MPL-chitosan is a potential new protective mucosal TB vaccine adjuvant.
Keywords: tuberculosis, Th17/Th1 responses, mucosal immunity, adjuvants
Abstract
Pulmonary tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) is a leading cause of global morbidity and mortality. The only licensed TB vaccine, Mycobacterium bovis bacillus Calmette-Guerin (BCG), has variable efficacy in protecting against pulmonary TB. Thus, the development of more effective TB vaccines is critical to control the TB epidemic. Specifically, vaccines delivered through the mucosal route are known to induce Th17 responses and provide superior protection against Mtb infection. However, already tested Th17-inducing mucosal adjuvants, such as heat-labile enterotoxins and cholera toxins, are not considered safe for use in humans. In the current study, we rationally screened adjuvants for their ability to induce Th17-polarizing cytokines in dendritic cells (DCs) and determined whether they could be used in a protective mucosal TB vaccine. Our new studies show that monophosphoryl lipid A (MPL), when used in combination with chitosan, potently induces Th17-polarizing cytokines in DCs and downstream Th17/Th1 mucosal responses and confers significant protection in mice challenged with a clinical Mtb strain. Additionally, we show that both TLRs and the inflammasome pathways are activated in DCs by MPL-chitosan to mediate induction of Th17-polarizing cytokines. Together, our studies put forward the potential of a new, protective mucosal TB vaccine candidate, which incorporates safe adjuvants already approved for use in humans.
Introduction
Mtb, the causative agent of TB, causes an estimated 1.1 million deaths and 9 million new cases each year [1]. Approximately one-third of the world’s population is latently infected with Mtb [1], with 5–10% of infected individuals developing active TB disease [1]. In addition, this public health threat has been confounded by the HIV/AIDS co-pandemic and the emergence of multidrug-resistant and extensively drug-resistant Mtb [2]. The only licensed TB vaccine currently in use, BCG, is a live attenuated vaccine that was passaged by Calmette and Guerin almost 100 yr ago. In most TB-endemic countries, BCG is administered intradermally shortly after birth [2]. Whereas BCG effectively protects against disseminated childhood TB, it has variable efficacy in protecting adolescents and adults against pulmonary TB [2]. Thus, the development of a more effective vaccine is a fundamental step in controlling the TB epidemic.
The transmission of Mtb occurs primarily through inhalation of fine droplets containing the bacilli from an infected individual. As the respiratory tract is the natural route of Mtb infection, mucosal vaccination is known to induce superior protection against Mtb challenge, when compared with parenteral routes of vaccination [3–6]. Enhanced protection by mucosal vaccination is thought to be a result of preferential trafficking of antigen-specific, vaccine-induced T cells back to the site of vaccination [7], possibly localizing within the airway lumen [6]. Thus, the development of a novel vaccine formulation, comprised of an immunodominant Mtb antigen(s) with an appropriate and safe mucosal adjuvant for use in humans, is crucial to improve current levels of vaccine-induced immunity against TB.
CD4+ Th cells producing IFN-γ have been conventionally thought to mediate protection against Mtb infection [8]. Accordingly, most of the TB vaccines in human clinical trials have evaluated IFN-γ production as a readout for vaccine efficacy [8]. However, despite induction of a strong IFN-γ response in humans [9], the first modern vaccine, modified vaccinia Ankara 85A, tested in humans did not improve vaccine efficacy conferred by BCG vaccination [10]. In recent times, we [7, 11–13] and others [14–16] have put forward the new concept that Th17 cells are critical for vaccine-induced immunity against TB. Importantly, mucosal vaccination with mucosal adjuvants, such as Escherichia coli HLTs [12] or cholera toxin [15], induces potent Th17 mucosal responses and mediates vaccine-induced protection. However, despite the ability of these mucosal adjuvants to induce protective Th17 responses [12, 15], there are serious concerns regarding the safety of using toxin subunits as mucosal adjuvants in humans. For example, Bell’s palsy has been observed following intranasal application of the vaccine Nasalflu, which contains E. coli HLTs as an adjuvant [17]. Therefore, we need to identify safe mucosal adjuvants that can induce Th17 mucosal responses and be incorporated into TB vaccines for mucosal delivery in humans.
Adjuvants can enhance the immunogenicity of vaccine antigens by eliciting a proinflammatory response by the recruitment of APCs to the mucosal site or exert their immunopotentiating effects by enhancing antigen presentation or inducing cytokine expression or by activation of mucosal APCs. In this study, we rationally screened adjuvants alone or in combination, for their ability to induce Th17-polarizing cytokines and function in a protective mucosal TB vaccine. Among these, adjuvants, such as chitosan [18], MPL [19], curdlan [20], poly I:C [21], R848 [22], CpG [23], and PGN [24, 25], have been shown to induce Th cell responses. MPL, curdlan, poly I:C, CpG, and R848 have also been tested for use in humans [26, 27]. Our new studies, described here, show that MPL (TLR-4 agonist), when used in combination with chitosan (inflammasome stimulator), potently induces Th17-polarizing cytokines in DCs, downstream Th17 mucosal responses and confers significant protection upon challenge with a clinical HN878. Importantly, we have also identified that both TLRs and the inflammasome pathways together are required for MPL-chitosan to induce Th17-polarizing cytokines to mediate adjuvant activity. Therefore, our studies have identified MPL-chitosan as a novel Th17-inducing mucosal adjuvant for use in a protective TB vaccine. As these individual adjuvant components have already been tested for human use, we propose the development of MPL-chitosan as a novel mucosal adjuvant amendable for future use in TB vaccines for humans.
MATERIALS AND METHODS
Mice
C57BL/6J, IFN-γ−/− (The Jackson Laboratory, Bar Harbor, ME, USA), and IL-17−/− [28] mice were bred under specific pathogen-free conditions at the Washington University in St. Louis. ESAT-6 TCR Tg mice on the recombination-activating gene-deficient background [29] were obtained from the Trudeau Institute (Saranac Lake, NY, USA) and bred in-house. The NLRP3−/− and ASC−/− mice were provided by Dr. Uma Nagarajan from the University of North Carolina (Chapel Hill, NC, USA). The TLR-4−/− and TLR-2−/− mice were a generous gift from Drs. Kory Lavine and Laura Schuttpelz (Washington University in St. Louis, St. Louis, MO, USA), respectively. Mice maintained were used at 6–8 wk of age and sex matched for all experiments. All animal experiments were performed in accordance with national and institutional guidelines for animal care under approved protocols.
