Abstract
Introduction
Obesity accelerates the development and progression of pancreatic cancer, though the mechanisms underlying this association are unclear. Adipocytes are biologically active, producing factors such as hepatocyte growth factor (HGF) that may influence tumor progression. We therefore sought to test the hypothesis that adipocyte-secreted factors including HGF accelerate pancreatic cancer cell proliferation.
Material and methods
Murine pancreatic cancer cells (Pan02 and TGP-47) were grown in a) conditioned medium (CM) from murine F442A preadipocytes, b) HGF-knockdown preadipocyte CM, c) recombinant murine HGF at increasing doses, and d) CM plus HGF-receptor (c-met) inhibitor. Cell proliferation was measured using the MTT assay. ANOVA and t-test were applied; p < 0.05 considered significant.
Results
Wild-type preadipocyte CM accelerated Pan02 and TGP-47 cell proliferation relative to control (59 ± 12% and 34 ± 12%, p < 0.01, respectively). Knockdown of preadipocyte HGF resulted in attenuated proliferation vs. wild type CM in Pan02 cells (35 ± 5% vs. 68 ± 14% greater than control; p < 0.05), but proliferation in TGP-47 cells remained unchanged. Recombinant HGF dose-dependently increased Pan02, but not TGP-47, proliferation (p < 0.05). Inhibition of HGF receptor, c-met, resulted in attenuated proliferation versus control in Pan02 cells, but not TGP-47 cells.
Conclusions
These experiments demonstrate that adipocyte-derived factors accelerate murine pancreatic cancer proliferation. In the case of Pan02 cells, HGF is responsible, in part, for this proliferation.
Keywords: Adipocytes, Pancreatic cancer, Hepatocyte growth factor, Obesity
1. Introduction
Obesity portends a poor outcome in numerous cancers, including breast, colon, prostate, and others [1]. These poor outcomes include increased cancer development and poorer survival among obese individuals relative to lean cohorts of patients. Recent data have shown that pancreatic cancer is also negatively affected by obesity. Epidemiologic studies show an increased risk of developing pancreatic cancer in obese patients [2–4], and clinical data clearly demonstrate that obesity leads to poor outcomes in pancreatic cancer patients [5–8]. We recently reported that pancreatic fat is associated with higher rates of lymphatic invasion and higher mortality [9]. Despite this substantial body of clinical evidence, virtually no basic science investigation has explained the mechanisms underlying the relationship between obesity and pancreatic cancer. Our group recently reported the first in vivo model of pancreatic cancer in obesity [10]. In this model, lean mice and congenitally obese mice were inoculated with murine pancreatic cancer (Pan02) cells. We observed larger, more aggressive tumors, as well as higher mortality in the obese mice when compared with the lean animals, all of whom developed tumors.
Adipose tissue is a biologically active endocrine organ, secreting numerous cytokines, adipokines, and growth factors [11]. Adipocytes and preadipocytes, both present in adipose tissue, secrete angiogenic, anti-apoptotic, and mitogenic molecules [12–16] that could enhance tumor growth. One of these molecules is hepatocyte growth factor (HGF). Hepatocyte growth factor is known in the endocrine literature as a potent mitogenic and angiogenic growth factor for adipose tissue [15], working synergistically with vascular endothelial growth factor (VEGF) to induce endothelial cell migration [17]. HGF production from adipocytes and preadipocytes is significant, leading to elevated systemic levels of HGF in obesity [18]. In the cancer literature, HGF is known to stimulate tumor proliferation in several types of cancer, including pancreatic cancer, through its only known receptor, c-met [19–23]. Stromal fibroblasts, which are important in pancreatic adenocarcinoma biology, also have been shown to secrete HGF [19]. Based on this background and the striking results of our in vivo model of obesity and pancreatic cancer, we developed an in vitro model to investigate potential mechanisms of obesity’s effect on pancreatic cancer. We hypothesized that adipocyte-derived factors, including HGF, would accelerate pancreatic cancer cell growth.
