Activation of neutrophils by Ferula iliensis essential oils enriched in (E/Z)-propenyl sec-butyl disulfides is mediated by activation of transient receptor potential V1 channels.
Keywords: calcium flux, molecular modeling, neutrophil, reactive oxygen species, transient receptor potential vanilloid 1 channel
Abstract
Essential oil extracts from Ferula iliensis have been used traditionally in Kazakhstan for treatment of inflammation and other illnesses. Because little is known about the biologic activity of these essential oils that contributes to their therapeutic properties, we analyzed their chemical composition and evaluated their phagocyte immunomodulatory activity. The main components of the extracted essential oils were (E)-propenyl sec-butyl disulfide (15.7–39.4%) and (Z)-propenyl sec-butyl disulfide (23.4–45.0%). Ferula essential oils stimulated [Ca2+]i mobilization in human neutrophils and activated ROS production in human neutrophils and murine bone marrow phagocytes. Activation of human neutrophil [Ca2+]i flux by Ferula essential oils was dose-dependently inhibited by capsazepine, a TRPV1 channel antagonist, indicating that TRPV1 channels mediate this response. Furthermore, Ferula essential oils stimulated Ca2+ influx in TRPV1 channel–transfected HEK293 cells and desensitized the capsaicin-induced response in these cells. Additional molecular modeling with known TRPV1 channel agonists suggested that the active component is likely to be (Z)-propenyl sec-butyl disulfide. Our results provide a cellular and molecular basis to explain at least part of the beneficial therapeutic properties of FEOs.
Introduction
The innate immune system is essential for host defense and provides immediate defense against infection. Among the earliest cell types responding to invasion by potentially pathogenic organisms are innate immune cells known as phagocytes, such as neutrophils and monocyte/macrophages [1]. These cells perform a variety of complex microbicidal functions, including phagocytosis, chemotaxis, and biochemical destruction of targeted organisms [2]. Phagocytes also perform important roles in the resolution of inflammation, including the clearance of dead tissue and the promotion of wound healing [3].
Several small molecules, including natural products, have been shown to exhibit immunomodulatory activity via regulation of phagocyte function [4, 5]. For example, we recently found that essential oils have phagocyte immunomodulatory activity and, depending on the source and composition of the essential oil, can activate or inhibit human neutrophil function [6, 7]. Essential oils are plant extracts that contain mixtures of terpenes and have a wide range of pharmacological activities [8]. For example, essential oils extracted from various Ferula species have been reported to exhibit antioxidant, anti-inflammatory, anti-mutagenic, antimicrobial, and antifungal activities [9–15]. Although essential oils from various Ferula species have been shown to contain monoterpenes, sesquiterpenes, and polysulfides [14, 16–22], the chemical composition of essential oils isolated from many native Ferula spp. have not yet been evaluated. Likewise, even less is known about their pharmacological and biologic properties. Clearly, a better understanding of the active components in plant essential oils and their mechanisms of action is necessary for assessing the therapeutic potential of these natural products [23].
Ferula iliensis Krasn. ex Korovin is a native plant of Kazakhstan. Once the plant reaches maturity, it produces numerous 5–10 cm round umbels (flower clusters) on branched stems with pale-yellow petals. This plant has been used in traditional medicine because of its reported anti-inflammatory activity [24]. Although several unique secondary metabolites have been isolated from F. iliensis [25], the chemical composition and biologic properties of FEOs have not yet been analyzed.
In the present study, we evaluated the composition and phagocyte immunomodulatory activity of essential oils obtained from various parts of F. iliensis (flowers, umbels+seeds, leaves, stems, and roots) at 2 vegetation stages (flowering and fruiting). We showed that FEOs have a high content of sulfur organic compounds and activate human neutrophils and mouse phagocytes, resulting in increased [Ca2+]i and the production of ROS. Activation of human neutrophil [Ca2+]i flux by FEOs was dose-dependently inhibited by capsazepine, a TRPV1 channel antagonist, indicating TRPV1 channels mediated this response. Furthermore, the oil with the highest amount of (E/Z)-propenyl sec-butyl disulfides activated TRPV1 channels with subsequent desensitization. Additional molecular modeling suggested that the active component is likely to be (Z)-propenyl sec-butyl disulfide. Given the critical role played by phagocytes in innate immunity against pathogens, our data support the possibility that (E/Z)-propenyl sec-butyl disulfides could be an effective therapeutic modulator of innate immune responses.
MATERIALS AND METHODS
Plant material
F. iliensis was collected in 2015 during the flowering and fruiting stages from the Almaty region of Kazakhstan. Voucher specimens were deposited at the Institute of Plant Biology and Biotechnology (Almaty, Kazakhstan). Flowers, umbels+seeds, leaves, stems, and roots were air-dried for 7–10 d at room temperature away from direct sunlight before hydrodistillation.
Materials
DMSO, fMLF, capsaicin, Histopaque 1077, SOD from bovine erythrocytes, xanthine oxidase from bovine milk, EGTA, and manganese (II) chloride (MnCl2) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Fluo-4 AM dye was from Thermo Fisher Scientific (Carlsbad, CA, USA), and Fura-2/AM was from TEFLabs (Austin, TX, USA). Cytochrome c from equine heart was purchased from Calbiochem (San Diego, CA, USA); 8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4(2H,3H)-dione (L-012) from Wako Chemicals (Richmond, VA, USA); capsazepine from Cayman Chemical Co. (Ann Arbor, MI, USA); ionomycin from EMD Biosciences (San Diego, CA, USA); capsaicin from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan); DMEM and penicillin–streptomycin solution from Mediatech (Herndon, VA, USA); and FBS from Atlas Biologicals (Fort Collins, CO, USA). HBSS [0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 5.56 mM glucose, and 10 mM HEPES (pH 7.4)] was from Thermo Fisher Scientific (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ is designated HBSS−; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 is designated HBSS+.
Essential oil extraction
Essential oils were obtained by hydrodistillation of dried plant material in a Clevenger type apparatus [7]. We used conditions accepted by the European Pharmacopoeia (European Directorate for the Quality of Medicines, Council of Europe, Strasbourg, France, 2014) to avoid artifacts. Stock solutions of the essential oils were prepared in DMSO (10 mg/ml) for biologic evaluation and in n-hexane (10% w/v) for GC analysis.
GC-MS analysis
GC-MS analysis was performed on a 5975 GC-MSD system (Agilent Technologies, Santa Clara, CA, USA) [26]. An Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness; Agilent Technologies) was used, with He as the carrier gas (0.8 ml/min). The GC oven temperature was kept at 60°C for 10 min, increased to 220°C at a rate of 4°C/min, kept constant at 220°C for 10 min, and then increased to 240°C at a rate of 1°C/min. The split ratio was adjusted to 40:1, and the injector temperature was 250°C. MS spectra were monitored at 70 eV with a mass range of 35 to 450 m/z.
GC analysis was performed on a 6890N GC system (Agilent). To obtain the same elution order as with GC-MS, we performed simultaneous injection with the same column and appropriate operational conditions. The FID temperature was 300°C. The essential oil components were identified by coinjection with standards (whenever possible), which were purchased from commercial sources or isolated from natural sources. In addition, compound identities were confirmed by comparison of their mass spectra with those in the Wiley GC/MS Library (John Wiley & Sons, New York, NY, USA), MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, and NIST Library. A C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of RRI. Relative percentage amounts of the separated compounds were calculated from FID chromatograms.
Cell culture
Cultured HEK293 cells were placed in a 6-well plate for 24 h and then transfected with human TRPV1 cDNA, by TransIT-2020 (Mirus Bio LLC, Madison, WI, USA), according to the manufacturer’s protocol. After transfection, the cells were suspended in DMEM supplemented with 10% FBS at 37°C and used for [Ca2+]i measurements.
Isolation of human neutrophils
For isolation of human neutrophils, blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University. Neutrophils were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells [5]. Isolated neutrophils were washed twice and resuspended in HBSS. Neutrophil preparations were routinely >95% pure, as determined by light microscopy, and >98% viable, as determined by Trypan blue exclusion. Neutrophils were obtained from multiple donors (n = 8); however, the cells from different donors were never pooled during experiments.
Isolation of murine bone marrow leukocytes
All animal use was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee at Montana State University. Bone marrow leukocytes were flushed from tibias and femurs of BALB/c mice with HBSS, filtered through a 70-μm nylon cell strainer (BD Biosciences, Franklin Lakes, NJ, USA) to remove cell clumps and bone particles, and resuspended in HBSS at 1 × 106 cells/ml.