Generation and stimulation of BMDCs
BMDCs were generated from the bone marrow cells of mice, as we described previously [11]. In brief, cells were extracted from femurs and cultured in cDMEM containing 20 ng/ml rmGM-CSF (PeproTech, Rocky Hill, NJ, USA). Cells were cultured for 3 d, after which, an additional 10 ml fresh cDMEM containing 20 ng/ml rmGM-CSF was added. On d 7, the nonadherent cells (BMDCs) were harvested by vigorous pipetting and enriched by centrifugation. Cells were resuspended (1 × 106 cells/ml) in cDMEM, and 500 µl aliquots were seeded into 24-well tissue-culture plates and rested overnight. DCs were subsequently cultured in the presence of various adjuvants (chitosan 25 µg/ml or/and MPL 25 µg/ml, curdlan 25 µg/ml, poly I:C 25 µg/ml, R848 25 µg/ml, CpG 25 µg/ml, PGN 20 µg/ml) or in medium alone. Culture supernatants were harvested for cytokine analysis at 48 h post-treatment.
Generation of ESAT-6-Tg T cells
A single-cell suspension from lymph nodes and spleens from Mtb-specific ESAT-6 T cell Tg (ESAT-6 TCR Tg) mice were prepared as described [11]. The single-cell suspension was treated with Gey’s solution to remove any residual RBC, and CD4+ T cells were isolated using a CD4+ T Cell Isolation Kit (Miltenyi Biotec, San Diego, CA, USA). For response of resting T cells from ESAT-6 TCR Tg mice, the isolated naive T cells were subsequently cultured in complete IMDM containing IL-2 (10 U/ml) and ESAT-61–20 (10 µg/ml) and cocultured with BMDCs stimulated with MPL-chitosan for 6 d. Supernatants were harvested for cytokine analysis.
Detection of cytokines in culture supernatants
Culture supernatants were assayed for multiple cytokines, either using Milliplex (EMD Millipore, Billerica, MA, USA) or for single cytokine proteins, using DuoSet ELISA (R&D Systems, Minneapolis, MN, USA), according to recommended standard protocols.
Vaccinations, Mtb infection, and determination of bacterial load
Adjuvants were obtained from commercial vendors: MPL (Avanti Polar Lipids, Alabaster, AL, USA); poly I:C (Sigma, St. Louis, MO, USA); chitosan, curdlan, R848, and CpG (InvivoGen, San Diego, CA, USA); and PGN (BEI Resources, Manassas, VA). ESAT-61–20 peptide was obtained from New England Peptide (Gardner, MA, USA). For intranasal vaccinations, vaccine suspension was prepared with chitosan and MPL (50 μg each), mixed along with ESAT-61–20 peptide (133 μg), and delivered by the intranasal route. MPL-chitosan-ESAT-6 vaccine thus prepared was delivered to 6–8 wk old C57BL/6J, IL-17−/−, or IFN-γ−/− mice, 3 times at 2 wk intervals, whereas mock-immunized mice received PBS as control. Four weeks after the last booster immunization, mice were challenged by aerosol with Mtb strain HN878 (BEI Resources). BCG (BCG Pasteur; Trudeau Institute) and Mtb strain HN878 (BEI Resources) were grown to mid-log phase in Proskauer Beck medium containing 0.05% Tween 80 and frozen in 1 ml aliquots at −80°C. Mice were vaccinated with 1 × 106 CFUs BCG subcutaneously as relevant controls [11]. Four weeks after challenge, unvaccinated and vaccinated mice were euthanized by CO2 asphyxiation, and the lungs were aseptically excised and individually homogenized in physiologic saline solution. Serial dilutions of lung homogenates were plated on 7H11 agar for CFU and counted after 3 wk of incubation at 37°C, as described before [28].
ELISPOT assay
Antigen-specific IFN-γ- and IL-17-producing cells in immunized lungs were detected by ELISPOT assay, as described [11]. In brief, 2 wk after the last immunization, lung single-cell suspension from immunized mice was seeded in antibody-coated plates at an initial density of 5 × 105/well. Irradiated syngeneic spleen cells (2000 RADS), IL-2 (final concentration of 10 U/ml), in the presence or absence of ESAT-61–20 peptide (10 µg/ml), were added to the cultures. After 18 h, the cells secreting IFN-γ or IL-17 were detected using 5-bromo-4-chloro-3-indolyl phosphate/NBT chloride (Sigma), according to the manufacturer’s instructions. The frequency of responding cells was calculated using ImmunoSpot software, and the total number of cytokine-producing cells was determined (Cellular Technology Limited, Shaker Heights, OH, USA).
Evaluation of inflammatory lesions and formation of B cell follicles in vaccinated mice by bright field and fluorescent microscopy
Lungs from vaccinated and unvaccinated Mtb-infected mice were perfused with 10% neutral-buffered formalin and embedded in paraffin. Paraffin lung sections (5 μm) were stained with H&E, and percentage of area occupied by inflammatory cell infiltrates was calculated with an automated tool of the Zeiss Axioplan microscope. Serial sections of 5 μm paraffin-embedded lung tissues were also stained with primary antibodies specific for CD3 (clone M-20; Santa Cruz Biotechnology, Dallas, TX, USA) and biotinylated antibodies against CD45R/B220 (clone RA3-6B2; BD Biosciences, San Jose, CA, USA). To visualize the B cell follicles and T cells inside TB granulomas, we incubated lung sections with Alexa Fluor 568 donkey anti-goat IgG (A11057; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 488 streptavidin (S11223; Thermo Fisher Scientific). After washing slides, they were mounted with ProLong Gold Antifade with DAPI (P36931; Thermo Fisher Scientific), and representative pictures were taken with a Zeiss Axioplan microscope and recorded with a Hamamatsu camera. Morphometric analysis of B cell follicles was performed with the outline automated tool of the Zeiss Axioplan microscope.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). For experiments with 2 groups, 2-tailed Student’s t-tests were performed. For 2 or more groups, a one-way ANOVA was used.