2. Material and methods
2.1. Cell lines
Two murine pancreatic cancer cell lines, Pan02 (gift of Dr. David Linehan, Washington University, St. Louis, MO) and TGP-47 (ATCC, Manassas, VA) were studied. Pan02 cells were grown in high glucose (4 g/l) Dulbecco’s Modified Essential Medium (DMEM; Sigma, St. Louis, MO) + 10% Fetal Bovine Serum (FBS; Cellgro, Manassas, VA) containing 1% penicillin/streptomycin (Thermo Scientific, Waltham, MA). TGP-47 cells were grown in DMEM + 10% FBS + pen/strep + growth factor F-12 (Sigma). TGP-47 cells were switched to the Pan02 medium 1 passage prior to each experiment. Murine 3T3-F442A preadipocytes (a generous gift from Howard Green, Harvard University, Cambridge, MA) were maintained as previously published [15] in high glucose DMEM+ 10% bovine calf serum (BCS, Sigma) +0.2% penicillin/streptomycin. Wild type 3T3-F442A preadipocytes were induced to differentiate into mature adipocytes by switching to medium containing 10%FBS and adding 0.01 μg/ml insulin (Sigma).
2.2. HGF knockdown
In order to create an HGF knockdown cell line, 3T3-F442A cells at ~70% confluence were infected with commercially available puromycin resistance-containing lentivirus shRNA reagents targeting murine HGF (MissionTM TRC shRNA Lentiviral Target Set, Sigma Aldrich). Scrambled shRNA was used as control. Following treatment with puromycin, surviving clones were picked and screened for HGF knockdown by mRNA expression and protein production. A clonal line was established that had 93% knockdown of HGF mRNA (1.89 vs 25.3 relative units) and 99% reduction in protein secretion (0.13 ng/ml/24 h vs 9.6 ng/ml/24 h). Specific details regarding the knockdown methodology have been published previously [15].
2.3. Preparation of conditioned medium
To prepare conditioned medium, 3T3-F442A cells with and without HGF knockdown and Pan02 cells (as a control) were grown to 70–80% confluence for 72 h. Medium was collected, centrifuged to remove debris, aliquotted, and frozen at −80 °C. Conditioned medium from F442A preadipocytes is referred to as “wild type” medium, that collected from HGF knockdown cells is referred to as “knockdown” medium, and medium conditioned by Pan02 cells is considered “control” unless otherwise specified.
2.4. Measurement of pancreatic cancer cell growth
To assess cancer cell proliferation under various conditions, Pan02 cells were plated onto a 96 well plate (Corning, Corning, NY) at a concentration of 5000 cells/well and allowed to adhere for 24 h. TGP-47 cells were plated at a concentration of 5000 cells/well and allowed to adhere for 8 h. Total volume of medium in the well was 100 μl. At the end of the adherence period 50 μl of medium was removed from each well, 50 μl of the various conditioned medium was added, and the cells were permitted to proliferate for 24 h. This time point was chosen based on our previous work [15]. During the last 4 h of the growth period, cell proliferation was measured using the 3-[4,5-dimethyl-thazol-2-yl]-2,5-diphneyltetrazolium-bromide (MTT) assay (Roche Applied Diagnostics, Manheim, Germany). Each proliferation condition was tested in quadruplicate wells or greater, and each experiment was repeated at least four separate times with each cell line at a different passage number.
2.5. HGF dose-response
Recombinant HGF (R&D Systems, Minneapolis, MN) was added to Pan02 (n = 5 repeat experiments) and TGP-47 cells (n = 4 repeat experiments) plated as described above, at the following doses: 0, 0.1, 1, 10, and 100 ng/ml. After 24 h of growth, the MTT proliferation assay was performed as above.
2.6. Inhibition of the HGF receptor, c-met
In order to evaluate the effect of HGF in the conditioned medium, the MTT assay was performed using wild-type conditioned medium in combination with 100 nmol of PF-02341066 (Selleck Chem, Houston, TX), a specific inhibitor of the HGF receptor, c-met.
2.7. Detection of the HGF receptor, c-met
To examine the presence of c-met on pancreatic cancer cells, RT-PCR and immunohistochemistry were performed. Total RNA was isolated from cells and tissues by guanidinium thiocyanate-phenol-chloroform extraction, and cDNA was synthesized from 0.5 ng of total RNA using random hexamers (Applied Biosystems, Foster City, CA) in a 100 μl reaction. mRNA levels of all genes investigated were determined by Real-Time RT-PCR using iTaq SYBR Green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA) in an ABI Prism 7700 Sequence Detection System (Applied Biosystems). Five microliters of cDNA (25 ng of total RNA) were amplified in a reaction volume of 25 μl, and cycling conditions were one cycle for 2 min at 50 °C and one cycle for 3 min at 95 °C, followed by 40 cycles of 15 s of denaturation at 95 °C and 1 min of annealing/extension at 60 °C. Primer sets (forward 5′ GAATGTCGTCCTACACGGCC-3′ and reverse 5′-CACTACACAGTCAGGACACTGC-3′; Invitrogen, Carlsbad, CA) were used at a concentration of 200 nM. Expression of all genes was normalized to 36B4 (mouse acidic ribosomal phosphoprotein) expression using the delta CT method.