Calcium mobilization assay
Changes in neutrophil [Ca2+]i concentrations were measured with a FlexStation 3 scanning fluorometer (Molecular Devices, Sunnyvale, CA, USA). In brief, human neutrophils were suspended in HBSS, loaded with Fluo-4 AM (Thermo Fisher Scientific) at a final concentration of 1.25 μg/ml and incubated for 30 min in the dark at 37°C. After dye loading, the cells were washed with HBSS−, resuspended in HBSS+, separated into aliquots, and placed into the wells of flat-bottomed, half-area–well black microtiter plates (2 × 105 cells/well). Essential oils diluted in DMSO were added to the wells (final concentration of DMSO was 1%), and changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature after addition of the test compound/oil. The maximum change in fluorescence observed after subtracting the background signal from DMSO-treated cells, was used to determine the agonist response. Calibration of the fluorescence signals to determine absolute Ca2+ concentrations was performed with ionomycin (2 µM), to obtain the maximum fluorescence, followed by MnCl2 (20 mM) to obtain the minimum fluorescence [27]. For this calculation, we used a dissociation constant (Kd) of 335 nM for the Fluo-4/Ca2+ complex (Thermo Fisher Scientific, Waltham, MA, USA).
To calculate EC50 values, individual [Ca2+]i flux responses were normalized to the maximum response in a given experiment, which was assigned a value of 100%. Efficacy was determined by comparing individual responses activated by essential oil samples to that induced by a positive control (5 nM fMLF), which was assigned a value of 100%. Curve fitting (at least 5 or 6 points) and calculation of EC50 were performed by nonlinear regression analysis of the dose–response curves generated (Prism 7; GraphPad Software, Inc., San Diego, CA, USA).
Transient Ca2+ influx in HEK293 cells (transfected with TRPV1 and nontransfected cells) was evaluated [28]. HEK293 cells were incubated in growth medium containing Fura-2 (2 μM) for 30 min at 37°C and 5% CO2. Coverslips containing the Fura-2-loaded HEK293 cells were mounted on the stage of an IX-81 inverted fluorescence microscope (Olympus America, Lake Success, NY, USA), and the cells were perfused continuously with serum-free medium at a flow rate of 2 ml/min. Cells were exposed to essential oils under investigation by switching from control medium to essential oil–containing medium for 10 s followed by a 1 or 5 min wash with control buffer before addition of the next dose of essential oil. Ca2+ influx measurements and data acquisition were simultaneously performed on multiple individual cells using a fluorescence imaging system (Easy Ratio Pro; Horiba Scientific, Edison, NJ, USA) equipped with a multiwavelength spectrofluorometer (DeltaRAM X; Photon Technology International Inc., Lawrence Township, NJ, USA) and a QuantEM 512SC electron multiplying charge-coupled device camera (Photometrics, Tucson, AZ, USA). Representative traces were acquired with an alternating excitation wavelength protocol (340, 380 nm/20 Hz) and emission wavelength of 510 nm.
Analysis of ROS production
ROS production was determined by monitoring L-012-enhanced chemiluminescence, which represents a sensitive and reliable method of detecting superoxide anion (O2−.) production [29]. Mouse bone marrow leukocytes or human neutrophils were resuspended at 106 cells/ml in HBSS containing Ca2+ and Mg2+ (HBSS+) and supplemented with 40 μM L-012. Cells (100 μl) were placed in aliquots into wells of 96-well flat-bottomed white microtiter plates containing test essential oil (final DMSO concentration of 1%). Luminescence was monitored for 30 min (2 min intervals) at 37°C with a Fluroscan Ascent FL microtiter plate reader (Thermo Electron, Waltham, MA, USA). The curve of light intensity (in relative luminescence units) was plotted against time, and the area under the curve was calculated as total integrated chemiluminescence (CL). L-012-enhanced chemiluminescence was calibrated to quantify absolute O2−. levels with a xanthine/xanthine oxidase system, to generate known amounts of O2−., as measured by the reduction of ferricytochrome c [30, 31]. To calculate EC50, individual O2−. responses were normalized to the maximum response in a given experiment, which was assigned a value of 100%. Curve fitting (at least 5 or 6 points) and calculation of EC50 were performed by nonlinear regression analysis of the dose–response curves (Prism 7; GraphPad Software, Inc., San Diego, CA, USA).
Molecular modeling
We used a ligand-based approach for molecular modeling employing field-point methodology [32]. The structures of capsaicin, N-geranyl cyclopropylcarboxamide, and (Z)-N-(2,7-dimethylocta-2,6-dienyl)-2-phenylacetamide (compound 2k) [33, 34] were imported for constructing a TRPV1 channel ligand-binding–site template, as described elsewhere [6]. Using the built-in capabilities of FieldTemplater software (Cresset Biomolecular Discovery Ltd., Hertfordshire, UK), we generated representative sets of conformations for the compound corresponding to local energy minima calculated within the extended electron distribution force field [32]. For the generation of field point patterns, probe atoms having positive, negative, and zero charge were placed in the vicinity of a given conformation, and the energy of their interaction with the molecular field was calculated with the extended electron distribution parameter set. Positions of energy extrema for positive probes represent “negative” field points, and energy extrema for negative and neutral probe atoms correspond to “positive” and steric field points, respectively. Hydrophobic field points were also generated with neutral probes capable of penetrating into the molecular core and reaching extrema in the centers of hydrophobic regions. A detailed description of the field point calculation procedure has been published elsewhere [32]. The structures of 4 isomers of propenyl sec-butyl disulfides were imported into the FieldAlign program (FieldAlign Version 2.0.1; Cresset Biomolecular Discovery Ltd.) in Tripos MOL2 format. Conformational search and field point calculation were performed, as described above for template building. Conformations with the best fit to the geometry and field points of the TRPV1 channel template were identified, and their superimpositions were refined by the simplex optimization algorithm and visualized using instruments incorporated in FieldAlign.
Statistical analysis
One way ANOVA was performed on the data sets, followed by Fisher’s uncorrected least-significant difference test for multiple comparisons. Pair-wise comparisons with differences at P < 0.05 were considered to be statistically significant.
RESULTS
Essential oil composition
The chemical composition of essential oils can be affected by many factors, including harvesting time and which part of the plant is used for essential oil isolation [35]. Thus, essential oils were obtained by hydrodistillation of FEOFl, FEOLfl, FEOSfl, FEORfl, FEOFr, FEOSfr, and FEORfr. (The abbreviations are defined in the footnote of Table 1.) The yields (v/w) were: 0.9% (FEOFl), 0.4% (FEOLfl), 1.1% (FEOSfl), 0.7% (FEORfl), 0.9% (FEOFr), 0.6% (FEOSfr), and 0.9% (FEORfr). The chemical composition of the oils was evaluated by using GC-FID and GC/MS simultaneously; Table 1 summarizes the identified compounds, percentage composition, and their retention indices. Compounds are listed in order of their elution.
TABLE 1.