RESULTS
MPL and chitosan synergistically induce Th17-polarizing cytokines, while inducing low levels of the anti-inflammatory cytokine IL-10
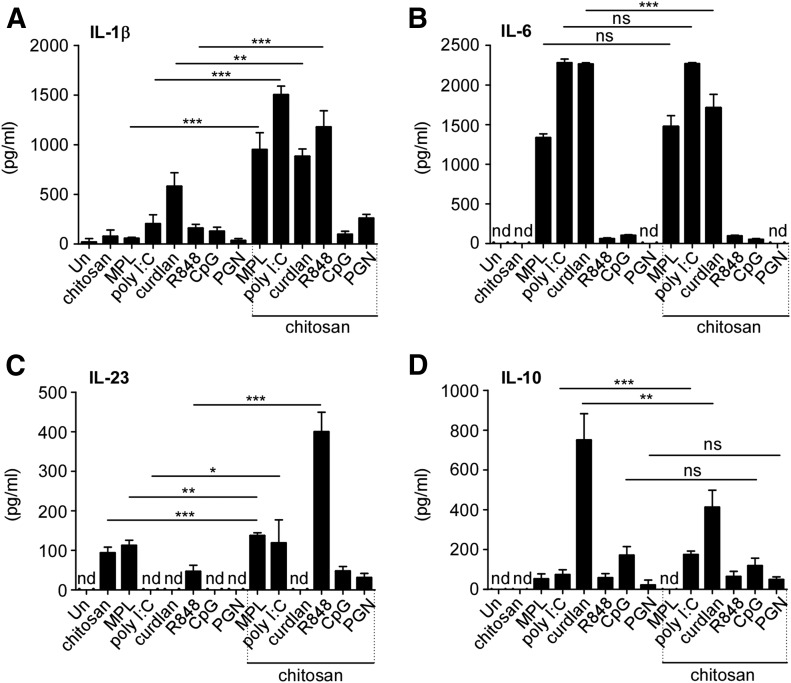
We [7, 12] and others [16] have demonstrated that mucosal vaccine-induced protection against Mtb may be IL-17 dependent. However, as a result of safety concerns, no currently available mucosal adjuvants that potently induce Th17 responses in animal models are licensed for use in human TB vaccines. As mucosal DCs are likely the key APCs upon mucosal vaccination, we first rationally screened adjuvants known to induce Th17 cell-driving, polarizing cytokines, such as IL-1β, IL-6, and IL-23, in DCs in vitro. Among these, adjuvants, such as chitosan [18], MPL [19], curdlan [20], poly I:C [21], R848 [22], CpG [23], and PGN [24, 25], are thought to induce Th17-polarizing cytokines in DCs. Chitosan has both mucoadhesive and adjuvant properties and has been tested in human vaccine formulations [27]. Thus, we treated BMDCs with MPL, poly I:C, curdlan, R848, CpG, PGN, or chitosan individually and determined whether treatment induced the production of Th17-polarizing cytokines, such as IL-1β, IL-6, and IL-23. Our results show that treatment with MPL, poly I:C, curdlan, R848, CpG, and PGN alone did not consistently induce high levels of IL-1β. However the adjuvants MPL, poly I:C, and curdlan induced high levels of IL-6 production in DCs (Fig. 1A and B). Incidentally, we found that treatment of DCs with chitosan by itself did not induce either high levels of IL-1β or IL-6. However, treating with either chitosan or MPL alone induced IL-23 levels in treated DCs (Fig. 1C). Thus, we next addressed whether cotreatment with chitosan, along with MPL, curdlan, or poly I:C, induced higher levels of Th17-polarizing cytokines than individually tested adjuvants. Whereas individual treatment with the above adjuvants induced low levels of IL-1β production, we found that cotreatment with chitosan induced significantly higher production of IL-1β in culture supernatants (Fig. 1A). In contrast, treatment with different adjuvants, along with chitosan, had no effect on IL-6 production, as individual adjuvants by themselves induced high levels of IL-6 production (Fig. 1B). However, whereas chitosan or MPL treatment alone induced IL-23 production in treated DCs, when poly I:C and R848 treatment was combined with chitosan, these treatments also induced IL-23 production in DCs (Fig. 1C). Although R848 treatment, in combination with chitosan, induced a significant increase in IL-1β and IL-23 levels in DCs, neither R848 treatment alone nor in combination with chitosan induced high IL-6 levels (Fig. 1A–C). IL-12p70 levels were below the threshold of detection for all samples tested. Together, our data suggest that cotreatment of TLR adjuvants, along with chitosan, may benefit the induction of Th17-polarizing cytokines, such as IL-1β and IL-23.
Figure 1. Synergistic induction of Th17-polarizing cytokines by MPL and chitosan in DCs.
BMDCs were generated from C57BL/6J mice, and 1 × 106 DCs were cultured in the presence or absence of various adjuvants (chitosan 25 µg/ml or/and MPL 25 µg/ml, poly I:C 25 µg/ml, curdlan 25 µg/ml, R848 25 µg/ml, CpG 25 µg/ml, PGN 20 µg/ml), alone or in combination, as indicated for 48 h. Culture supernatants were harvested for cytokine analysis: (A) IL-1β, (B) IL-6, (C) IL-23, (D) IL-10 by ELISA. The data points represent the means (sd) of values from 3 to 5 samples. *P < 0.05, **P < 0.005, ***P < 0.0005. ns, Not significant; nd, not detected; Un, untreated.
IL-10 is an anti-inflammatory cytokine that dampens Th17 responses in vaccine-induced immunity to Mtb infection [11, 30]. Thus, an ideal TB vaccine adjuvant should not induce high levels of IL-10. Therefore, we also measured IL-10 levels in culture supernatants of adjuvant-treated DCs. Curdlan treatment produced a significant increase in IL-10 levels in DCs, but this effect was dampened in combination with chitosan (Fig. 1D). Treatment of DCs with poly I:C, R848, CpG, and PGN resulted in the induction of low levels of IL-10. Combined treatment of DCs with chitosan and poly I:C produced a marginal increase of IL-10 (Fig. 1D). However, MPL, despite induction of strong Th17-polarizing cytokines (Fig. 1A–C), did not induce high IL-10 production in DCs alone or in combination with chitosan (Fig. 1D). Therefore, treatment with chitosan and MPL, in a synergistic manner, induced IL-1β and IL-23 production by DCs, whereas induction of IL-6 was solely dependent on MPL. Importantly, this treatment combination did not induce the anti-inflammatory cytokine IL-10 in DCs. Thus, we rationally selected MPL-chitosan as an adjuvant combination that may favor subsequent Mtb-specific Th17 responses in vitro and in vivo.
