutrophil dysfunction, which also affects newly produced neutrophils, is not well understood [21], but is implicated in delayed or poor recovery of immune homeostasis in sepsis survivors.
We have recently shown that appearance of DAMP proteins, in particular the HMGB1 protein, diminishes neutrophil-dependent bacterial killing in models of sepsis [17]. HMGB1, originally described as a nuclear nonhistone DNA-binding protein, has subsequently been shown to be an alarmin, and involved in the inflammatory response [22–24]. HMGB1 released from dying cells promotes proinflammatory activation of immune cells contributing to organ injury in polymicrobial sepsis and sterile inflammatory conditions associated with trauma and hemorrhage [23, 25–28]. Although plasma levels of HMGB1 are increased during sepsis, substantial amounts of HMGB1 are also present in the circulation of patients after severe infection for extended periods [23, 27]. We hypothesize that such prolonged accumulation of extracellular HMGB1 promotes development of immunosuppression related to dysfunction of neutrophil NADPH oxidase and diminished bacterial clearance in mice subjected to sepsis and in patients who survive septic shock.
MATERIAL AND METHODS
Patients and control participants
This study was performed in the infectious diseases department and ICU at Rennes University Hospital. The study design was approved by our ethics committee (CHU Rennes, no. 13-8 and no. 15.44-2) and informed consent was obtained. Patients admitted to our ICU for septic shock (septic patients) or for other types of shock (control patients) were included. Pregnant women, patients who were younger than 18 yr, patients with malignancy, HIV-infected patients, and patients receiving immunosuppressive agents were excluded. Standard criteria were used for the diagnosis of septic shock (a clinical construct of infection with persisting hypotension requiring vasopressors to maintain mean arterial pressure >65 mm Hg) [1]. Patients under vasopressor therapy admitted for reasons other than septic shock were also included as the control. The following data were recorded: age, reason for admission, length of stay, mortality, and occurrence of nosocomial infection. Nosocomial infections were defined as already described [29]. To investigate the late phase of sepsis, patients were included when vasopressor therapy (mainly norepinephrine) was stopped and blood samples were collected. Thus, postsepsis is defined as weaning from vasopressor therapy. The decision to include patients after weaning of vasopressor therapy is based on clinical data that demonstrate that patients admitted for septic shock could be divided into 2 groups in relation to mortality: early mortality (until d 5) and late mortality [30]. Furthermore, ROS/RNS generation in neutrophils is significantly higher in patients with sepsis at admission and decreases dramatically after 1 wk [12, 31].
Human PMN isolation and culture.
PMNs were purified with whole blood CD15 microbeads (Miltenyi Biotec, Gladbach, Germany) as previously described [32]. The purified fraction, ≥95% cell purity as evaluated by flow cytometry (Galios; Beckman Coulter, Roissy, France), was used for further experiments. PMNs were cultured in RPMI 1640 containing 10% FBS. In investigating the late phase of sepsis, we included patients when vasopressor therapy was stopped and blood samples were collected.
Measurement of ROS.
PMNs were incubated with or without plasma followed by stimulation with PMA (10 µM, Sigma-Aldrich, Lyon, France) for 15 min. To detect ROS production, PMNs were incubated with H2DCFDA probes (10 µM; Thermo Fisher Scientific, Waltham, MA, USA) for 30 min. Quantification of ROS production was determined by flow cytometry. Results were expressed as the ratio of mean fluorescence intensity obtained from PMA-treated and untreated PMNs. An HMGB1-neutralizing Ab was purchased from IBL International GmbH (Hamburg Germany).
Expression of RAGE and TLR4 receptors on change in PMN count during the course of sepsis.
Expression of RAGE and TLR-4 was measured at admission and after resolution of sepsis by real-time quantitative PCR. cDNA synthesis was performed with Superscript II reverse transcriptase and random hexamers (Thermo Fisher Scientific). For real-time qPCR, we used assay-on-demand primers and probes, and Taqman Universal Master Mix (Thermo Fisher Scientific). Gene expression was measured with StepOnePlus (Thermo Fisher Scientific) based on the ∆Ct calculation method. 18S was determined to be the appropriate internal standard gene by TaqMan Endogenous Control Assays (Thermo Fisher Scientific). Quantification of RAGE and TLR4 was performed with appropriate assay-on-demand primers and probes. For each sample, the Ct for the gene of interest was determined and normalized to its respective value for 18S, and results were then standardized by comparison to gene expression of PMNs at admission.
PMN bacterial killing assay.
PMNs (106 cells) were incubated with Escherichia coli (2 × 106) or MSSA (2 × 106) for 16 h. Cells were lysed with Triton X-100 (0.1%), and serial dilutions were incubated on agar plates overnight at 37°C. The amounts of viable bacteria colonies were calculated as colony-forming units (CFU).
Mice
Male C57BL/6 mice were purchased from the U. S. National Cancer Institute (Frederick, MD, USA). Male mice, 8–10 wk of age, were used for the experiments. The mice were kept on a 12 h light–dark cycle with free access to food and water. All experiments were conducted in accordance with protocols approved by the University of Alabama at Birmingham Animal Care and Use Committee.
Reagents.