Chemical composition of FEOs
| Compound number | RRI | Compound name | Concentration in FEOs (%)a |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FEOFl | FEOLfl | FEOSfl | FEORfl | FEOFr | FEOSfr | FEORfr | |||
| 1 | 1032 | α-Pinene | 2.3 | 1.9 | 0.7 | 1.1 | 2.2 | 4.8 | 3.5 |
| 2 | 1035 | α-Thujene | t | t | t | t | t | t | t |
| 3 | 1076 | Camphene | t | t | |||||
| 4 | 1118 | β-Pinene | 0.4 | t | t | 0.2 | 0.3 | t | |
| 5 | 1132 | Sabinene | 0.5 | t | |||||
| 6 | 1174 | Myrcene | 0.6 | 0.4 | 0.3 | 0.2 | 0.5 | t | |
| 7 | 1176 | α-Phellandrene | t | t | 0.1 | t | |||
| 8 | 1203 | Limonene | 0.7 | 0.5 | 0.5 | 1.1 | 0.5 | 1.2 | 0.6 |
| 9 | 1218 | β-Phellandrene | 0.2 | t | t | 0.1 | 0.4 | ||
| 10 | 1246 | (Z)-β-Ocimene | 1.5 | 0.9 | 0.3 | 1.1 | 0.8 | 1.3 | t |
| 11 | 1255 | γ-Terpinene | t | t | |||||
| 12 | 1266 | (E)-β-Ocimene | 2.1 | 1.6 | 0.6 | 1.8 | 1.9 | 2.0 | t |
| 13 | 1271 | 3,4-Dimethylthiophene | t | t | |||||
| 14 | 1272 | Methyl cis-propenyl disulfide | t | t | |||||
| 15 | 1280 | p-Cymene | 0.3 | t | 0.3 | 0.4 | 0.6 | 0.5 | |
| 16 | 1288 | Methyl sec-butyl disulphide | t | ||||||
| 17 | 1290 | Terpinolene | t | ||||||
| 18 | 1294 | Methyl trans-propenyl disulfide | t | t | t | ||||
| 19 | 1368 | 2,3,4-Trimethylthiophene | 0.7 | 0.3 | 1.1 | 1.1 | 0.4 | 0.6 | 1.4 |
| 20 | 1382 | cis-Alloocimene | t | ||||||
| 21 | 1393 | Dimethyl trisulfide | t | t | |||||
| 22. | 1428 | n-Propyl sec-butyl disulfide | 0.6 | 0.6 | 0.6 | 0.3 | 0.9 | 0.6 | t |
| 23 | 1457 | sec-Butyl disulfide | 0.5 | 0.6 | 0.4 | 1.3 | 2.4 | ||
| 24 | 1463 | 2,3,4,5-Tetramethyl thiophene | 0.9 | 0.9 | 1.1 | 1.6 | 0.5 | 0.7 | |
| 25 | 1475 | (E)-Propenyl sec butyl disulfide | 39.4 | 21.5 | 32.8 | 28.0 | 24.2 | 34.9 | 15.7 |
| 26 | 1493 | (Z)-Propenyl sec butyl disulfide | 23.4 | 47.9 | 25.4 | 30.4 | 45.0 | 31.0 | 30.8 |
| 27 | 1590 | Bornyl acetate | t | t | t | t | t | t | t |
| 28 | 1600 | β-Elemene | t | t | 0.1 | t | t | ||
| 29 | 1611 | Terpinen-4-ol | t | ||||||
| 30 | 1612 | β-Caryophyllene | t | ||||||
| 31 | 1614 | Carvacrol methyl ether | 0.5 | t | 0.1 | ||||
| 32 | 1650 | γ-Elemene | t | t | 0.1 | ||||
| 33 | 1688 | 4,11-Eudesmadiene | t | t | |||||
| 34 | 1690 | Cryptone | t | t | t | ||||
| 35 | 1694 | Drima-7,9(11)-diene | t | t | t | ||||
| 36 | 1719 | Borneol | t | ||||||
| 37 | 1740 | Valencene | 0.2 | ||||||
| 38 | 1742 | β-selinene | 0.2 | ||||||
| 39 | 1744 | β-Dihydroagarofuran | 1.2 | 0.9 | 1.1 | 1.2 | 0.6 | 0.8 | 2.7 |
| 40 | 1758 | (E,E)-α-Farnesene | t | t | t | t | |||
| 41 | 1798 | α-Dihydroagarofuran | t | t | 0.1 | 0.1 | t | t | |
| 42 | 1830 | 2,6-Dimethyl-3(E),5(E),7-octatriene-2-ol | t | 0.1 | |||||
| 43 | 1854 | Germacrene-B | 0.3 | 0.3 | 0.3 | 0.4 | 0.2 | 0.7 | |
| 44 | 1878 | 2,5-Dimethoxy-p-cymene | 0.2 | ||||||
| 45 | 1907 | α-Agarofuran | 0.1 | 0.5 | |||||
| 46 | 1932 | 1-Methylthiopropyl propyl disulfide | 0.4 | 3.7 | 8.0 | 0.1 | 0.4 | 0.5 | |
| 47 | 1947 | 1-Methylthiopropyl propyl disulfide isomerb | 1.4 | 0.3 | 0.2 | ||||
| 48 | 1969 | cis-Jasmone | t | 0.4 | 0.1 | ||||
| 49 | 2061 | Guaiol acetate | t | ||||||
| 50 | 2096 | Elemol | 0.3 | 0.4 | 0.2 | t | 0.5 | ||
| 51 | 2103 | Guaiol | 1.2 | 0.9 | 1.3 | 0.9 | 0.4 | 0.7 | 2.0 |
| 52 | 2127 | 10-Epi-γ-eudesmol | 5.2 | 2.9 | 6.7 | 4.4 | 0.1 | 2.9 | 10.3 |
| 53 | 2144 | Rosifoliol | t | t | t | ||||
| 54 | 2157 | 5-Epi-7-epi-α-eudesmol | 0.5 | 0.9 | 0.4 | 0.3 | 0.3 | 1.2 | |
| 55 | 2185 | γ-Eudesmol | 0.9 | t | |||||
| 56 | 2212 | Agarospirol | 1.9 | 2.9 | 1.4 | 0.8 | 0.9 | 4.0 | |
| 57 | 2224 | α-Guaiol | 0.6 | 0.5 | 1.1 | 0.4 | 0.3 | 0.7 | 1.3 |
| 58 | 2237 | Valerianol | 1.9 | 1.2 | 2.8 | 1.4 | 0.8 | 0.8 | 3.6 |
| 59 | 2240 | α-Guaiol isomerb | 3.3 | 0.9 | 1.0 | ||||
| 60 | 2245 | Elemicine | 0.6 | ||||||
| 61 | 2250 | α-Eudesmol | 0.4 | ||||||
| 62 | 2257 | β-Eudesmol | 0.9 | 0.7 | t | 0.8 | 0.3 | 0.7 | |
| 63 | 2287 | (2Z,6Z)-Farnesol | 0.4 | 0.3 | 0.6 | ||||
| 64 | 2655 | Benzyl benzoate | t | 0.1 | |||||
| 65 | 2900 | Nonacosane | t | 0.2 | |||||
| 66 | 2931 | Hexadecanoic acid | t | t | |||||
| Total | 87.1 | 84.0 | 91.7 | 86.0 | 86.1 | 90.0 | 85.3 | ||
| Monoterpene hydrocarbons | 8.6 | 5.3 | 2.4 | 5.4 | 6.4 | 11.1 | 4.6 | ||
| Oxygenated monoterpenes | t | 0.5 | 0.4 | t | 0.5 | t | t | ||
| Sesquiterpene hydrocarbons | 0.3 | - | 0.3 | 0.3 | 0.5 | 0.2 | 1.2 | ||
| Oxygenated sesquiterpenes | 12.8 | 7.7 | 22.1 | 10.4 | 5.5 | 8.7 | 27.4 | ||
| Sulfur containing compounds | 65.4 | 70.3 | 66.4 | 69.5 | 72.9 | 70.1 | 51.5 | ||
| Miscellaneous compounds | t | - | - | - | 0.3 | - | 0.6 | ||
The data are presented as relative percentage by weight for each component that was isolated from F. iliensis flowers (FEOFl), leaves in the flowering stage (FEOLfl), stems in the flowering stage (FEOSfl), roots in the flowering stage (FEORfl), fruits (seeds+umbels) (FEOFr), stems in the fruiting stage (FEOSfr), and roots in the fruiting stage (FEORfr). RRI, relative retention index calculated on the basis of retention of n-alkanes; %, calculated from FID data. Trace amounts (t) were present at <0.1%. bTentatively identified using Wiley and MassFinder mass spectra libraries and published RRI. All other compounds were identified by comparison with coinjected standards.
Analysis of the essential oils demonstrated 25 (FEOLfl) to 46 (FEOSfr) different constituents, with sulfur-containing compounds being predominant. Specifically, (E)-propenyl sec-butyl disulfide (range, 15.7–39.4%) and (Z)-propenyl sec-butyl disulfide (range, 23.4–45.0%) were found to be the predominant essential oil constituents from all parts of the plant (structures of these compounds are shown in Fig. 1). Note, however, that the ratio of R/S enantiomers (based on the chiral center at the butyl group carbon) comprising the (E/Z)-propenyl sec-butyl disulfides was not defined. Nevertheless, essential oil extracted from flowers had the highest levels of (E)-propenyl sec-disulfide, whereas essential oils obtained from umbels with seeds and from leaves harvested during full flowering were highest in (Z)-propenyl sec-butyl disulfide. Other sulfur-containing compounds present in minor percentages included 3,4-dimethylthiophene, methyl cis-propenyl disulfide, methyl sec-butyl disulfide, 2,3,4-trimethylthiophene, 2,3,4,5-tetramethyl thiophene, dimethyl trisulfide, n-propyl sec-butyl disulfide, and 1-methylthiopropyl propyl disulfide. Thus, sulfur-containing compounds represented 51.5 (FEORFr) to 72.9% (FEOFr) of the total oil composition (Table 1). Six eudesmane-type sesquiterpenes were also present in FEOs, although at much lower concentrations.
Figure 1. Chemical structures of (E/Z)-propenyl sec-butyl disulfides found in FEOs.
*Location of the chiral center for (R/S) enantiomers.