MPL-chitosan treatment induces Th17-polarizing cytokines in DCs through a TLR-2/4-dependent and NLRP3-independent inflammasome pathway
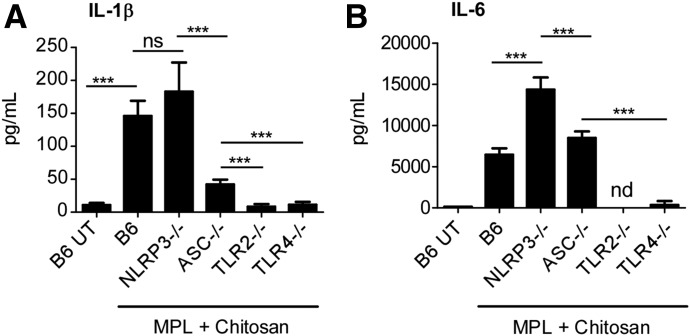
MPL is a potent TLR-4 agonist [31], whereas chitosan is thought to activate the inflammasome in LPS-stimulated macrophages by a mechanism dependent on phagocytosis [32]. To address mechanistically the signaling pathways in DCs that were involved in induction of Th17-polarizing cytokines upon MPL-chitosan treatment, we generated BMDCs from TLR-2−/− and TLR-4−/− mice, as well as mice deficient in inflammasome pathways, namely ASC−/− and NLRP3−/− [33]. We found that upon treatment with MPL-chitosan, NLRP3−/− BMDCs still produced substantial IL-1β, but IL-1β responses were substantially blunted in ASC−/− DCs (Fig. 2A). These results indicate that MPL-chitosan activates ASC inflammasome in an NLRP3-independent manner to induce IL-1β secretion. In addition, both TLR-2 and TLR-4 are critical for induction of IL-1β production following MPL-chitosan treatment, suggesting TLR-mediated activation of inflammasome (Fig. 2A). IL-6 production, induced following treatment with MPL-chitosan, was inflammasome independent, as both NLRP3−/− and ASC−/− DCs induced high levels of IL-6 upon treatment with MPL-chitosan (Fig. 2B). In sharp contrast, IL-6 production, following treatment with MPL-chitosan, was dependent on the presence of TLR-2 and TLR-4. In TLR-2−/− as well as TLR-4−/− BMDCs, IL-6 production was decreased significantly (Fig. 2B), suggesting that MPL-chitosan signals through both TLR-2 and TLR-4, and the loss of 1 receptor was sufficient to result in loss of IL-6 induction. Thus, our studies provide support that IL-6, induced upon MPL-chitosan treatment, is TLR-2/4 dependent and independent of the inflammasome, whereas IL-1β secretion is dependent on the ASC inflammasome activation, as well as through the TLR-2/4 pathways.
Figure 2. MPL-chitosan treatment activates the inflammasome and TLR-2/4 pathways for induction of Th17-polarizing cytokines.
BMDCs were generated from TLR-2−/−, TLR-4−/−, ASC−/−, and NLRP3−/− mice and 1 × 106 DCs cultured with MPL-chitosan (25 µg/ml each) or with media alone for 48 h. The culture supernatants were harvested and analyzed for the presence of (A) IL-1β and (B) IL-6 by ELISA. The data points represent the means (sd) of values from 3 to 5 samples. ***P < 0.0005. B6, C57BL/6J; UT, untreated.
MPL and chitosan treatment in DCs induces IL-17 and IFN-γ production in T cells
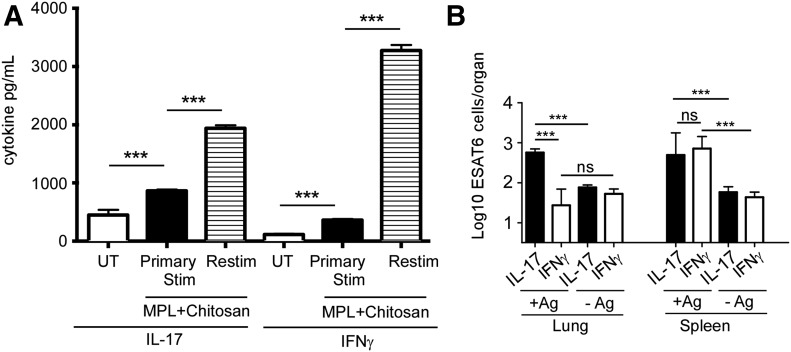
As MPL-chitosan treatment induces Th17-polarizing cytokines, such as IL-1β, IL-6, and IL-23, in DCs, we next determined whether the treatment of DCs with the adjuvant combination will drive the differentiation of naive CD4+ T cell toward a Th17-differentiation pathway. Thus, DCs treated with MPL-chitosan were cocultured with naive CD4+ T cells isolated from Mtb-specific TCR Tg mice (ESAT-6 TCR Tg mice) [29], and ESAT-6 antigen in vitro. When naive CD4+ TCR Tg T cells were cultured with MPL-chitosan-primed DCs, we found that MPL-chitosan-treated DCs induced substantial IL-17 production, whereas IFN-γ levels were lower in T cell culture supernatants (Fig. 3A). However, when previously primed ESAT-6 TCR Tg T cells were rested in vitro and then restimulated with MPL-chitosan-treated DCs, we found that IFN-γ and IL-17 levels were substantially increased in restimulated Mtb-specific TCR Tg T cells (Fig. 3A). Thus, as MPL-chitosan treatment of DCs was able to drive the differentiation of naive T cells toward the Th17-differentiation pathway, along with induction of Th1 responses, we next determined whether MPL-chitosan, when used as an adjuvant, along with ESAT-61–20 antigen, will drive mucosal Th17 responses in vivo in vaccinated mice. C57BL/6J mice were mucosally vaccinated with the ESAT-61–20 MHC class II-restricted peptide, along with MPL-chitosan, and then boosted twice. We found that mucosal vaccination of ESAT-6 in MPL-chitosan potently induced a population of Th17 mucosal responses, whereas induction of ESAT-6-specific Th1 responses in the lung was limited (Fig. 3B). In contrast, both Mtb-specific Th1 and Th17 responses were induced in the spleen (Fig. 3B). Unvaccinated mice did not induce any responses (data not shown). Cells isolated from lungs and spleens and not stimulated with antigen in vitro did not induce cytokine responses (Fig. 3B). These in vivo results are consistent with our in vitro data showing that MPL-chitosan treatment of DCs can drive both Th1 and Th17 responses. Importantly, mucosal vaccination with ESAT-6 in MPL-chitosan preferentially induced Th17 responses, specifically in the lung, whereas inducing Th17/Th1 responses in the spleen.
Figure 3. MPL-chitosan treatment of DCs induces IL-17 and IFN-γ production in T cells.
(A) C57BL/6J BMDCs were left untreated (UT) or treated with MPL-chitosan for 48 h, following which DCs were cocultured with naive CD4+ ESAT-6 TCR Tg T cells (Primary Stim) in the presence of ESAT-61–20 antigen. Additionally, previously primed CD4+ ESAT-6 TCR Tg T cells were rested in vitro, following which they were restimulated with MPL-chitosan-treated DCs (Restim). The supernatants from T cell cocultures were collected for cytokine analysis by ELISA. (B) C57BL/6J mice were vaccinated with ESAT-61–20 peptide (133 µg/mouse), formulated in MPL-chitosan adjuvant (50 µg each /mouse), intranasally 3 times with 2 wk intervals. On d 14 post-last booster, lungs and spleens were harvested and ESAT-6-specific IL-17, and IFN-γ-producing cells were enumerated by antigen-driven ELISPOT assay, with and without antigen (+Ag and −Ag, respectively). The data points represent the means (sd) of values from 3 to 5 samples (A) or 4 to 5 mice (B). ***P < 0.0005.