Murine c-met immunohistochemistry was performed using C-28 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Pan02, TGP-47, and HEK-293 cells (as a positive control) grown on glass slides, were fixed with methanol at −10 °C for 5 min, and washed with PBS 3 times. The remainder of the processing was performed as specified by the HRP rabbit ImmunoCruz Staining System (Santa Cruz Biotechnology, Santa Cruz, CA). Briefly, endogenous peroxidase activity was quenched using peroxidase block, followed by incubation in normal goat serum. Primary c-met antibody (C-28; Santa Cruz Biotechnology) was diluted 1:50 in normal goat serum for a concentration of 4 μg/ml, then added to slides and incubated overnight. Negative control slides were not exposed to primary antibody. Specimens were then probed with secondary antibody for 30 min, followed by HRP-streptavidin complex for 30 min. The HRP substrate was then added for 25 min and slides were counterstained with hematoxylin (Invitrogen) for 10 s before dehydration and mounting.
2.8. Statistical analyses
All data are expressed as mean ± SEM. Statistical comparisons of conditioned medium experiments used paired t-tests, and statistical comparisons of HGF dose response and c-met inhibition experiments used ANOVA with post-hoc Tukey comparison. A P value of <0.05 was considered significant. All statistics were performed using GraphPad Prism Software (La Jolla, CA).
3. Results
3.1. Conditioned medium enhances pancreatic cancer cell proliferation
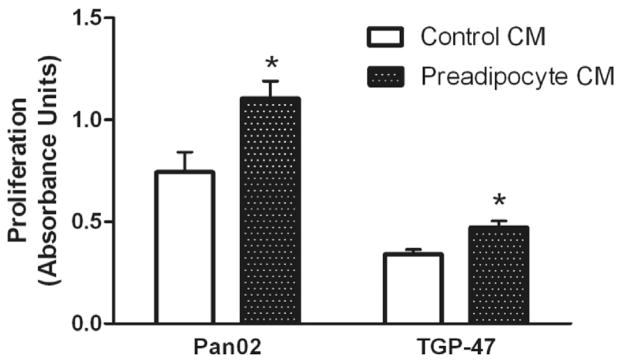
Pan02 murine pancreatic cancer cells were cultured in conditioned medium (CM) from undifferentiated (preadipocyte) F442A cells and in CM from F442A cells induced to differentiate into mature adipocytes. Undifferentiated preadipocyte medium significantly increased Pan02 proliferation over control by 59 ± 12% (p < 0.01). Similarly, differentiated adipocyte CM significantly increased Pan02 proliferation by 34 ± 12% over control (p < 0.01). Further experiments were conducted using F442A preadipocytes. Preadipocyte CM also significantly enhanced the proliferation of a second murine pancreatic cancer cell line, TGP-47, by 39 ± 5% over control medium (p < 0.01). Thus, preadipocyte CM significantly enhanced proliferation of two discrete murine pancreatic cancer cell lines (Fig. 1).
Fig. 1.
Murine pancreatic cancer cells, Pan02 and TGP-47 were incubated with conditioned medium (CM) from F442A preadipocytes. Proliferation was measured using the MTT assay. Values represent mean ± SEM for 8 separate experiments in Pan02 cells and 7 separate experiments in TGP-47 cells. *p < 0.01 vs. control medium.
3.2. HGF knockdown medium attenuates adipocyte-mediated pancreatic cancer proliferation
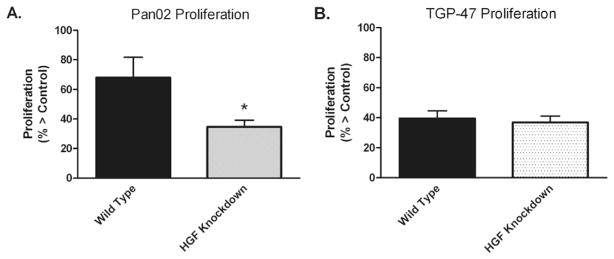
Hepatocyte growth factor (HGF) is produced by preadipocytes and is known to be important in pancreatic cancer growth [22,23]. Using shRNA techniques, F442A HGF protein expression was knocked down by 99%. As shown in Fig. 2A, Pan02 proliferation was significantly decreased when grown in the presence of HGF knockdown medium relative to growth in wild-type conditioned medium (35 ± 5% vs. 68 ± 14% growth > control; p < 0.05). In contrast, no significant difference was seen in TGP-47 proliferation when grown in HGF knockdown versus wild-type CM (37 ± 4% vs. 40 ± 5% growth > control; p = NS) (Fig. 2B).