RPMI 1640 was purchased from BioWhittaker (Walkersville, MD, USA). FBS and penicillin-streptomycin were obtained from Gemini Bioproducts (Calabasas, CA, USA). HBSS was purchased from Thermo Fisher Scientific. Custom Ab mixtures and negative-selection columns for PMN isolation were from StemCell Technologies (Vancouver, BC, Canada). Anti-phospho-p40phox (Thr154) Ab was purchased from Cell Signaling Technology (Danvers, MA, USA). Polyclonal Abs to neutralize HMGB1 were prepared as described [33]. PMA, E. coli 0111:B4 endotoxin (LPS), and IgG were purchased from Sigma-Aldrich.
CLP-induced sepsis.
Animals underwent CLP or sham laparotomy. In brief, a midline abdominal incision was made in animals under isoflurane anesthesia. In animals that underwent CLP, the cecum was exposed and ligated in the middle, below the ileocecal valve and punctured once with a 21-gauge needle. A small amount of fecal matter was squeezed out of the cecum to induce polymicrobial peritonitis [34]. The cecum was then returned to the abdominal cavity, and the abdominal wall was closed in layers. Antibiotic therapy (25 mg/kg imipenem) was initiated 4 h after CLP or sham surgery and administered by subcutaneous injection every 12 h for 5 d. Saline for fluid resuscitation was administered to create a more clinically relevant sepsis model, as this is standard care for humans. Mice were then euthanized 7 d after surgery and bone marrow PMNs were isolated (Fig. 1A). In a second set of experiments, mice underwent CLP, and neutralizing anti-HMGB1 Ab (125 µg, 500 µl PBS) or IgG was administrated with antibiotics for 5 d. Mice were then euthanized 7 d after surgery, and bone marrow PMNs were isolated.
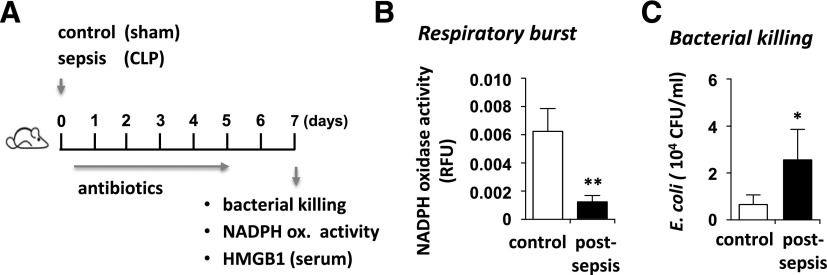
Figure 1. Mice subjected to intra-abdominal polymicrobial sepsis developed neutrophil dysfunction.
(A) Mice subjected to CLP or sham surgery were treated with antibiotics for 5 d, followed by measurement of neutrophil function at d 7. (B) Extent of NADPH oxidase activation in PMA-stimulated bone marrow neutrophils obtained from control (sham) or CLP mice. (C) Bacterial clearance was determined after culture of neutrophils (CLP or control) with E. coli for 16 h (n = 6). Means ± sd. *P < 0.05; **P < 0.01.
PMN isolation and culture.
Bone marrow PMNs were isolated as described previously [17]. PMNs were cultured in RPMI 1640 medium containing 5% FBS and treated as indicated in the figure legends. PMN viability under experimental conditions was determined with trypan blue staining and was consistently greater than 95%.
Assay for NADPH activity.
NADPH oxidase activity was measured with a standard cytochrome c reduction assay [17]. Briefly, PMNs (5 × 105/ml) were incubated with cytochrome c (10 µM) in the presence or absence of E. coli (106/ml) or HMGB1 (300 ng/ml) in 1 ml HBSS. The rate of cytochrome c reduction was recorded with a spectrophotometer (UV-2501PC; Shimadzu; Shimadzu, Japan) for 15 min.
In vitro killing activity assay.
PMNs (0.5 × 106) were incubated with ampicillin-resistant E. coli DH5α (1 × 106) in RPMI 1640 medium (1 ml) without serum for 2, 6, and 16 h at 37°C. Next, 20 µl of cell/bacterial suspension was incubated with 480 µl Triton X-100 (0.1%) for 10 min to lyse the PMNs. Serial dilutions were then plated on agar plates with ampicillin and incubated overnight at 37°C. The number of bacterial colonies on agar plates was determined with colony counting software (Bio-Rad, Hercules, CA, USA).
Western blot analysis.
Western blot analysis was performed as described elsewhere [33]. In brief, equal amounts of protein were resolved in 8 or 12% SDS polyacrylamide gels and transferred onto PVDF membranes (Immobilon-P; Millipore, Billerica, MA). To measure the amount of total and phosphorylated proteins, membranes were probed with specific antibodies followed by detection with HRP-conjugated goat anti-rabbit IgG. Bands were visualized by ECL (ECL Plus; GE Health Care, Vélizy-Villacoublay, France) and quantified by AlphaEase FC software (Alpha Innotech, San Leandro, CA, USA). Each experiment was performed 2 or more times.
Statistical analyses
Statistical analyses were performed with Prism 6.0 software (Graph Pad, San Diego, CA, USA) with the nonparametric Wilcoxon test for matched pairs or the Mann-Whitney nonparametric U test, as appropriate for continuous variables. Proportions were compared between groups by the χ2 test or Fisher exact test, when required.