MPL-chitosan mucosal TB vaccine protects upon challenge with HN878
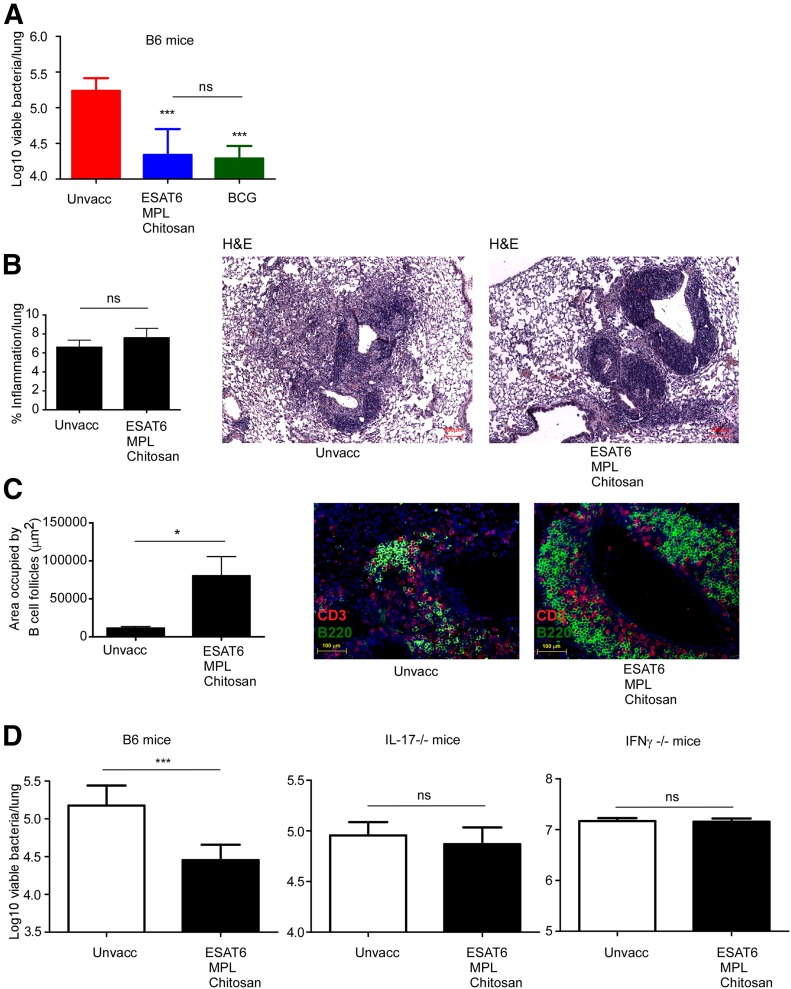
Our rationalized screening of adjuvants has identified MPL-chitosan as a potential mucosal adjuvant candidate for use in TB vaccines. Thus, we mucosally vaccinated C57BL/6J mice with the immunodominant Mtb antigen ESAT-61–20 in MPL-chitosan, followed by 2 boosts. In addition, a control group of mice was parenterally vaccinated with the gold standard TB vaccine, BCG. Vaccinated and unvaccinated mice were rested for 4 wk and then challenged with a low dose of aerosolized hypervirulent clinical Mtb strain HN878. As expected, BCG vaccination resulted in significant protective efficacy upon Mtb challenge (Fig. 4A). Importantly, we found that mucosal vaccination with ESAT-61–20 in MPL-chitosan also induced significant protection in the lung upon Mtb challenge compared with protection induced with BCG vaccination (Fig. 4A). In addition, mice mucosally vaccinated with ESAT-61–20 in MPL-chitosan, upon Mtb challenge, did not show increased lung inflammation compared with unvaccinated mice (Fig. 4B). In contrast, mice vaccinated with ESAT-61–20 in MPL-chitosan developed larger aggregates of B cell follicle-containing granulomas (Fig. 4C), a correlate associated with protective outcomes during vaccine responses following Mtb challenge [12, 34].
Figure 4. Mucosal TB vaccine comprising MPL-chitosan protects mice upon challenge with clinical Mtb HN878 strain.
C57BL/6J mice were vaccinated with ESAT-61–20 peptide (133 µg/mouse), formulated in MPL-chitosan adjuvant (50 µg each/mouse), delivered intranasally 3 times with 2 week intervals. Unvaccinated and BCG vaccinated mice were also included as relevant controls. Vaccinated mice received 1 × 106 CFU BCG subcutaneously. All groups of mice were rested for 30 d after the vaccinations and then challenged with aerosolized Mtb HN878 (100 CFU). (A) Lung bacterial burden was determined on d 30 post-Mtb infection in C57BL/6J mice. (B) Pulmonary inflammation was assessed on formalin-fixed, paraffin-embedded lung sections from unvaccinated (Unvacc) and MPL-chitosan-vaccinated mice on H&E-stained sections. Representative images are shown. (C) B Cell lymphoid follicle formation was determined by CD3 (red) and B220 (green) staining on formalin-fixed, paraffin-embedded sections by immunofluorescent staining. The total area occupied by B cell follicles per lobe was quantified using the morphometric tool of the Zeiss Axioplan microscope. Representative images of B cell follicles are shown. (D) Lung bacterial burden was determined on d 30 post-Mtb infection in C57BL/6J, IL-17−/−, IFN-γ−/− unvaccinated, and ESAT-61–20 MPL-chitosan-vaccinated mice. The data points represent the means (sd) of values from 4 to 5 mice. *P < 0.05, ***P < 0.0005.
IL-17 has been shown to be important in mediating immunity against TB in mucosal vaccination models [12, 16]. To address if MPL-chitosan mucosal vaccine-induced protection was dependent on the production of IL-17 or IFN-γ, IL-17−/− and IFN-γ −/− mice were vaccinated with ESAT-61–20 in MPL-chitosan, rested, and challenged with Mtb HN878. Whereas vaccine-induced protection occurred in C57BL6/J-vaccinated mice, vaccine-induced protection was lost in the absence of IFN-γ or IL-17, suggesting an important role for both cytokines in MPL-chitosan TB vaccine-mediated, vaccine-induced protection (Fig. 4D). Thus, our study has identified use of MPL-chitosan as a new and safe mucosal adjuvant for use in a protective TB vaccine and mediates protection through the Th17/Th1 pathways.