Fig. 2.
Murine pancreatic cancer cells Pan02 (A) and TGP-47 (B) were incubated with wild type preadipocyte conditioned medium (CM) and HGF knockdown CM. Values represent mean ± SEM for 6 separate experiments with Pan02 cells and 7 separate experiments with TGP-47.*p < 0.05 vs. wild-type.
3.3. Effect of recombinant HGF on pancreatic cancer cell proliferation
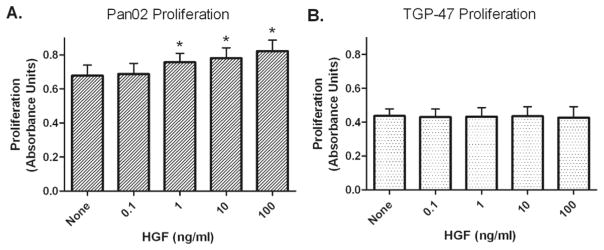
Treatment of Pan02 cells with exogenous recombinant HGF resulted in a dose-dependent increase in proliferation that was significantly greater than control at doses of 1, 10, and 100 ng/ml (p < 0.01; Fig. 3A). Exogenous HGF administration did not change TGP-47 proliferation at the doses tested (Fig. 3B).
Fig. 3.
Murine pancreatic cancer cells Pan02 (A) and TGP-47 (B) were incubated with increasing concentrations of exogenous HGF. Values represent mean ± SEM of 5 separate experiments with Pan02 and 4 separate experiments with TGP-47 cells. *p < 0.01 vs. “none” (control).
3.4. Pharmacologic inhibition of HGF receptor c-met
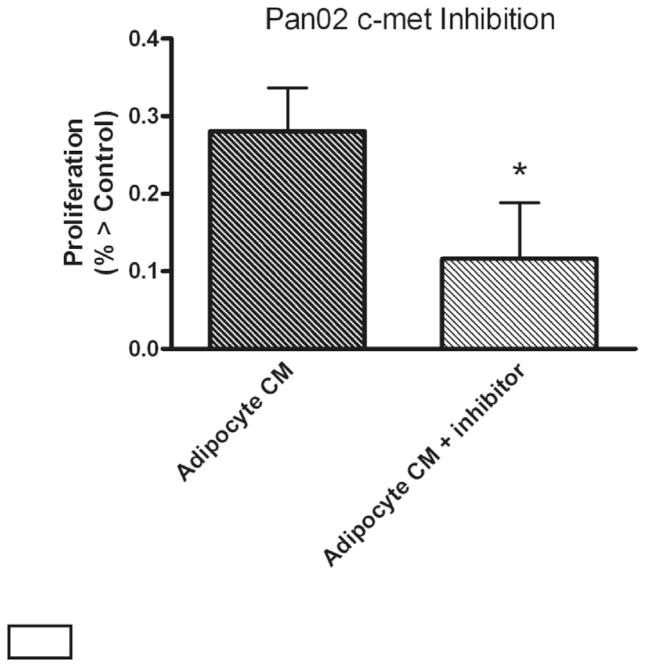
The tyrosine kinase inhibitor PF02341066 specifically blocks the HGF receptor, c-met. C-met inhibition significantly decreased Pan02 proliferation (0.81 ± 0.06 vs. 0.71 ± 0.07 absorbance units; p < 0.05; Fig. 4). Incubation with inhibitor alone did not affect proliferation of Pan02 cells (data not shown). TGP-47 cell proliferation was not affected by inhibition of the HGF receptor, c-met (p = NS).
Fig. 4.
Proliferative effects of preadipocyte conditioned medium (CM) on Pan02 cells are reduced in the presence of the c-met inhibitor, PF-02341066. Values represent mean ± SEM for 4 separate experiments. *p < 0.05 vs. Adipocyte CM.