Effect of Ferula essential oils on neutrophil Ca2+ mobilization
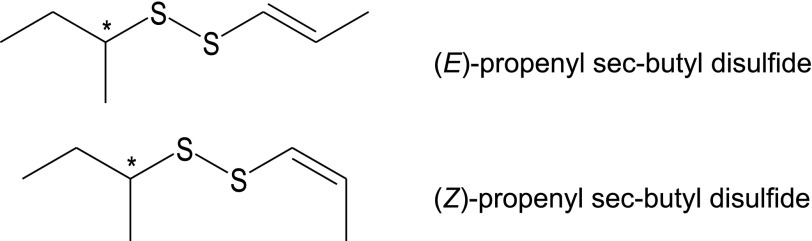
Essential oils and their components have been reported previously to modulate [Ca2+]i levels in neutrophils and other cell types [7]. We found that FEOs activated human neutrophil Ca2+ flux (Table 2). Representative kinetic curves for FEORfl and FEOFr are shown in Fig. 2. Although FEOFr and FEOLfl contain up to ∼70% (E/Z)-propenyl sec-butyl disulfides (Table 1), FEOFr had a much higher yield during essential oil isolation (see above). Thus, we used FEOFr as a representative sample of FEOs in most of the following experiments.
TABLE 2.
Effect of FEOs on Ca2+ flux in human neutrophils and ROS production in mouse bone marrow phagocytes and human neutrophils
| FEO | Ca2+ flux EC50 (µg/ml) and efficacy (%) |
ROS production EC50 (µg/ml) |
|
|---|---|---|---|
| Neutrophils | BM cells | Neutrophils | |
| FEOFl | 18.5 ± 7.8 (90 ± 12.3) | 12.0 ± 4.6a,b,c | 17.9 ± 3.3a,b,c,d |
| FEOLfl | 12.4 ± 2.7 (100 ± 13.3) | 25.1 ± 2.6a,d | 33.6 ± 4.1a,e,f,g,h,i |
| FEOSfl | 25.9 ± 14.6 (80 ± 9.1) | 22.7 ± 5.7b,f | 20.5 ± 3.9e,j |
| FEORfl | 11.9 ± 5.2 (100 ± 11.8) | 16.6 ± 1.9 | 26.3 ± 3.6b,f,k |
| FEOFr | 17.5 ± 8.6 (100 ± 12.9) | 24.6 ± 9.2c,g | 26.9 ± 3.8c,g,l |
| FEOSfr | 17.2 ± 9.1 (100 ± 7.6) | 15.7 ± 5.7d | 26.3 ± 4.3d,h,m |
| FEORfr | 20.1 ± 8.8 (90 ± 10.4) | 8.7 ± 1.6e,f,g | 13.9 ± 2.8i,j,k,l,m |
EC50 values were determined by nonlinear regression analysis of the dose-response curves. Efficacy (in brackets) is expressed as % of the response induced by 5 nM fMLF. EC50 and efficacy values are presented as the mean ± sd of 3 independent experiments. No statistically significant differences were found between the Ca2+ flux measurements. a–mStatistically significant differences between the data in each column are indicated by superscript letters (samples with the same letter indicate that significant differences were found; P < 0.05).
Figure 2. FEOs activate neutrophil Ca2+ mobilization.
(A) Human neutrophils were treated with 50 µg/ml FEORfl, 50 µg/ml FEOFr, 5 nM fMLF (positive control), or 1% DMSO (negative control), and [Ca2+]i flux was monitored for the indicated times (arrow indicates when treatment was added). Data are from 1 experiment, representative of 3 independent experiments. (B) Human neutrophils were treated with the indicated concentrations of FEORfl, FEOFr, or 1% DMSO (negative control), and [Ca2+]i flux was monitored. The data are presented as the maximum change in [Ca2+]i (max–min) induced by the indicated essential oil concentrations. For both panels the data are from 1 experiment, representative of 3 independent experiments.
We also performed experiments in the absence of extracellular Ca2+ to explore the relative contribution of intracellular Ca2+ pools to the overall response induced by FEOFr. Assuming that no extracellular Ca2+ could enter the cells, any increase in [Ca2+]i would have to arise from Ca2+ released from the intracellular stores. Addition of the extracellular Ca2+ chelator, EGTA (10 mM) [36], reduced the increase in [Ca2+]i induced by 50 µg/ml FEOFr by ∼67.3 ± 4.6%, indicating that most [Ca2+]i flux was related to the entry of extracellular Ca2+ (data not shown).
Effect of Ferula essential oils on neutrophil ROS production
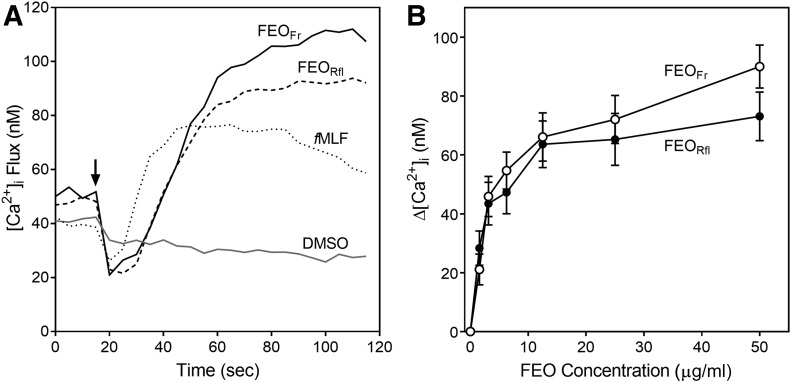
ROS production was analyzed in purified human neutrophils, as well as mouse bone marrow phagocytes. Note that ROS generation in mouse bone marrow preparations is primarily from neutrophils and macrophages [37]. Similar to the effect on Ca2+ mobilization, we found that the FEOs activated SOD-inhibitable O2−. production in human neutrophils and mouse bone marrow phagocytes (Table 2). O2−. production exhibited unimodal kinetics, which is similar to that observed for other phagocyte-activating agents [5], and representative kinetic curves for O2−. induced by FEOFr and FEORfr are shown in Fig. 3A. This response was inhibited by SOD (e.g., Fig. 3A shows inhibition of the FEOFr induced response by SOD). Note that addition of FEOFr to cells pretreated with 0.1% Triton X100 (lysed cells) also did not result in a luminescence signal, demonstrating that the O2−. signal originated from the phagocytes and was not an interfering artifact associated with FEOs.
Figure 3. FEOs activate ROS production by phagocytic leukocytes.
(A) Kinetics of bone marrow leukocyte ROS production activated by FEOFr and FEORfr. Bone marrow leukocytes were treated with 1% DMSO (negative control), FEOFr (12.5 µg/ml), or FEORfr (12.5 µg/ml), and the kinetics of ROS production was monitored with an L-012-amplified assay system. Additional specificity controls include samples containing 100 U SOD and 0.1% Triton X100, as indicated. Data from 1 experiment of 3 independent experiments is shown. (B and C) Mouse bone marrow phagocytes (B) and human neutrophils (C) were treated with the indicated concentrations of FEOFl, FEOFr, or 1% DMSO (negative control), and ROS production was monitored. Data are the mean ± sd of triplicate samples from 1 experiment, representative of 3 independent experiments.
Dose-dependent enhancement of O2−. production was observed in human and mouse leukocytes, with FEOLfl and FEOFr being the most active (Table 2), and representative concentration-dependent responses for stimulation of O2−. production in mouse bone marrow phagocytes and human neutrophils treated by FEOFl and FEOFr are shown in Fig. 3B and C, respectively.