RESULTS
Deficient oxidative burst and diminished bacterial killing is associated with neutrophil dysfunction in experimental sepsis
To examine the nature of neutrophil dysfunction during sepsis, mice were subjected to CLP or sham surgery. Similar to antibiotic regimens administered to critically ill patients, the mice were treated with imipenem (25 mg/kg) each day for 5 d (Fig. 1A). Early administration of antibiotics and fluid resuscitation resulted in marked improvement of viability, give that 100% of mice on imipenem survived at least 7 d after CLP, whereas those without antibiotics incurred 80% mortality within 48 h [34] (data not shown). The extent of immunosuppression was determined by measurement of neutrophil oxidative burst (e.g., NADPH oxidase-derived superoxide formation), in PMA-stimulated bone marrow neutrophils that were isolated from mice 7 d after the sham procedure or CLP. Significant reduction in NADPH oxidase activation was found in postsepsis neutrophils, as compared with those obtained from the sham group (Fig. 1B). Along with dysfunctional oxidative burst, postsepsis neutrophils also had diminished capacity to kill bacteria (Fig. 1C).
HMGB1 inhibits neutrophil NADPH oxidase activation and bacterial killing
Development of postsepsis immunosuppression is associated with prolonged accumulation of HMGB1 in plasma (Fig. 2A). We further examined whether HMGB1 affects oxidative burst activity in the presence of bacteria. Inclusion of HMGB1 effectively reduced NADPH oxidase activation in the presence of E. coli (Fig. 2B). Next, to establish the importance of HMGB1 in regulating sepsis-mediated neutrophil dysfunction, septic mice were injected with HMGB1-neutralizing Ab or isotype-specific (control) IgG. We found that the HMGB1-neutralizing Ab effectively prevented inactivation of NADPH oxidase in mice subjected to sepsis (Fig. 2C).
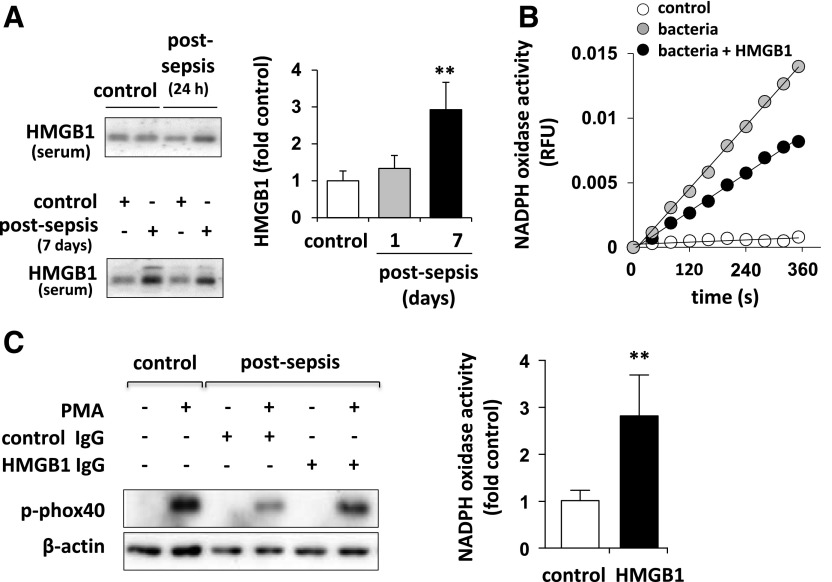
Figure 2. HMGB1 diminished neutrophil oxidative burst.
(A) Western blot and quantitative analysis showed the amount of HMGB1 in mouse sera obtained before or 24 h or 7 d after CLP or sham (n = 5). Means ± sd; **P < 0.01. (B) Representative rates of cytochrome c reduction showed NADPH oxidase inactivation in neutrophils treated with HMGB1 (0 or 300 ng/ml) for 15 min. The oxidative burst was determined after inclusion of E. coli (106/ml) for 15 min. (C) Sham mice and mice with CLP-induced sepsis were treated with HMGB1-neutralizing Ab (125 µg) or control isotype-specific IgG for (5 d). NADPH oxidase subunit p-phox40 (left) or oxidative burst (right) was than determined in bone marrow neutrophils stimulated with PMA (0 or 10 nM) for 15 min (n = 3). Means ± sd; **P < 0.01.
NADPH oxidase requires phosphorylation and assembly of several subunits, including activatory phosphorylation of p40phox [19]. A robust increase of p40phox phosphorylation occurs promptly after stimulation of neutrophils (control) with PMA. However, there was little to no p40phox phosphorylation in neutrophils isolated 7 d after CLP (Fig. 2C). Of note, PMA-induced p40phox phosphorylation was preserved in neutrophils isolated from mice treated with CLP and anti-HMGB1 Ab, but not in neutrophils obtained from mice subjected to sepsis and control IgG (Fig. 2C). These results suggest that HMGB1 plays an important role in acquisition of the neutrophil immunosuppressive phenotype that is characterized by diminished oxidative burst.