3.5. Detection of c-met
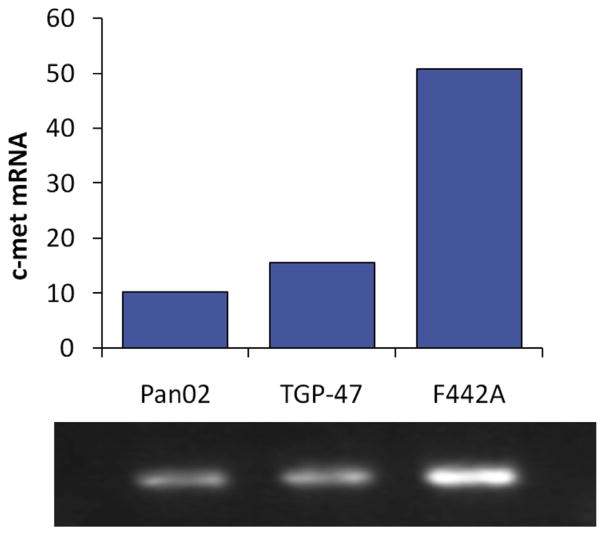
The only known receptor for HGF is the tyrosine kinase c-met. Using Real Time RT-PCR, mRNA for the c-met receptor was detected in both Pan02 and TGP-47 cells (CT = 23.5 and 22.1, respectively), as well as F442A cells (positive control); Fig. 5. Immunohistochemical staining demonstrated the presence of c-met receptors on the cell surface of both Pan02 and TGP-47 cells (data not shown).
Fig. 5.
rtPCR documents presence of receptor c-met on Pan02 and TGP47 cells (as well as F442A cells as positive control).
4. Discussion
Obesity significantly affects the growth of pancreatic cancer, as shown in epidemiologic, clinical [1,6,9], and in vivo biological studies [10]. The mechanisms underlying this association, however, remain unknown. The current experiments show that preadipocyte conditioned medium significantly increases proliferation of two distinct murine pancreatic cell lines, Pan02 and TGP-47. In Pan02 cells, this accelerated proliferation is reduced when hepatocyte growth factor has been attenuated, either by shRNA knockdown or HGF receptor inhibition. Exogenous HGF also dose-dependently accelerated the proliferation of the Pan02 pancreatic cancer cells. In contrast, exogenous HGF did not increase proliferation in TGP-47 cells; and in turn, TGP-47 proliferation was not affected by decreasing HGF (by shRNA knockdown and receptor inhibition). Taken in aggregate, these data suggest that preadipocyte-secreted HGF is responsible, in part, for obesity-related accelerated murine pancreatic cancer growth. We have previously documented the presence of HGF message as well as protein in F442A cells [15]. In addition, silencing of HGF resulted in 83% decrease in HGF production [15].
The fact that obesity exerts a negative influence on numerous malignancies, including pancreatic cancer, is now well-accepted [1,2]. A substantial body of epidemiologic data supports this concept; in addition, several group have reported that obesity increases perioperative complications in patients undergoing pancreatectomy for cancer [5,7,8]. Moreover, fat in the pancreas has been associated with an increased incidence of pancreatic fistula after pancreaticoduodenectomy [24].
Obesity also appears to impact survival of patients with pancreatic cancer. Fleming et al. found that relative to lean patients, obese patients with resected pancreatic cancer were significantly more likely to develop lymphatic metastases, with decreased disease free and overall survival [6]. In addition to generalized adiposity, local pancreatic fat content may play a role in this process. In patients matched for BMI (as well as tumor size and grade), those with a greater volume of pancreatic fat had more lymphatic metastasis and poorer overall survival [9]. A striking observation in our murine model of pancreatic cancer in obesity was that tumors developing in obese mice had a greater volume of intratumoral fat than tumors growing in lean animals [10]. Preliminary observations have confirmed the presence of adipocytes in human pancreatic cancers (unpublished data). Obesity may therefore influence pancreatic cancer development and growth at several levels, including systemically, locally, and at the level of the tumor microenvironment.
Contemporary understanding of adipocyte biology has shed light on potential mechanisms by which obesity influences cancer growth. Once thought to be a storage depot for triglycerides, adipocytes are now recognized as active metabolic cells, producing nearly 100 proteins that affect metabolism, inflammation, and cell growth [11]. A major finding in the current study is that co-culture with preadipocyte conditioned medium significantly accelerated the proliferation of two discrete murine pancreatic cancer cell lines. Similar observations have been reported in other cancer cell systems [25,26]; to our knowledge, this is the first such report involving pancreatic cancer. These data provide strong support for the concept that adipocyte-derived factors directly influence the proliferation of pancreatic cancer.