Effect of FEOFr on TRPV1 channel activity
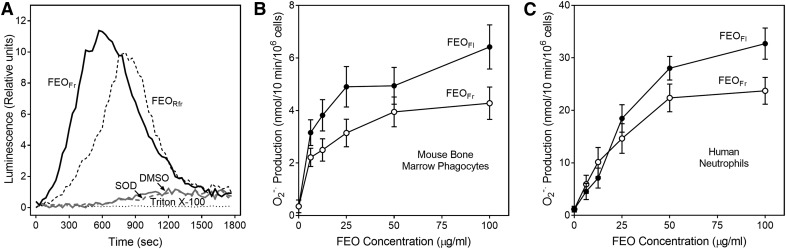
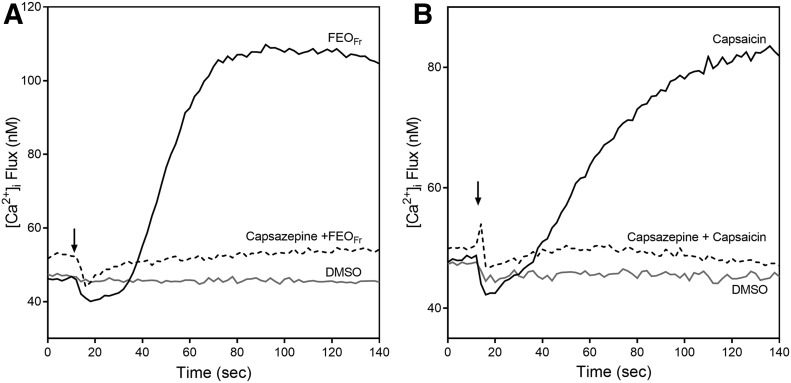
Various essential oil constituents have been reported to be agonists or antagonists of temperature-sensitive TRP channels, including TRPA1, TRPV1/3, and TRPM8 [6, 38], and it is also known than several TRP channels, including TRPV1, are expressed on neutrophils [39, 40]. Indeed, we found that capsaicin, a TRPV1 channel agonist, directly activated neutrophil Ca2+ flux [6]. Thus, we evaluated effects of the TRPV1 channel antagonist capsazepine on neutrophil [Ca2+]i flux induced by FEO. [Ca2+]i flux stimulated by FEOFr or control capsaicin was completely inhibited by pretreatment of the cells with capsazepine (Fig. 4). Capsazepine dose-dependently inhibited neutrophil [Ca2+]i flux induced by capsaicin or FEOFr with IC50 of 20.2 ± 2.3 and 27.0 ± 1.5 µM, respectively, suggesting that TRPV1 is the primary neutrophil cellular target for FEOFr.
Figure 4. Capsazepine inhibits neutrophil Ca2+ mobilization induced by FEOFr and capsaicin.
(A) Human neutrophils were pretreated with 50 µM of capsazepine for 15 min, followed by treatment with 50 µg/ml FEOFr. (B) Human neutrophils were pretreated with 50 µM of capsazepine for 15 min, followed by treatment with 50 µM capsaicin. For both experiments, [Ca2+]i flux was monitored for the indicated times (arrow indicates when treatment was added). A vehicle control (1% DMSO) was included in all experiments. The data are from 1 experiment, representative of 2 independent experiments.
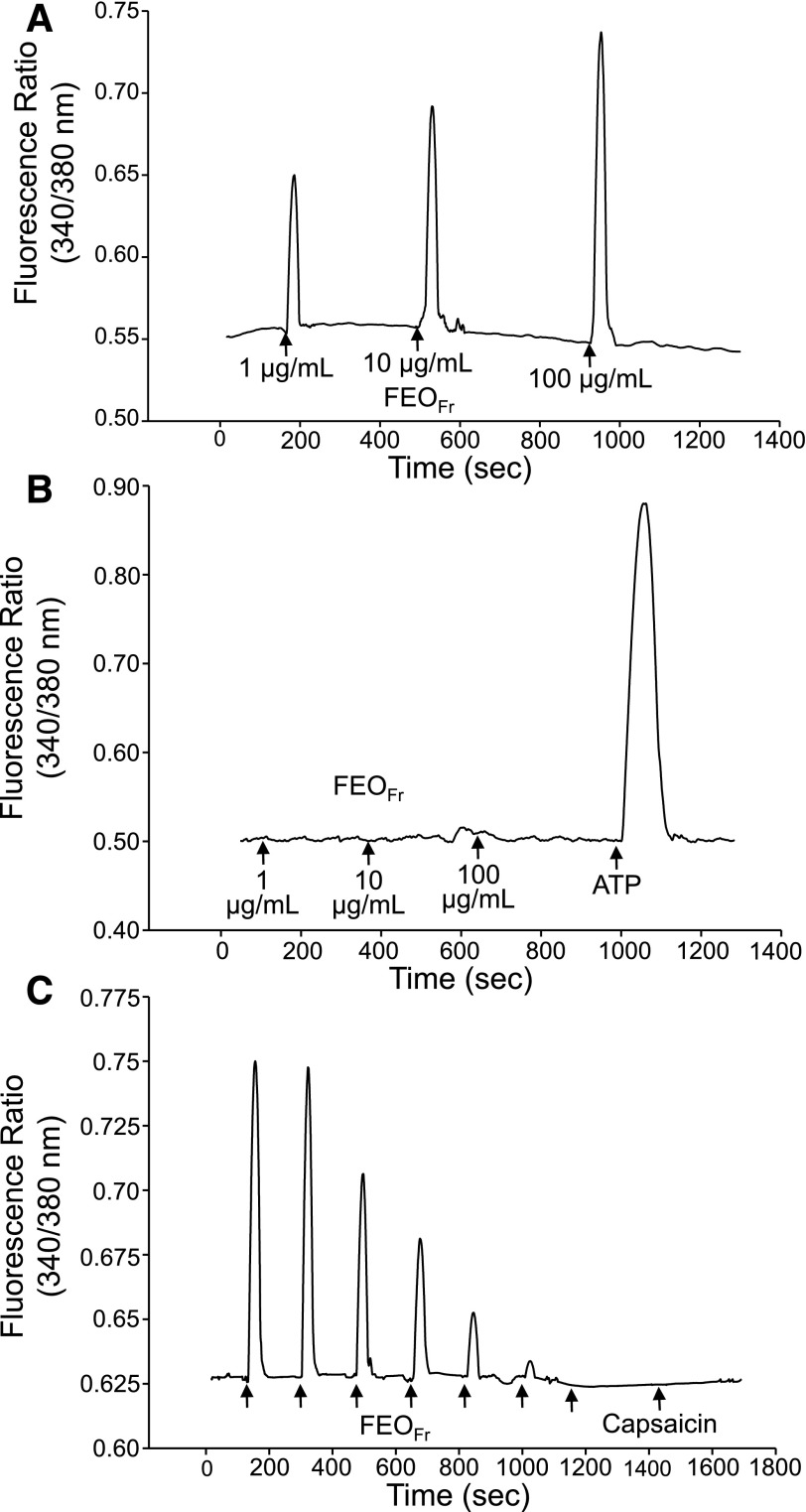
To confirm the role of TRPV1 in cellular activation by FEOs, we evaluated the effects of FEOFr in HEK293 cells transfected with TRPV1 channels. Consistent with our conclusions, FEOFr dose dependently induced a transient increase in [Ca2+]i in TRPV1 channel-transfected HEK293 cells (Fig. 5A). We also performed the reverse dose–response experiment, starting with the high dose of FEOFr (100 µg/ml), and observed a decrease in the amplitude of the Ca2+ transients with decreasing concentrations of the oil (Supplemental Fig. S1). Note, however, that the essential oil compounds are lipophilic and likely remain in the membranes after washes, thereby affecting the dose–response behavior (regardless of whether the dose goes from high to low or low to high), resulting in a hysteresis effect. FEOFr did not induce a response in nontransfected HEK293 cells at all concentrations tested (Fig. 5B), demonstrating the specific requirement of TRPV1 channels for this response. Furthermore, repetitive application of 10 µg/ml FEOFr desensitized TRPV1 channels, and no response to the specific TRPV1 channel agonist capsaicin was observed after desensitization (Fig. 5C). Thus, these data confirm that FEOs stimulate TRPV1 channels, resulting in neutrophil activation.
Figure 5. TRPV1 channel-dependent stimulation of Ca2+ influx by FEOFr.
HEK293 cells transfected with TRPV1 channels (V1-HEK293) (A) and nontransfected cells (B) were exposed to the indicated concentrations of FEOFr, and real-time Ca2+ recordings were collected. ATP (10 µM) was used as a positive control in nontransfected cells, to demonstrate receptor viability. (C) V1-HEK293 cells were subjected to repetitive treatments of FEOFr (10 µg/ml) to demonstrate desensitization of the TRPV1 channel, followed by a single application of capsaicin (100 nM) to assess postdesensitization responses. Representative traces from 5 experiments are shown.
DISCUSSION
Plant-derived organosulfur compounds exhibit a broad spectrum of pharmacological properties, including immunomodulation [41]. For example, organosulfur compounds contribute to the medicinal properties of garlic [42]. Ferula spp. and essential oils derived from these species are another important source of unique organosulfur compounds [14, 16, 43–45]. In the present study, we defined the chemical profile of essential oils extracted from different parts of F. iliensis and evaluated the effect of these oils on phagocyte functions in vitro.
A total of 66 constituents were identified in the FEOs, with organosulfur compounds (E)-propenyl sec-butyl disulfide and (Z)-propenyl sec-butyl disulfide being the major components. Previously, sec-butyl disulfide derivatives of sulfur-containing compounds were found in essential oils and/or ole-gum resins obtained from various Ferula spp [14, 16, 43–45]. It should be noted that the sec-butyl feature of sulfur-containing compounds is considered to be the chemotaxonomic marker of the family Apiaceae [44]. All FEOs, with the exception of FEOFr, also had reasonable levels of 10-epi-γ-eudesmol (2.9–10.3%) and guaiol-type sesquiterpenes (up to 8.5%). Among Ferula spp., eudesmane-type sesquiterpenes have been reported in essential oils of F. communis L., F. assa-foetida L., and F. alliacea Boiss [14, 44, 46].