Deficient oxidative burst and bacterial killing persist in PMNs of patients who survive septic shock
To establish whether loss of bacterial killing is associated with dysfunctional NADPH oxidase, oxidative burst was measured in PMNs obtained from patients after septic shock or from either healthy donors or critically ill patients without sepsis (control patient) (Fig. 3A). A total of 33 patients, including 21 with septic shock [infective endocarditis (n = 6; 29%), pneumonia (n = 5; 23%), urinary tract infection (n = 3; 14%), intra-abdominal infection (n = 3; 14%), bacteremia (n = 2; 10%), and meningitis (n = 2; 10%)] and 12 critically ill without sepsis (control patients) [adult respiratory distress syndrome (ARDS; n = 5; 42%), cardiogenic shock (n = 2; 17%), status epilepticus (n = 2; 17%), cardiac arrest (n = 2; 17%), and stroke (n = 1; 8%)], were prospectively enrolled and compared with 16 healthy control participants. The duration of vasopressor therapy in septic and control patients was (median [IQR]) 6 d [5–10] in septic patients and 4 d [3.75–8.75] in control patients. Characteristics of patients included in the study are shown in Table 1.
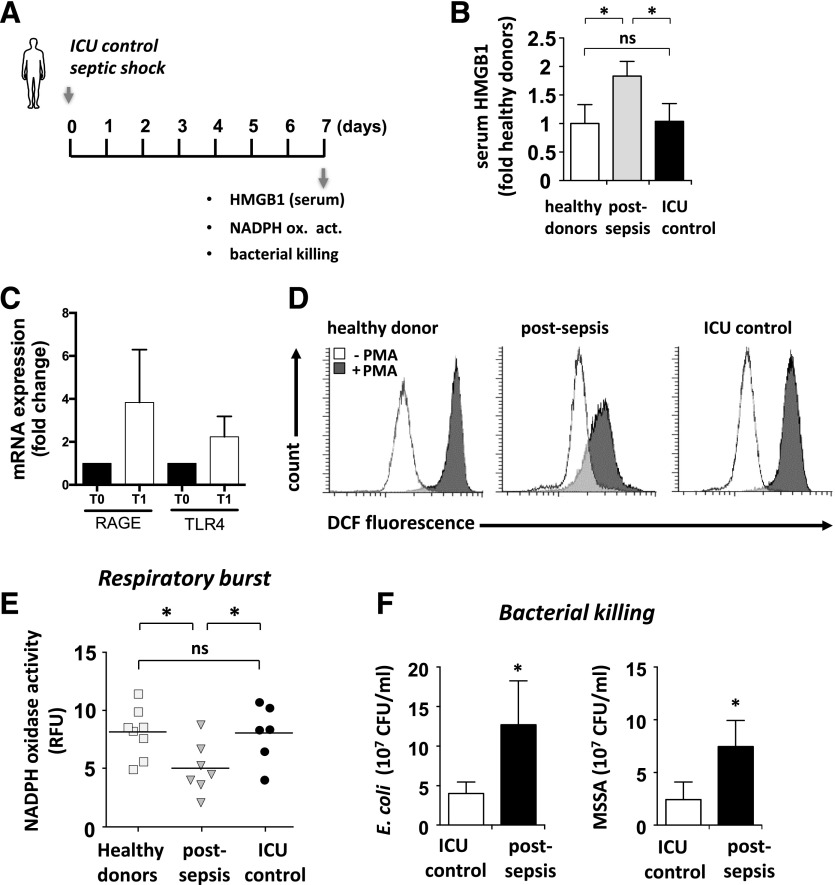
Figure 3. Human PMNs have diminished NADPH oxidase activation and ability to kill bacteria in patients who survived septic shock.
(A) PMNs purified from blood of normal patients or of patients in ICU without sepsis or 7 d after septic shock. (B) The amount of HMGB1 was determined in plasma of healthy donors, patients without sepsis, or patients after septic shock (n = 6–8). Means ± sd; *P < 0.05. (C) The extent of RAGE and TLR4 mRNA expression was determined in PMNs of control and postsepsis patients. RQ-PCR was performed on neutrophils at admission (T0) and when vasopressor therapy was stopped (T1) to analyze RAGE and TLR4 expression. Each sample was normalized to 18S expression and compared to expression levels on T0 neutrophils. (D) PMNs purified from blood of normal patients or patients in ICU without sepsis or 7 d after septic shock were loaded with H2DCF-DA and treated with PMA (0 or 10 μM) for 30 min. Measurement of ROS production occurred before and after stimulation with PMA (10 µM) for 15 min. Representative FACS analyses (left) showed the extent of ROS production in unaltered (white) or stimulated (gray) PMNs. (E) Measurement of ROS production after stimulation with PMA (10 nM) for 15 min. PMA-stimulated NADPH oxidase activation was determined in PMNs isolated from patients with septic shock, patients without sepsis, and healthy donors (right) (n = 6–7). Means ± sd; *P < 0.05. (F) PMNs (106 cells) obtained from healthy or postsepsis patients were incubated with E. coli or MSSA for 16 h and the amount of viable bacteria determined with a CFU assay (n = 6–7). Means ± sd; *P < 0.05).
TABLE 1.