DISCUSSION
TB continues to persist as a global health concern, and the emergence of drug-resistant strains of Mtb has further heightened the spectre of TB. The currently licensed BCG vaccine provides variable efficacy against adult pulmonary TB [35]. Thus, there have been concerted efforts to develop novel vaccines for TB that will provide improved protection upon Mtb challenge. Especially important in our search for novel TB vaccines is the development of potent mucosal TB vaccines, as mucosal routes of vaccination confer superior protection upon Mtb challenge [3–6]. Thus, the identification of safe mucosal adjuvants that can be incorporated into TB vaccines for use in humans is an important focus area of vaccine research. In the current study, we show that the use of rationalized screening of Th17-inducing adjuvants resulted in the identification of MPL-chitosan as an adjuvant that can drive potent induction of Th17-polarizing cytokines in DCs, subsequent mucosal Mtb-specific Th17 responses in the lung, and mixed Th17/Th1 responses in the spleens of vaccinated mice. In addition, we show that vaccination with Mtb-specific antigen in MPL-chitosan confers protection in a mouse model of TB to levels of protection comparable with the gold standard vaccine, BCG. As both MPL and chitosan have already been individually tested safe for use in humans, our study has identified a novel use for MPL and chitosan as a combined mucosal adjuvant for use in TB vaccines, which can be developed for human use.
In an attempt to identify mucosal adjuvants that can induce T cell responses, we found that combined use of MPL and chitosan together can induce strong Th17/Th1 responses both in vitro and in vivo. Chitosan has both mucoadhesive and adjuvant properties [27], whereas MPL, a nontoxic derivative of LPS, exhibits adjuvant properties similar to those of the parent LPS molecule [36]. MPL functions as an adjuvant through the induction of chemokines, such as CCL-2 and CCL-3, resulting in the recruitment of APCs for antigen presentation [36]. In use by itself, MPL is considered a potent Th1-inducing adjuvant [37]. Furthermore, MPL-induced IL-6 in APCs is also thought to promote T cell differentiation into T follicular helper-like cells and drive antibody responses [38]. Our data show that combined use of MPL and chitosan together drives improved induction of IL-1β, IL-6, and IL-23, key cytokines involved in Th17 cell differentiation. In contrast, MPL and chitosan, individually and in combination, do not induce the anti-inflammatory cytokine IL-10, which can adversely limit Th17/Th1 responses [11]. Accordingly, DCs treated with MPL-chitosan are potent at driving naive T cells to differentiate into Th17/Th1 cells, both in vitro and in vivo, when formulated and delivered as a mucosal vaccine. Consistent with a role for MPL in driving Th1 responses [37], we observed IFN-γ induction in T cells cocultured with MPL-chitosan-treated DCs. Indeed, in vivo, we found that mucosal delivery of Mtb antigen in MPL-chitosan also induced Th1 responses in the spleen but not in the lung. This differential localization of Th1 and Th17 cells may be associated with differential expression of chemokine receptors, such as CCR4, that allow homing of Th17 cells but not Th1 cells to mucosal sites [7]. Thus, our studies, in a rationalized manner, have identified that combined use of both MPL and chitosan together can serve as potent Th17/Th1-inducing adjuvants for use in TB vaccines. As IL-17−/− and IFN-γ−/− vaccinated mice are not protected upon challenge with Mtb, our data suggest that Th17/ Th1 responses are both required to mediate vaccine-induced protection against TB.
Chitosan has been reported to activate the inflammasome through an NLRP3-dependent pathway following phagocytosis in macrophages [32]. This activation of the inflammasome in macrophages was dependent on its acetylation and particle size, with smaller particles inducing a greater response [32]. However, we observe that in DCs, chitosan treatment by itself fails to induce IL-1β. However, combined treatment of chitosan with MPL induces substantial IL-1β induction in wild-type and NLRP3−/− DCs, whereas ASC−/− DCs show reduced levels of IL-1β induction. These results could be a result of differential inflammasome activation in DCs by MPL-chitosan when compared with activation in macrophages by chitosan [32]. Thus, our data support a role for an NLRP3-independent, ASC-dependent inflammasome pathway in IL-1β induction when both TLR-2/4 engagement is activated through the combined use of MPL-chitosan. These effects could be a result of direct activation of the inflammasome pathway by TLR signaling without priming by IL-1R-associated kinase 1 [39] or through the involvement of another NLR protein. In contrast, induction of IL-6 by combined MPL-chitosan treatment does not depend on either NLRP3- or ASC-dependent inflammasome pathways. Instead, induction of IL-6 is mediated via the engagement of TLR-2 and TLR-4. Consistent with these results, although MPL is primarily considered a potent TLR-4 agonist, MPL can also engage TLR-2 to induce proinflammatory cytokines in human monocytes [40]. Thus, our studies project the involvement of both the inflammasome and TLR pathways in induction of Th17-polarizing cytokines upon MPL-chitosan treatment in DCs.
Our studies show that mucosal delivery of Mtb antigen in MPL-chitosan induces protective Th17/Th1 vaccine responses and upon challenge with a hypervirulent clinical Mtb strain, induces formation of protective TB granulomas, immune correlates that we have shown previously to be important for protective vaccine responses [12, 13]. MPL, used in this study, is a synthetic derivative but with a highly acceptable safety profile, making it suitable for use as a mucosal vaccine adjuvant in humans [41]. MPL has been approved as a component in an improved hepatitis B vaccine and other vaccines that are in various stages of clinical testing [41]. The intranasal administration of TLR-4 agonists has also shown that the mucosal route is safe and effective and may reduce possible safety concerns of systemic administration of TLR agonists. Chitosan has been designated as “generally recognized as safe” in the United States, Japan, and Italy, although it has not been used in any marketed vaccine formulations [42]. However, a few recent studies have elucidated upon the clinical applications of chitosan as an adjuvant. For example, the safety and immune-stimulatory capacity of a chitosan-glutamate intranasal delivery system for the diphtheria toxoid antigen CRM197, among healthy volunteers, has been reported [43]. Furthermore, a vaccine formulated with chitosan, Norovirus virus-like particle antigen and MPL as an immune enhancer, was administered intranasally in clinical trials, and no vaccine-related serious adverse effects were observed [44]. Based on these safety profiles, our studies project the combined use of MPL and chitosan as a potential adjuvant candidate for mucosal vaccine formulations against TB. Additionally, as Th17 vaccine responses are critical for protection against other significant pulmonary pathogens, such as Streptococcus pneumonia [45, 46], Bordetella pertussis [47, 48], Klebsiella pneumonia [49], and Pseudomonas aeruginosa [50], identification of safe and potent Th17-inducing adjuvants, such as MPL-chitosan, can be incorporated into effective mucosal vaccines against several important pulmonary diseases.