Evaluation of the immunomodulatory properties of FEOs revealed that all oils stimulated [Ca2+]i flux in human neutrophils and stimulated ROS production in human neutrophils and mouse phagocytes, regardless of the plant part or flowering stage used for essential oil extraction. Although various essential oils have been reported to modulate ROS production and Ca2+ mobilization in neutrophils, including oils from another Ferula spp. [6, 7, 47, 48], the current study is the first to report the phagocyte stimulatory effects of essential oils with a high content of organosulfur compounds.
Various TRP channels are functionally expressed in phagocytic cells, and a transient elevation in [Ca2+]i through activation of these channels can regulate various aspects of inflammatory and immune responses [39]. In addition, we found that TRPV1 channels were activated by geranylacetone and inhibited by myristicin, which are present in essential oil from a different species of Ferula [6]. In an attempt to investigate the molecular mechanisms of the immunomodulatory effects of FEOs, we evaluated the effects of capsazepine, a TRPV1 antagonist, on neutrophil activation by FEOFr and showed that the response was completely inhibited. In addition FEOFr activated a transient increase in [Ca2+]i in TRPV1 channel-transfected HEK293 cells, but not in nontransfected cells. Together, these data clearly demonstrate that similar to capsaicin the molecular target of FEOs is TRPV1 and that activation of TRPV1 leads to phagocyte activation. Although it may seem counterintuitive that neutrophil activation could contribute to anti-inflammatory activity, it should be noted that capsaicin is a well-known TRPV1 agonist with anti-inflammatory activity (reviewed in [49]). For example, capsaicin can ameliorate experimental arthritis and it has become clear that this natural compound could also exert a protective effect against different forms of arthritis and pain-related processes in humans [49]. Thus, our finding that the molecular target for FEO is TRPV1 channels can explain in part the molecular mechanism of anti-inflammatory activity of traditional remedies prepared from this plant [24].
Because the extracted essential oils represent mixtures and the primary components are very difficult to isolate and are not commercially available, it is difficult to identify the specific compound responsible for TRPV1 activation and thus phagocyte activation. However, it should be noted that the primary components in all of the extracts were (E/Z)-propenyl sec-butyl disulfides (e.g., these compounds represent 69.2% of the total FEOFr composition). Thus, it is reasonable to hypothesize that (E/Z)-propenyl sec-butyl disulfides are the main bioactive components of these essential oils. Indeed, other organosulfur compounds have been shown to activate TRPA1 and TRPV1 channels [50, 51]. As essential oils extracted from various parts of the plant had different concentrations of (E)- and (Z)-propenyl sec-butyl disulfides, we used this feature to consider which might be the active compound and isomer based on a correlation of individual isomer concentration with ROS activating capacity. Comparison of EC50 values for each FEO sample for stimulation of neutrophil ROS production (Table 2) vs. the content of (E)- and (Z)-propenyl sec-butyl disulfide present in that sample (Table 1) demonstrated a relatively high linear correlation when comparing (Z)-propenyl sec-butyl disulfide content to ROS-activating capacity (r = 0.740; n = 7), whereas no correlation was found for (E)-propenyl sec-butyl disulfide (r = −0.06; n = 7). Similar results were obtained when plotting the EC50 for ROS production by murine phagocytes vs. content of (Z)- and (E)-propenyl sec-butyl disulfides (r = 0.653 and −0.06, respectively) in each sample (data not shown). Thus, these data suggest that (Z)-propenyl sec-butyl disulfide is the main bioactive component in FEO, although further research with isolated individual isomers is needed to verify this conclusion.
Previously, we developed a pharmacophore model of the TRPV1 ligand binding site that was based on conformations of TRPV1 channel agonists, including capsaicin, N-geranyl cyclopropylcarboxamide, and compound 2k [6]. In the current study, we used molecular modeling with this pharmacophore model to consider whether (E/Z)-sec-butyl disulfides did indeed fit with the structural requirements of a TRPV1 ligand and which of the 4 possible isomers might have the best fit. Thus, we overlaid all 4 isomers of propenyl sec-butyl disulfides separately onto the TRPV1 channel pharmacophore model to determine their geometric and field similarities. Superimposition of the isomers onto the template showed a relatively higher quality of alignment in terms of geometric similarity (Sim) for (Z) compared to (E) isomers: (S)(Z)-propenyl sec-butyl disulfide (Sim = 0.637) > (R)(Z)-propenyl sec-butyl disulfide (Sim = 0.588) > (S)(E)-propenyl sec-butyl disulfide (Sim = 0.587) > (R)(E)-propenyl sec-butyl disulfide (Sim=0.561). As shown in Supplemental Fig. S2, overlay of the (S) enantiomers [(S)(Z)-propenyl sec-butyl disulfide and (S)(E)-propenyl sec-butyl disulfide] on a pharmacophore model of the TRPV1 channel ligand binding site indicated that the (Z) configuration was preferred over the (E) configuration. Specifically, the —SCH = CHCH3 group of the (Z) isomer fit better into the corresponding region of the scaffold. This high-quality fit was geometrically unattainable for the (E) isomer, which may result in steric repulsion by the TRPV1 channel ligand-binding site and, hence, less affinity to the receptor. Likewise, overlay of the (R) enantiomers showed that the (Z) configuration was preferred over the (E) configuration (not shown). Thus, these data, together with the correlation analysis above, suggest that (Z)-propenyl sec-butyl disulfide is likely the bioactive molecule responsible for modulation of phagocytic responses and TRPV1 channel activity. However, further studies with purified compounds are necessary to address this question.
Despite the evidence implicating (Z)-propenyl sec-butyl disulfide as the active component, we also cannot absolutely exclude a modulatory effect of other minor constituents of the essential oils. For example, FEOFr also contains α-pinene, myrcene, limonene, (E/Z)-β-ocimene, and p-cymene. Note, however, that our previous analysis of all of these compounds showed that they were not able to stimulate human neutrophil Ca2+ flux [6, 7], indicating that they could not be contributors to the responses observed in the present study for FEOs. Although β-pinene can activate Ca2+ flux in human neutrophils [7], this monoterpene was only present in very small amounts (0.2%). Additional components of FEOFr were not found even in trace amounts in essential oils from other parts of this plant (Table 1); thus, they probably can be excluded from the list of bioactive compound involved in the specific phagocyte-modulatory effects measured.
Essential oil of Calycorectes sellowianus containing a high amount of guaiol (13.1%) has been reported to inhibit neutrophil chemotaxis [52]. Although there are no direct data on the immunomodulatory activity of 10-epi-γ-eudesmol, molecular modeling by Yadav et al. [53] suggests a high binding affinity of this compound to major anti-inflammatory and immunomodulatory receptors [53]. It should be noted that ferutinin, a sesquiterpenic compound isolated from Ferula spp., has Ca2+ ionophore properties [54] and can also induce Ca2+-dependent mitochondrial depolarization [55]. Another sesquiterpene, eudesmol, was recently reported as an activator of TRPA1 channels [56]. Thus, because the tested essential oils, with the exception of FEOFr, contain eudesmane-type sesquiterpenes, we also cannot exclude roles of these sesquiterpenes in modulation of Ca2+ flux in phagocytes by those essential oil extracts.
Various disulfides and trisulfides derived from garlic and onion have been shown to modulate immune functions and inflammation [57, 58]. Other reports have demonstrated that some garlic-derived organosulfur compounds, including diallyl sulfide, diallyl disulfide, diallyl trisulfide, can induce Ca2+ flux in several cell types, such as human glioblastoma cells, human colon cancer cells, and Madin-Darby canine kidney renal tubular cells [59–61]. Moreover, diallyl disulfide activated TRPA1 channels [50]. However, there are no reports on the immunomodulatory effects of (E/Z)-propenyl sec-butyl disulfides, as these compounds are probably unique in Ferula spp. In our study, we found that essential oils enriched in (E/Z)-propenyl sec-butyl disulfides were highly active, although we were not able isolate the individual isomers to conclusively determine whether one isomer configuration was more bioactive. Thus, further studies are needed to isolate the individual isomers and evaluate their immunomodulatory effects.