Patient characteristics
| Characteristic | Postsepsis patients (n = 21) | Critically ill nonseptic patients (n = 12) | P |
|---|---|---|---|
| Age, median years [IQR] | 63 [49–69] | 62 [57.8–71.8] | 0.79 |
| Outcome, n (%) | 0.37 | ||
| Survivor | 15 (71) | 10 (83) | |
| Nonsurvivor | 6 (29) | 2 (17) | |
| Nosocomial infections, n (%) | 6 (29) | 3 (25) | 0.58 |
| Length of stay in ICU, median days [IQR] | 11 [8–29] | 13 [8.8–28.5] | 0.63 |
Circulating levels of HMGB1 are elevated for prolonged periods in survivors of sepsis and in preclinical models of polymicrobial infection [23, 35] and in patients in our study (Fig. 3B). Expression of RAGE products and TLR4, 2 receptors for HMGB1 on neutrophils, was increased during the course of sepsis (Fig. 3C). PMNs loaded with the redox-sensitive H2DCFDA fluorogenic probe [36, 37] were treated with PMA to stimulate NADPH oxidase, and DCF fluorescence was measured by flow cytometry. Neutrophils of healthy or critically ill patients without sepsis had robust activation of NADPH oxidase (Fig. 3D). However, this response was reduced in neutrophils of patients after septic shock. A decrease in NADPH oxidase activity was also associated with diminished bacterial killing; this effect was shown in neutrophils of patients who had sepsis or a nonseptic critical illness, cultured with E. coli or MSSA (Fig. 3E).
HMGB1 promotes dysfunction of neutrophils after sepsis
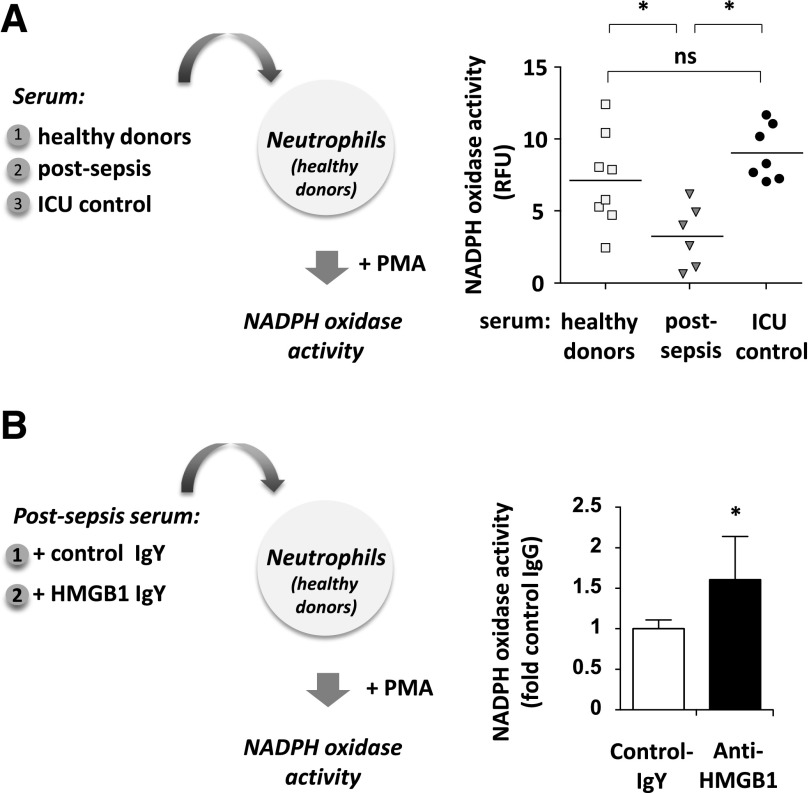
It is not known whether prolonged accumulation of extracellular HMGB1 contributes to complications after sepsis, including increased susceptibility to secondary infections. Neutrophils isolated from healthy donors had a substantial loss of oxidative burst when cultured with plasma from patients after sepsis, as compared to exposure to plasma from healthy donors or critically ill patients without sepsis (Fig. 4A). Because HMGB1 effectively diminished NADPH oxidase activation in mice subjected to polymicrobial sepsis, we also examined whether similar immunosuppressive effects mediated by HMGB1 are found in patients who survive septic shock. To test this possibility, neutrophils of healthy donors were cultured with plasma obtained from patients after sepsis in the presence or absence of HMGB1 neutralizing Ab or control isotype-specific IgY. Anti-HMGB1 Ab increased the ability of neutrophils to activate NADPH oxidase when compared to control IgY (Fig. 4B). These results suggest that postsepsis circulating levels of HMGB1 in patients are implicated in the development of immunosuppression characterized by diminished activity of neutrophil NADPH oxidase.
Figure 4. HMGB1 in postsepsis plasma promoted dysfunction of PMN oxidative burst.
(A) Healthy donor PMNs were incubated for 30 min with plasma of healthy patients or plasma of those with or without sepsis, and then oxidative burst was determined after exposure to PMA (n = 6–8) Means ± sd; *P < 0.05. (B) Plasma obtained from postsepsis patients was treated with HMGB1-neutralizing Ab (50 µg) or IgG (isotype control) for 2 h, and the treated plasma was used in culture with healthy donor PMNs for an additional 30 min. The oxidative burst was measured after inclusion of PMA (0 or 10 µM) for 15 min (n = 6). Means ± sd; *P < 0.05.
DISCUSSION
In the present studies, increased amounts of circulating HMGB1 have substantial impact on the development of neutrophil immunosuppressive phenotypes in a mouse model of intra-abdominal polymicrobial sepsis and in patients who survive septic shock. In particular, we found that exposure of neutrophils to HMGB1 directly suppressed the PMA- or E. coli-induced oxidative burst and the ability of neutrophils to eradicate bacteria. In turn, neutralization of HMGB1 effectively prevented dysfunction of neutrophils and preserved their capacity for bacterial clearance. We also demonstrated that circulating amounts of HMGB1 were sufficient to diminish neutrophil oxidative burst in patients who survived septic shock. Moreover, our data indicate that neutrophils obtained from healthy or critically ill patients without sepsis developed a phenotype consistent with immunosuppression, as defined by diminished oxidative burst and bacterial killing, after culture with plasma from patients recovering from sepsis, despite an increase in the expression of RAGE and TLR4 in PMNs of patients who survive septic shock. The inhibitory effects of septic plasma on neutrophil activation were preventable upon inclusion of anti-HMGB1-neutralizing Ab.