In summary, in the current study, we have rationally screened adjuvants and identified combined use of MPL and chitosan as a novel formulation for use as a mucosal adjuvant to generate protective immune responses against Mtb infection. The fact that this vaccine formulation is protective against emerging, clinically relevant Mtb strains, such as the HN878 strain, further supports the use of MPL-chitosan as a potent Th17-inducing adjuvant for mucosal use in human TB vaccines. Further investigations and trials with primate models will be very crucial toward achieving higher goals and translation of MPL-chitosan for use in a human TB vaccine.
AUTHORSHIP
M.A., H.J., and S.A.K. designed the experiments. M.A., H.J., R.D.G., and S.D. performed the experiments. M.A., R.D.G., S.D., J.R-M., and S.A.K. performed analyses. U.M.N. provided reagents. M.A. and S.A.K. wrote the paper. M.A., S.D., U.M.N., and S.A.K. edited the paper. S.A.K. provided funding.
ACKNOWLEDGMENTS
This work was supported by Washington University in St. Louis and U.S. National Institutes of Health Grant HL105427 (to S.A.K.); American Lung Association Senior Research Training Fellowship RT-30592 and Department of Molecular Microbiology, Washington University in St. Louis and Alexander and Gertrude Berg Fellowship (to K.L.G.). J.R-M. was supported by funds of the Department of Medicine, University of Rochester. The authors thank Sarah Squires (Washington University in St. Louis) and John Allen (University of North Carolina) for animal breeding. The authors also thank Drs. Kory Lavine (Washington University in St. Louis) and Laura Schuttpelz (Washington University in St. Louis) for the TLR-4−/− and TLR-2−/− mice, respectively.
Glossary
- −/−
deficient
- ASC
adaptor protein apoptosis-associated speck-like protein containing caspase activation and recruitment domain
- BCG
Mycobacterium bovis bacillus Calmette-Guerin
- BMDC
bone marrow-derived dendritic cell
- C57BL/6J
C57BL/6
- CD
cluster of differentiation
- cDMEM
DMEM with FBS
- DC
dendritic cell
- ESAT-6
early secreted antigenic target 6 kDa protein
- HLT
heat labile enterotoxin
- HN878
hypervirulent Mycobacterium tuberculosis strain
- IMDM
Iscove’s modified Dulbecco’s medium
- MPL
monophosphoryl lipid A
- Mtb
Mycobacterium tuberculosis
- NLR
nucleotide-binding oligomerization domain-like receptor
- NLRP3
nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3
- PGN
peptidoglycan
- poly I:C
polyinosinic:polycytidylic acid
- R848
Resiquimod
- rm
recombinant murine
- TB
tuberculosis
- Tg
transgenic
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.World Health Organization (2015) Global Tuberculosis Report 2015 WHO, Geneva, Switzerland. [Google Scholar]
- 2.Dye C., Glaziou P., Floyd K., Raviglione M. (2013) Prospects for tuberculosis elimination. Annu. Rev. Public Health 34, 271–286. [DOI] [PubMed] [Google Scholar]
- 3.Goonetilleke N. P., McShane H., Hannan C. M., Anderson R. J., Brookes R. H., Hill A. V. (2003) Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171, 1602–1609. [DOI] [PubMed] [Google Scholar]
- 4.Chen L., Wang J., Zganiacz A., Xing Z. (2004) Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72, 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Thorson L., Stokes R. W., Santosuosso M., Huygen K., Zganiacz A., Hitt M., Xing Z. (2004) Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173, 6357–6365. [DOI] [PubMed] [Google Scholar]
- 6.Santosuosso M., Zhang X., McCormick S., Wang J., Hitt M., Xing Z. (2005) Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 174, 7986–7994. [DOI] [PubMed] [Google Scholar]
- 7.Khader S. A., Bell G. K., Pearl J. E., Fountain J. J., Rangel-Moreno J., Cilley G. E., Shen F., Eaton S. M., Gaffen S. L., Swain S. L., Locksley R. M., Haynes L., Randall T. D., Cooper A. M. (2007) IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths K. L., Khader S. A. (2014) Novel vaccine approaches for protection against intracellular pathogens. Curr. Opin. Immunol. 28, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tameris M., Geldenhuys H., Luabeya A. K., Smit E., Hughes J. E., Vermaak S., Hanekom W. A., Hatherill M., Mahomed H., McShane H., Scriba T. J. (2014) The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS One 9, e87340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tameris M., Hokey D. A., Nduba V., Sacarlal J., Laher F., Kiringa G., Gondo K., Lazarus E. M., Gray G. E., Nachman S., Mahomed H., Downing K., Abel B., Scriba T. J., McClain J. B., Pau M. G., Hendriks J., Dheenadhayalan V., Ishmukhamedov S., Luabeya A. K., Geldenhuys H., Shepherd B., Blatner G., Cardenas V., Walker R., Hanekom W. A., Sadoff J., Douoguih M., Barker L., Hatherill M. (2015) A double-blind, randomised, placebo-controlled, dose-finding trial of the novel tuberculosis vaccine AERAS-402, an adenovirus-vectored fusion protein, in healthy, BCG-vaccinated infants. Vaccine 33, 2944–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal R., Lin Y., Obermajer N., Slight S., Nuthalapati N., Ahmed M., Kalinski P., Khader S. A. (2012) IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopal R., Rangel-Moreno J., Slight S., Lin Y., Nawar H. F., Fallert Junecko B. A., Reinhart T. A., Kolls J., Randall T. D., Connell T. D., Khader S. A. (2013) Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 6, 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monin L., Griffiths K. L., Slight S., Lin Y., Rangel-Moreno J., Khader S. A. (2015) Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol. 8, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desel C., Dorhoi A., Bandermann S., Grode L., Eisele B., Kaufmann S. H. (2011) Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 204, 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths K. L., Stylianou E., Poyntz H. C., Betts G. J., Fletcher H. A., McShane H. (2013) Cholera toxin enhances vaccine-induced protection against Mycobacterium tuberculosis challenge in mice. PLoS One 8, e78312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilo N., Alvarez-Arguedas S., Uranga S., Marinova D., Monzon M., Badiola J., Martin C. (2016) Pulmonary but not subcutaneous delivery of BCG vaccine confers protection to tuberculosis-susceptible mice by an interleukin 17-dependent mechanism. J. Infect. Dis. 213, 831–839. [DOI] [PubMed] [Google Scholar]
- 17.Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R. T., Linder T., Spyr C., Steffen R. (2004) Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 350, 896–903. [DOI] [PubMed] [Google Scholar]
- 18.Xu J., Dai W., Wang Z., Chen B., Li Z., Fan X. (2011) Intranasal vaccination with chitosan-DNA nanoparticles expressing pneumococcal surface antigen a protects mice against nasopharyngeal colonization by Streptococcus pneumoniae. Clin. Vaccine Immunol. 18, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garçon N., Chomez P., Van Mechelen M. (2007) GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6, 723–739. [DOI] [PubMed] [Google Scholar]