In conclusion, we found that FEOs contain a high amount of (E/Z)-propenyl sec-butyl disulfides and showed that these oils activated human neutrophils and murine phagocytic leukocytes, and that phagocyte activation by Ferula essential oils was inhibited by a TRPV1 antagonist. Moreover, the essential oil containing the highest amount of (E/Z)-propenyl sec-butyl disulfides activated TRPV1-tranfected cells, with subsequent desensitization after repetitive stimulation with the oil. Thus, our data provide a molecular basis to explain at least part of the beneficial therapeutic effects of F. iliensis extracts and suggest that stimulation of phagocytic leukocytes by organosulfur components from this plant may enhance resistance to infection. Studies are now in progress to determine precisely the active component and evaluate its potential as a therapeutic treatment for various disorders with immune and inflammatory mechanisms.
AUTHORSHIP
G.Ö., I.A.S., S.V.K., A.I.K., and M.T.Q. participated in research design. G.Ö., I.A.S., G.A.U., L.N.K., S.R.A., K.T.A., and A.I.K. conducted the experiments. K.H.C.B., A.I.K., and D.S.D. contributed new reagents or analytical tools. G.Ö., I.A.S., L.N.K., S.R.A., T.Ö., K.H.C.B., A.I.K., D.S.D., and M.T.Q. performed data analysis. T.Ö., I.A.S., G.A.U., K.T.A., A.I.K., D.S.D., and M.T.Q. wrote or contributed to writing the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported in part by U.S. National Institutes of Health IDeA Program COBRE Grant GM110732 (M.T.Q.); Ministry of Education and Science, Kazakhstan Grants 0504/GF3 and 2117/GF4 (S.V.K.); USDA National Institute of Food and Agriculture Hatch project 1009546 (M.T.Q.); Montana University System Research Initiative 51040-MUSRI2015-03 (M.T.Q.); and the Montana State University Agricultural Experiment Station. We thank Dr. Edgar Kooijman (Kent State University) for expert advice on lipophilic compounds and membrane interactions.
Glossary
- FEO
essential oil of F. iliensis
- FID
flame ionization detector
- fMLF
N-formyl-Met-Leu-Phe
- GC
gas chromatography
- HEK
human embryonic kidney (cell)
- MS
mass spectrometry
- ROS
reactive oxygen species
- RRI
relative retention indices
- Sim
geometric similarity
- SOD
superoxide dismutase
- TRPV1
transient receptor potential vanilloid 1 (Channel)
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Beutler B. (2004) Innate immunity: an overview. Mol. Immunol. 40, 845–859. [DOI] [PubMed] [Google Scholar]
- 2.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. (2000) Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. 80, 617–653. [DOI] [PubMed] [Google Scholar]
- 3.Su Y., Richmond A. (2015) Chemokine regulation of neutrophil infiltration of skin wounds. Adv. Wound Care (New Rochelle) 4, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher S., Steffy K., Averett D. (2006) Masked oral prodrugs of toll-like receptor 7 agonists: a new approach for the treatment of infectious disease. Curr. Opin. Investig. Drugs 7, 702–708. [PubMed] [Google Scholar]
- 5.Schepetkin I. A., Kirpotina L. N., Khlebnikov A. I., Quinn M. T. (2007) High-throughput screening for small-molecule activators of neutrophils: identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 71, 1061–1074. [DOI] [PubMed] [Google Scholar]
- 6.Schepetkin I. A., Kushnarenko S. V., Özek G., Kirpotina L. N., Sinharoy P., Utegenova G. A., Abidkulova K. T., Özek T., Başer K. H., Kovrizhina A. R., Khlebnikov A. I., Damron D. S., Quinn M. T. (2016) Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 64, 7156–7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schepetkin I. A., Kushnarenko S. V., Özek G., Kirpotina L. N., Utegenova G. A., Kotukhov Y. A., Danilova A. N., Özek T., Başer K. H., Quinn M. T. (2015) Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 63, 4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Cássia da Silveira e Sá R., Andrade L. N., Dos Reis Barreto de Oliveira R., de Sousa D. P. (2014) A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 19, 1459–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadraei H., Asghari G. R., Hajhashemi V., Kolagar A., Ebrahimi M. (2001) Spasmolytic activity of essential oil and various extracts of Ferula gummosa Boiss. on ileum contractions. Phytomedicine 8, 370–376. [DOI] [PubMed] [Google Scholar]
- 10.Maggi F., Cecchini C., Cresci A., Coman M. M., Tirillini B., Sagratini G., Papa F. (2009) Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy). Fitoterapia 80, 68–72. [DOI] [PubMed] [Google Scholar]
- 11.Al-Ja’fari A. H., Vila R., Freixa B., Tomi F., Casanova J., Costa J., Cañigueral S. (2011) Composition and antifungal activity of the essential oil from the rhizome and roots of Ferula hermonis. Phytochemistry 72, 1406–1413. [DOI] [PubMed] [Google Scholar]
- 12.Zellagui A., Gherraf N., Rhouati S. (2012) Chemical composition and antibacterial activity of the essential oils of Ferula vesceritensis Coss et Dur. leaves, endemic in Algeria. Org. Med. Chem. Lett. 2, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Znati M., Jabrane A., Hajlaoui H., Harzallah-Skhiri F., Bouajila J., Casanova J., Ben Jannet H. (2012) Chemical composition and in vitro evaluation of antimicrobial and anti-acetylcholinesterase properties of the flower oil of Ferula lutea. Nat. Prod. Commun. 7, 947–950. [PubMed] [Google Scholar]
- 14.Kavoosi G., Rowshan V. (2013) Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assa-foetida oleo-gum-resin: effect of collection time. Food Chem. 138, 2180–2187. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan H., Yanmis D., Karadayi M., Bal T., Baris O., Gulluce M. (2014) Determination of genotoxic and antigenotoxic properties of essential oil from Ferula orientalis L. using Ames/Salmonella and E. coli WP2 bacterial test systems. Toxicol. Ind. Health 30, 714–723. [DOI] [PubMed] [Google Scholar]
- 16.Zhi-da M., Qi-Fi M., Mizuno M., Tanaka T., Iinuma M. (1987) Polysulfanes in the volatile oils of Ferula species. Planta Med. 53, 300–302. [DOI] [PubMed] [Google Scholar]
- 17.Duan H., Takaishi Y., Tori M., Takaoka S., Honda G., Ito M., Takeda Y., Kodzhimatov O. K., Kodzhimatov K., Ashurmetov O. (2002) Polysulfide derivatives from Ferula foetida. J. Nat. Prod. 65, 1667–1669. [DOI] [PubMed] [Google Scholar]
- 18.Yousefi M., Mohammadi M., Habibi Z. (2011) Disulphides in the volatile oil of Ferula behboudiana Rech. f. & Esfand. Nat. Prod. Res. 25, 1629–1634. [DOI] [PubMed] [Google Scholar]
- 19.Ye B., Wang S., Zhang L. (2011) Studies on the detoxification effects and acute toxicity of a mixture of cis-sec-butyl-1-propoenyl disulphide and trans-sec-butyl-1-propoenyl disulphide isolated from crude essential oil of Ferula sinkiangensis K.M. Shen, a Chinese traditional herbal medicine. Nat. Prod. Res. 25, 1161–1170. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Wang Y., Zhu J., Xiao Q. (2011) Essential oil composition analysis of three cultivars seeds of Resina ferulae from Xinjiang, China. Pharmacogn. Mag. 7, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kavoosi G., Tafsiry A., Ebdam A. A., Rowshan V. (2013) Evaluation of antioxidant and antimicrobial activities of essential oils from Carum copticum seed and Ferula assafoetida latex. J. Food Sci. 78, T356–T361. [DOI] [PubMed] [Google Scholar]
- 22.Alipour Z., Taheri P., Samadi N. (2015) Chemical composition and antibacterial activity of the essential oils from flower, leaf and stem of Ferula cupularis growing wild in Iran. Pharm. Biol. 53, 483–487. [DOI] [PubMed] [Google Scholar]
- 23.Izzo A. A., Hoon-Kim S., Radhakrishnan R., Williamson E. M. (2016) A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 30, 691–700. [DOI] [PubMed] [Google Scholar]
- 24. Sinitsin, G. S. (1982) New Medicinal Plants of Kazakhstan. Nauka, Alma-Ata, Kazakhstan, p. 127.