Although recent studies have shown improved survival of patients in the first several days after sepsis [38], there remain multiple long-term complications that accompany this condition, including frailty, cognitive impairment, and diminished ability to contain ongoing infections, as well as an increased risk of acquiring nosocomial bacterial, viral, and fungal infections [39, 40]. Although most studies have focused on the detrimental effects of activated PMNs during the proinflammatory phase of sepsis, less is known about mechanisms linked to loss of antibacterial action by existing and newly produced PMNs at later times after severe infection. Of note, experimental studies have shown impairment of innate immune responses, especially dysfunction of neutrophil oxidative burst and increased susceptibility to secondary infections (e.g., P. aeruginosa) after the induction of severe infection [41].
Our previous study suggested that HMGB1 interferes with NADPH oxidase functional assembly, including phosphorylation of p40phox and showed that interaction between HMGB1 and RAGE effectively suppresses NADPH oxidase activation in neutrophils [17]. Such findings are consistent with the results obtained in our current study, which showed the ability of HMGB1 to diminish oxidative burst and bacterial clearance by neutrophils isolated from mice with sepsis or from patients recovering from septic shock. Of note, we found that RAGE expression was increased in neutrophils of patients who survived septic shock, which validates findings in a published study [42]. In addition to prompting a decrease in oxidative burst, a possible mechanism by which HMGB1 affects neutrophil function is related to impairment of neutrophil chemotaxis and failure to target infectious foci for bacterial eradication. In particular, modulation of neutrophil mitochondrial membrane potential is directly linked to dysfunctional neutrophil motility and diminished NADPH oxidase activation [43]. This concept is supported by distal localization of mitochondria in polarized neutrophils that was recently linked to ATP extracellular flux and enhanced directional motility by neutrophils [44].
Recently, HMGB1 was implicated in mitochondrial depolarization [45]. Although HMGB1 is released from dying cells, activated macrophages and other cell populations during inflammation, the pathophysiologic impact of late extracellular HMGB1accumulation is not well understood. In particular, elevated levels of HMGB1 in the circulation appear to be similar between survivors and nonsurvivors of severe infection and do not predict hospital mortality [27]. However, neutralization of HMGB1 with anti-HMGB1 Ab has favorable outcomes in experimental sepsis [23, 25, 46]. Furthermore, an improved clinical outcome has been observed in patients with sepsis that produced substantial amounts of anti-HMGB1 Ab [47]. Studies have shown that besides sepsis, trauma/hemorrhage is associated with development of immunosuppression accompanied by the release of HMGB1 [48, 49]. Elevated amounts of HMGB1 in circulation were also associated with reduced immune system function in diabetes, patients undergoing therapies for cancer, and chronic inflammatory conditions resulting from atherosclerosis or rheumatoid arthritis [23, 48]. It is important to note that acute organ injury associated with trauma and hemorrhage causes a rapid release of HMGB1 from necrotic cells. HMGB1 was a late mediator of morbility and mortality among the patients who initially survived severe infection. Similarly, we observed only a modest increase of HMGB1 in plasma of mice 24 h after CLP, as compared to sham, whereas significant increases were found 7 d after sepsis.
HMGB1 was initially described as a nuclear protein with important regulatory functions associated with gene expression, but more recent studies have shown that HMGB1 has potent proinflammatory actions and can be classified as a DAMP mediator. Therapeutic approaches aimed at inhibiting the actions of HMGB1 could be of interest in diminishing tissue injury and organ dysfunction. Our data suggest that HMGB1 accumulation in the late phase of sepsis contributes to the development of postsepsis immunosuppression through inhibiting neutrophil-dependent antimicrobial defense mechanisms. Blocking HMGB1 may be a promising therapeutic intervention to diminish adverse effects of sepsis-induced immunosuppression and improve recovery of immune homeostasis in this setting.
AUTHORSHIP
M.G., J.-M.T., C.P., N.B., J.-W.Z. performed the experiments; M.G., J.-M.T., F.U., A.G., J.-W.Z. wrote the report and designed the experiments; J.-M.T and F.U. included the patients; M.G., J.-M.T., F.U., A.G., K.T., E.A. and J.-W.Z. analyzed and discussed the results and read and discussed the report; M.G., A.G. and J.-W.Z. performed the statistical analyses; and M.G., J.-M.T., F.U., A.G., C.P., N.B., Y.L.-T., E.A., K.T. and J.-W.Z. read and approved the final report.