- 20.LeibundGut-Landmann S., Gross O., Robinson M. J., Osorio F., Slack E. C., Tsoni S. V., Schweighoffer E., Tybulewicz V., Brown G. D., Ruland J., Reis e Sousa C., Sousa C. (2007) Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8, 630–638. [DOI] [PubMed] [Google Scholar]
- 21.Garrett S., Fitzgerald M. C., Sullivan K. E. (2008) LPS and poly I:C induce chromatin modifications at a novel upstream region of the IL-23 p19 promoter. Inflammation 31, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roses R. E., Xu S., Xu M., Koldovsky U., Koski G., Czerniecki B. J. (2008) Differential production of IL-23 and IL-12 by myeloid-derived dendritic cells in response to TLR agonists. J. Immunol. 181, 5120–5127. [DOI] [PubMed] [Google Scholar]
- 23.Tokumasa N., Suto A., Kagami S., Furuta S., Hirose K., Watanabe N., Saito Y., Shimoda K., Iwamoto I., Nakajima H. (2007) Expression of Tyk2 in dendritic cells is required for IL-12, IL-23, and IFN-gamma production and the induction of Th1 cell differentiation. Blood 110, 553–560. [DOI] [PubMed] [Google Scholar]
- 24.Acosta-Rodriguez E. V., Napolitani G., Lanzavecchia A., Sallusto F. (2007) Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8, 942–949. [DOI] [PubMed] [Google Scholar]
- 25.Van Beelen A. J., Zelinkova Z., Taanman-Kueter E. W., Muller F. J., Hommes D. W., Zaat S. A., Kapsenberg M. L., de Jong E. C. (2007) Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27, 660–669. [DOI] [PubMed] [Google Scholar]
- 26.Rappuoli R., Mandl C. W., Black S., De Gregorio E. (2011) Vaccines for the twenty-first century society. Nat. Rev. Immunol. 11, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arca H. C., Günbeyaz M., Senel S. (2009) Chitosan-based systems for the delivery of vaccine antigens. Expert Rev. Vaccines 8, 937–953. [DOI] [PubMed] [Google Scholar]
- 28.Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. (2002) Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387. [DOI] [PubMed] [Google Scholar]
- 29.Reiley W. W., Calayag M. D., Wittmer S. T., Huntington J. L., Pearl J. E., Fountain J. J., Martino C. A., Roberts A. D., Cooper A. M., Winslow G. M., Woodland D. L. (2008) ESAT-6-specific CD4 T cell responses to aerosol Mycobacterium tuberculosis infection are initiated in the mediastinal lymph nodes. Proc. Natl. Acad. Sci. USA 105, 10961–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redford P. S., Murray P. J., O’Garra A. (2011) The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 4, 261–270. [DOI] [PubMed] [Google Scholar]
- 31.Romero C. D., Varma T. K., Hobbs J. B., Reyes A., Driver B., Sherwood E. R. (2011) The Toll-like receptor 4 agonist monophosphoryl lipid a augments innate host resistance to systemic bacterial infection. Infect. Immun. 79, 3576–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bueter C. L., Lee C. K., Rathinam V. A., Healy G. J., Taron C. H., Specht C. A., Levitz S. M. (2011) Chitosan but not chitin activates the inflammasome by a mechanism dependent upon phagocytosis. J. Biol. Chem. 286, 35447–35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latz E., Xiao T. S., Stutz A. (2013) Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaushal D., Foreman T. W., Gautam U. S., Alvarez X., Adekambi T., Rangel-Moreno J., Golden N. A., Johnson A. M., Phillips B. L., Ahsan M. H., Russell-Lodrigue K. E., Doyle L. A., Roy C. J., Didier P. J., Blanchard J. L., Rengarajan J., Lackner A. A., Khader S. A., Mehra S. (2015) Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat. Commun. 6, 8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colditz G. A., Brewer T. F., Berkey C. S., Wilson M. E., Burdick E., Fineberg H. V., Mosteller F. (1994) Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271, 698–702. [PubMed] [Google Scholar]
- 36.Alderson M. R., McGowan P., Baldridge J. R., Probst P. (2006) TLR4 agonists as immunomodulatory agents. J. Endotoxin Res. 12, 313–319. [DOI] [PubMed] [Google Scholar]
- 37.De Becker G., Moulin V., Pajak B., Bruck C., Francotte M., Thiriart C., Urbain J., Moser M. (2000) The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int. Immunol. 12, 807–815. [DOI] [PubMed] [Google Scholar]
- 38.Fazilleau N., McHeyzer-Williams L. J., Rosen H., McHeyzer-Williams M. G. (2009) The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nat. Immunol. 10, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin K. M., Hu W., Troutman T. D., Jennings M., Brewer T., Li X., Nanda S., Cohen P., Thomas J. A., Pasare C. (2014) IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. USA 111, 775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin M., Michalek S. M., Katz J. (2003) Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect. Immun. 71, 2498–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garçon N., Van Mechelen M. (2011) Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 10, 471–486. [DOI] [PubMed] [Google Scholar]
- 42.Smith A., Perelman M., Hinchcliffe M. (2014) Chitosan: a promising safe and immune-enhancing adjuvant for intranasal vaccines. Hum. Vaccin. Immunother. 10, 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mills K. H., Cosgrove C., McNeela E. A., Sexton A., Giemza R., Jabbal-Gill I., Church A., Lin W., Illum L., Podda A., Rappuoli R., Pizza M., Griffin G. E., Lewis D. J. (2003) Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect. Immun. 71, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Kamary S. S., Pasetti M. F., Mendelman P. M., Frey S. E., Bernstein D. I., Treanor J. J., Ferreira J., Chen W. H., Sublett R., Richardson C., Bargatze R. F., Sztein M. B., Tacket C. O. (2010) Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202, 1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malley R., Srivastava A., Lipsitch M., Thompson C. M., Watkins C., Tzianabos A., Anderson P. W. (2006) Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74, 2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z., Clarke T. B., Weiser J. N. (2009) Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Invest. 119, 1899–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higgins S. C., Jarnicki A. G., Lavelle E. C., Mills K. H. (2006) TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177, 7980–7989. [DOI] [PubMed] [Google Scholar]
- 48.Banus S., Stenger R. M., Gremmer E. R., Dormans J. A., Mooi F. R., Kimman T. G., Vandebriel R. J. (2008) The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen K., McAleer J. P., Lin Y., Paterson D. L., Zheng M., Alcorn J. F., Weaver C. T., Kolls J. K. (2011) Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Priebe G. P., Walsh R. L., Cederroth T. A., Kamei A., Coutinho-Sledge Y. S., Goldberg J. B., Pier G. B. (2008) IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 181, 4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]