- 25.Abd El-Razek M. H., Ohta S., Hirata T. (2003) Terpenoid coumarins of the genus Ferula. Heterocycles 60, 689–716. [Google Scholar]
- 26.Özek G., Ishmuratova M., Tabanca N., Radwan M. M., Göger F., Özek T., Wedge D. E., Becnel J. J., Cutler S. J., Can Başer K. H. (2012) One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 35, 650–660. [DOI] [PubMed] [Google Scholar]
- 27.Gelfand E. W., Cheung R. K., Grinstein S. (1986) Mitogen-induced changes in Ca2+ permeability are not mediated by voltage-gated K+ channels. J. Biol. Chem. 261, 11520–11523. [PubMed] [Google Scholar]
- 28.Sinharoy P., Zhang H., Sinha S., Prudner B. C., Bratz I. N., Damron D. S. (2015) Propofol restores TRPV1 sensitivity via a TRPA1-, nitric oxide synthase-dependent activation of PKCε. Pharmacol. Res. Perspect. 3, e00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daiber A., August M., Baldus S., Wendt M., Oelze M., Sydow K., Kleschyov A. L., Munzel T. (2004) Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 36, 101–111. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell V. B., Azzi A. (1996) High rates of extracellular superoxide generation by cultured human fibroblasts: involvement of a lipid-metabolizing enzyme. Biochem. J. 318, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Káldi K., Szászi K., Koncz G., Suszták K., Ligeti E. (1996) Arachidonic acid activatable electrogenic H+ transport in the absence of cytochrome b558 in human T lymphocytes. FEBS Lett. 381, 156–160. [DOI] [PubMed] [Google Scholar]
- 32.Cheeseright T., Mackey Phd M., Rose Phd S., Vinter Phd A. (2007) Molecular field technology applied to virtual screening and finding the bioactive conformation. Expert Opin. Drug Discov. 2, 131–144. [DOI] [PubMed] [Google Scholar]
- 33.Kim M. J., Son H. J., Kim Y., Kweon H. J., Suh B. C., Lyall V., Rhyu M. R. (2014) Selective activation of hTRPV1 by N-geranyl cyclopropylcarboxamide, an amiloride-insensitive salt taste enhancer. PLoS One 9, e89062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortar G., Schiano Moriello A., Morera E., Nalli M., Di Marzo V., De Petrocellis L. (2014) Effect of acyclic monoterpene alcohols and their derivatives on TRP channels. Bioorg. Med. Chem. Lett. 24, 5507–5511. [DOI] [PubMed] [Google Scholar]
- 35.Perry N. B., Anderson R. E., Brennan N. J., Douglas M. H., Heaney A. J., McGimpsey J. A., Smallfield B. M. (1999) Essential oils from dalmatian sage (Salvia officinalis L.): variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 47, 2048–2054. [DOI] [PubMed] [Google Scholar]
- 36.Forsman H., Dahlgren C. (2010) The FPR2-induced rise in cytosolic calcium in human neutrophils relies on an emptying of intracellular calcium stores and is inhibited by a gelsolin-derived PIP2-binding peptide. BMC Cell Biol. 11, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W., Chung S. C. (2003) Flow cytometric evaluation of leukocyte function in rat whole blood. In Vitro Cell. Dev. Biol. Anim. 39, 413–419. [DOI] [PubMed] [Google Scholar]
- 38.Premkumar L. S. (2014) Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 5, 1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heiner I., Eisfeld J., Lückhoff A. (2003) Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 33, 533–540. [DOI] [PubMed] [Google Scholar]
- 40.Köse S. A., Nazıroğlu M. (2014) Selenium reduces oxidative stress and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Biol. Trace Elem. Res. 158, 136–142. [DOI] [PubMed] [Google Scholar]
- 41.Schäfer G., Kaschula C. H. (2014) The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anticancer. Agents Med. Chem. 14, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iciek M., Kwiecień I., Włodek L. (2009) Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 50, 247–265. [DOI] [PubMed] [Google Scholar]
- 43.Iranshahi M., Amin G. R., Amini M., Shafiee A. (2003) Sulfur containing derivatives from Ferula persica var. latisecta. Phytochemistry 63, 965–966. [DOI] [PubMed] [Google Scholar]
- 44.Kasaian J., Asili J., Iranshahi M. (2016) Sulphur-containing compounds in the essential oil of Ferula alliacea roots and their mass spectral fragmentation patterns. Pharm. Biol. 54, 2264–2268. [DOI] [PubMed] [Google Scholar]
- 45.Iranshahi M., Mojarab M., Sadeghian H., Hanafi-Bojd M. Y., Schneider B. (2008) Polar secondary metabolites of Ferula persica roots. Phytochemistry 69, 473–478. [DOI] [PubMed] [Google Scholar]
- 46.Maggi F., Papa F., Dall’Acqua S., Nicoletti M. (2016) Chemical analysis of essential oils from different parts of Ferula communis L. growing in central Italy. Nat. Prod. Res. 30, 806–813. [DOI] [PubMed] [Google Scholar]
- 47.Basile A., Senatore F., Gargano R., Sorbo S., Del Pezzo M., Lavitola A., Ritieni A., Bruno M., Spatuzzi D., Rigano D., Vuotto M. L. (2006) Antibacterial and antioxidant activities in Sideritis italica (Miller) Greuter et Burdet essential oils. J. Ethnopharmacol. 107, 240–248. [DOI] [PubMed] [Google Scholar]
- 48.Cosentino M., Luini A., Bombelli R., Corasaniti M. T., Bagetta G., Marino F. (2014) The essential oil of bergamot stimulates reactive oxygen species production in human polymorphonuclear leukocytes. Phytother. Res. 28, 1232–1239. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes E. S., Cerqueira A. R., Soares A. G., Costa S. K. (2016) Capsaicin and its role in chronic diseases. Adv. Exp. Med. Biol. 929, 91–125. [DOI] [PubMed] [Google Scholar]
- 50.Bautista D. M., Movahed P., Hinman A., Axelsson H. E., Sterner O., Högestätt E. D., Julius D., Jordt S. E., Zygmunt P. M. (2005) Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salazar H., Llorente I., Jara-Oseguera A., García-Villegas R., Munari M., Gordon S. E., Islas L. D., Rosenbaum T. (2008) A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 11, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apel M. A., Lima M. E., Sobral M., Young M. C., Cordeiro I., Schapoval E. E., Henriques A. T., Moreno P. R. (2010) Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm. Biol. 48, 433–438. [DOI] [PubMed] [Google Scholar]
- 53.Yadav D. K., Mudgal V., Agrawal J., Maurya A. K., Bawankule D. U., Chanotiya C. S., Khan F., Thul S. T. (2013) Molecular docking and ADME studies of natural compounds of Agarwood oil for topical anti-inflammatory activity. Curr. Comput. Aided Drug Des. 9, 360–370. [DOI] [PubMed] [Google Scholar]
- 54.Macho A., Blanco-Molina M., Spagliardi P., Appendino G., Bremner P., Heinrich M., Fiebich B. L., Muñoz E. (2004) Calcium ionophoretic and apoptotic effects of ferutinin in the human Jurkat T-cell line. Biochem. Pharmacol. 68, 875–883. [DOI] [PubMed] [Google Scholar]
- 55.Abramov A. Y., Duchen M. R. (2003) Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium 33, 101–112. [DOI] [PubMed] [Google Scholar]
- 56.Ohara K., Fukuda T., Okada H., Kitao S., Ishida Y., Kato K., Takahashi C., Katayama M., Uchida K., Tominaga M. (2015) Identification of significant amino acids in multiple transmembrane domains of human transient receptor potential ankyrin 1 (TRPA1) for activation by eudesmol, an oxygenized sesquiterpene in hop essential oil. J. Biol. Chem. 290, 3161–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee S. H., Liu Y. T., Chen K. M., Lii C. K., Liu C. T. (2012) Effect of garlic sulfur compounds on neutrophil infiltration and damage to the intestinal mucosa by endotoxin in rats. Food Chem. Toxicol. 50, 567–574. [DOI] [PubMed] [Google Scholar]
- 58.Hashizume Y., Shirato K., Abe I., Kobayashi A., Mitsuhashi R., Shiono C., Sato S., Tachiyashiki K., Imaizumi K. (2012) Diallyl disulfide reduced dose-dependently the number of lymphocyte subsets and monocytes in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 58, 292–296. [DOI] [PubMed] [Google Scholar]
- 59.Das A., Banik N. L., Ray S. K. (2007) Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer 110, 1083–1095. [DOI] [PubMed] [Google Scholar]
- 60.Chen C. Y., Huang C. F., Tseng Y. T., Kuo S. Y. (2012) Diallyl disulfide induces Ca2+ mobilization in human colon cancer cell line SW480. Arch. Toxicol. 86, 231–238. [DOI] [PubMed] [Google Scholar]
- 61.Jan C. R., Lo H. R., Chen C. Y., Kuo S. Y. (2012) Effect of allyl sulfides from garlic essential oil on intracellular ca2+ levels in renal tubular cells. J. Nat. Prod. 75, 2101–2107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.