ACKNOWLEDGMENTS
This work was supported by grants from Comité de la Recherche Clinique et Translationnelle (CORECT) 2013, CHU Rennes, France. M.G. was a recipient of funds the Association pour la Recherche contre le Cancer (ARC). J.W.Z. was funded by U.S. National Institutes of Health, Heart, Lung, and Blood Institute Grant HL107585. The data were presented in abstract form at the 43rd Société de Réanimation de Langue Française (SRLF) Annual Congress, Paris 2016
Glossary
- CLP
cecal ligation and puncture
- DAMP
damage-associated molecular pattern
- H2DCFDA
2′,7′-dichlorohydrofluorescin diacetate
- HMGB1
high-mobility group box 1
- ICU
intensive care unit
- MSSA
methicillin-susceptible Staphylococcus aureus
- qPCR
quantitative PCR
- RAGE
receptor for advanced glycation end products
- RFU
relative fluorescence units
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
Footnotes
SEE CORRESPONDING EDITORIAL ON PAGE 1273
Disclosures
The authors declare no conflicts of interest.
REFERENCES
- 1.Angus D. C., van der Poll T. (2013) Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851. [DOI] [PubMed] [Google Scholar]
- 2.Boomer J. S., To K., Chang K. C., Takasu O., Osborne D. F., Walton A. H., Bricker T. L., Jarman S. D. II, Kreisel D., Krupnick A. S., Srivastava A., Swanson P. E., Green J. M., Hotchkiss R. S. (2011) Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306, 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss R. S., Monneret G., Payen D. (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamim C. F., Hogaboam C. M., Kunkel S. L. (2004) The chronic consequences of severe sepsis. J. Leukoc. Biol. 75, 408–412. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss R. S., Coopersmith C. M., McDunn J. E., Ferguson T. A. (2009) The sepsis seesaw: tilting toward immunosuppression. Nat. Med. 15, 496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Tulzo Y., Pangault C., Amiot L., Guilloux V., Tribut O., Arvieux C., Camus C., Fauchet R., Thomas R., Drénou B. (2004) Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am. J. Respir. Crit. Care Med. 169, 1144–1151. [DOI] [PubMed] [Google Scholar]
- 7.Ward P. A. (2011) Immunosuppression in sepsis. JAMA 306, 2618–2619. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J., Opal S., Calandra T. (2012) Sepsis studies need new direction. Lancet Infect. Dis. 12, 503–505. [DOI] [PubMed] [Google Scholar]
- 9.Vincent J. L., Opal S. M., Marshall J. C., Tracey K. J. (2013) Sepsis definitions: time for change. Lancet 381, 774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leentjens J., Kox M., Koch R. M., Preijers F., Joosten L. A., van der Hoeven J. G., Netea M. G., Pickkers P. (2012) Reversal of immunoparalysis in humans in vivo: a double-blind, placebo-controlled, randomized pilot study. Am. J. Respir. Crit. Care Med. 186, 838–845. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger R. P., Levy M. M., Rhodes A., Annane D., Gerlach H., Opal S. M., Sevransky J. E., Sprung C. L., Douglas I. S., Jaeschke R., Osborn T. M., Nunnally M. E., Townsend S. R., Reinhart K., Kleinpell R. M., Angus D. C., Deutschman C. S., Machado F. R., Rubenfeld G. D., Webb S. A., Beale R. J., Vincent J. L., Moreno R.; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. [DOI] [PubMed] [Google Scholar]
- 12.Stephan F., Yang K., Tankovic J., Soussy C. J., Dhonneur G., Duvaldestin P., Brochard L., Brun-Buisson C., Harf A., Delclaux C. (2002) Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit. Care Med. 30, 315–322. [DOI] [PubMed] [Google Scholar]
- 13.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 14.Grailer J. J., Kalbitz M., Zetoune F. S., Ward P. A. (2014) Persistent neutrophil dysfunction and suppression of acute lung injury in mice following cecal ligation and puncture sepsis. J. Innate Immun. 6, 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mantovani A., Cassatella M. A., Costantini C., Jaillon S. (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531. [DOI] [PubMed] [Google Scholar]
- 16.Segal A. W. (2005) How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadié J. M., Bae H. B., Banerjee S., Zmijewski J. W., Abraham E. (2012) Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am. J. Physiol. Cell Physiol. 302, C249–C256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panday A., Sahoo M. K., Osorio D., Batra S. (2015) NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 12, 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groemping Y., Rittinger K. (2005) Activation and assembly of the NADPH oxidase: a structural perspective. Biochem. J. 386, 401–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiszt M., Kapus A., Ligeti E. (2001) Chronic granulomatous disease: more than the lack of superoxide? J. Leukoc. Biol. 69, 191–196. [PubMed] [Google Scholar]
- 21.Demaret J., Venet F., Friggeri A., Cazalis M. A., Plassais J., Jallades L., Malcus C., Poitevin-Later F., Textoris J., Lepape A., Monneret G. (2015) Marked alterations of neutrophil functions during sepsis-induced immunosuppression. J. Leukoc. Biol. 98, 1081–1090. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi M. E. (2009) HMGB1 loves company. J. Leukoc. Biol. 86, 573–576. [DOI] [PubMed] [Google Scholar]
- 23.Andersson U., Tracey K. J. (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H., Antoine D. J., Andersson U., Tracey K. J. (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J. Leukoc. Biol. 93, 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H., Ward M. F., Sama A. E. (2009) Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock 32, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manganelli V., Signore M., Pacini I., Misasi R., Tellan G., Garofalo T., Lococo E., Chirletti P., Sorice M., Delogu G. (2010) Increased HMGB1 expression and release by mononuclear cells following surgical/anesthesia trauma. Crit. Care 14, R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibot S., Massin F., Cravoisy A., Barraud D., Nace L., Levy B., Bollaert P. E. (2007) High-mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 33, 1347–1353. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson S., Pettilä V., Tenhunen J., Laru-Sompa R., Hynninen M., Ruokonen E. (2008) HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 34, 1046–1053. [DOI] [PubMed] [Google Scholar]
- 29.Tadié J. M., Trinquart L., Jannière-Nartey C., Guerot E., Louis B., Fagon J. Y., Diehl J. L., Delclaux C. (2010) Prediction of nosocomial infection acquisition in ventilated patients by nasal nitric oxide: proof-of-concept study. Shock 34, 217–221. [DOI] [PubMed] [Google Scholar]
- 30.Otto G. P., Sossdorf M., Claus R. A., Rödel J., Menge K., Reinhart K., Bauer M., Riedemann N. C. (2011) The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 15, R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos S. S., Brunialti M. K., Rigato O., Machado F. R., Silva E., Salomao R. (2012) Generation of nitric oxide and reactive oxygen species by neutrophils and monocytes from septic patients and association with outcomes. Shock 38, 18–23. [DOI] [PubMed] [Google Scholar]
- 32.Grégoire M., Guilloton F., Pangault C., Mourcin F., Sok P., Latour M., Amé-Thomas P., Flecher E., Fest T., Tarte K. (2015) Neutrophils trigger a NF-κB dependent polarization of tumor-supportive stromal cells in germinal center B-cell lymphomas. Oncotarget 6, 16471–16487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadie J. M., Bae H. B., Deshane J. S., Bell C. P., Lazarowski E. R., Chaplin D. D., Thannickal V. J., Abraham E., Zmijewski J. W. (2012) Toll-like receptor 4 engagement inhibits adenosine 5′-monophosphate-activated protein kinase activation through a high mobility group box 1 protein-dependent mechanism. Mol. Med. 18, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotze M. T., Tracey K. J. (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342. [DOI] [PubMed] [Google Scholar]
- 36.Wrona M., Patel K., Wardman P. (2005) Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic. Biol. Med. 38, 262–270. [DOI] [PubMed] [Google Scholar]
- 37.Zmijewski J. W., Banerjee S., Bae H., Friggeri A., Lazarowski E. R., Abraham E. (2010) Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 285, 33154–33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevenson E. K., Rubenstein A. R., Radin G. T., Wiener R. S., Walkey A. J. (2014) Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit. Care Med. 42, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luyt C. E., Combes A., Deback C., Aubriot-Lorton M. H., Nieszkowska A., Trouillet J. L., Capron F., Agut H., Gibert C., Chastre J. (2007) Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am. J. Respir. Crit. Care Med. 175, 935–942. [DOI] [PubMed] [Google Scholar]
- 40.Limaye A. P., Kirby K. A., Rubenfeld G. D., Leisenring W. M., Bulger E. M., Neff M. J., Gibran N. S., Huang M. L., Santo Hayes T. K., Corey L., Boeckh M. (2008) Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z., Bone N., Jiang S., Park D. W., Tadie J. M., Deshane J., Rodriguez C. A., Pittet J. F., Abraham E., Zmijewski J. W. (2015) AMP-activated protein kinase and glycogen synthase kinase 3β modulate the severity of sepsis-induced lung injury [E-pub ahead of print]. Mol. Med. 10.2119/molmed.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achouiti A., de Vos A. F., van ’t Veer C., Florquin S., Tanck M. W., Nawroth P. P., Bierhaus A., van der Poll T., van Zoelen M. A. (2016) Receptor for advanced glycation end products (RAGE) serves a Protective Role during Klebsiella pneumoniae - Induced Pneumonia. PLoS One 11, e0141000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fossati G., Moulding D. A., Spiller D. G., Moots R. J., White M. R., Edwards S. W. (2003) The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 170, 1964–1972. [DOI] [PubMed] [Google Scholar]
- 44.Jiang S., Park D. W., Stigler W. S., Creighton J., Ravi S., Darley-Usmar V., Zmijewski J. W. (2013) Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J. Biol. Chem. 288, 26013–26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M. T., Billiar T. R., Wang H., Cao L., Tang D. (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Entezari M., Weiss D. J., Sitapara R., Whittaker L., Wargo M. J., Li J., Wang H., Yang H., Sharma L., Phan B. D., Javdan M., Chavan S. S., Miller E. J., Tracey K. J., Mantell L. L. (2012) Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas Aeruginosa pneumonia in cystic fibrosis. Mol. Med. 18, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnay-Verdier S., Fattoum L., Borde C., Kaveri S., Gibot S., Maréchal V. (2011) Emergence of autoantibodies to HMGB1 is associated with survival in patients with septic shock. Intensive Care Med. 37, 957–962. [DOI] [PubMed] [Google Scholar]
- 48.Sims G. P., Rowe D. C., Rietdijk S. T., Herbst R., Coyle A. J. (2010) HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388. [DOI] [PubMed] [Google Scholar]
- 49.LeBlanc P. M., Doggett T. A., Choi J., Hancock M. A., Durocher Y., Frank F., Nagar B., Ferguson T. A., Saleh M. (2014) An immunogenic peptide in the A-box of HMGB1 protein reverses apoptosis-induced tolerance through RAGE receptor. J. Biol. Chem. 289, 7777–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]