One likely component in the interaction between adipocytes and cancer cells is HGF. First isolated from hepatocytes, HGF is produced in significant quantity by preadipocytes and adipocytes [18]. Adipocyte-derived HGF has mitogenic and angiogenic properties, and HGF promotes the growth of several cancers, including pancreatic cancer [19–23]. Exogenous HGF within physiologic range increased the proliferation of Pan02 pancreatic cancer cells, and knockdown of adipocyte-derived HGF and specific blockade of the only known HGF receptor, c-met, both significantly attenuated adipocyte-induced excess Pan02 proliferation. The presence of c-met has been documented in pancreatic cancer cells, and understanding of it’s role in pancreatic carcinogenesis is evolving [22,23]. While possible that another adipocyte-derived protein may be responsible for this excess proliferation, this constellation of findings strongly suggests that accelerated Pan02 proliferation is mediated, in part, by adipocyte-derived HGF.
On the other hand, neither exogenous HGF administration nor preadipocyte HGF knockdown affected TGP-47 proliferation. One possible explanation for this observation is that TGP-47 cells do not express the HGF receptor, c-met. Another possible explanation is that HGF does not directly affect TGP-47 proliferation but acts through a discrete mechanistic pathway such as apoptosis arrest. In the larger picture, differences of HGF effect on Pan02 and TGP-47 reflect the innate variability inherent in biological systems. For example, while HGF is likely responsible for a portion of the excess adipocyte-induced proliferation of Pan02 cells, other adipocyte-derived factors clearly contribute as well. Similarly, the current experiments were designed as a logical extension of our murine in vivo pancreatic cancer studies, and thus investigated murine preadipocytes and pancreatic cancer cells.
In vitro cell culture work has inherent limitations, including differential proliferative response to immortalization. Continuation of the current work using human adipocytes and pancreatic cancer cells is clearly necessary. Fresh cultured human tumors in murine models are also attractive models. Furthermore, cellular proliferation represents just one piece of the carcinogenesis puzzle [27]. The influence of adipocyte-derived factors (including HGF) on pancreatic cancer migration and invasion remains to be defined.
5. Conclusions
These experiments clearly demonstrate that adipocyte-derived factors accelerate murine pancreatic cancer proliferation. In the case of Pan02 cells, adipocyte-induced excess proliferation is mediated, in part, by a HGF mechanism. Further elucidation of the pathways by which adipocytes influence pancreatic cancer growth may identify novel targets for directed therapy [28].
HIGHLIGHTS.
Adipocyte conditioned media accelerated pancreatic cancer cell proliferation.
Knockdown of adipocyte hepatocyte growth factor (HGF) attenuated pancreatic cell proliferation.
Recombinant HGF dose-dependently increased proliferation of Pan02 pancreatic cancer cells.
Inhibition of HGF receptor (c-met) attenuated proliferation of Pan02 pancreatic cancer cells.
Acknowledgments
Sources of funding
none.
Footnotes
Conflicts of interest
none.
Ethical approval
Indiana University Animal Care and Use Committee.
Author contribution
Ziegler – design, data collection, analysis, writing.
Considine – design, data collection, analysis, writing.
True – design, data collection, writing.
Swartz-Basile – design, analysis, writing.
Pitt – design, analysis, writing.
Zyromski – design, analysis, writing.
Guarantor
Nicholas J. Zyromski, MD.
References
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Stolzenberg-Solomon RZ, Adams K, Leitzmann M, Schairer C, Michaud DS, Hollenbeck A, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol. 2008;167(5):586–597. doi: 10.1093/aje/kwm361. [DOI] [PubMed] [Google Scholar]
- 3.Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89(3):519–523. doi: 10.1038/sj.bjc.6601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.House MG, Fong Y, Arnaoutakis DJ, Sharma R, Winston CB, Protic M, et al. Preoperative predictors for complications after pancreaticoduodenectomy: impact of BMI and body fat distribution. J Gastrointest Surg. 2008;12(2):270–278. doi: 10.1007/s11605-007-0421-7. [DOI] [PubMed] [Google Scholar]
- 6.Fleming JB, Gonzalez RJ, Petzel MQ, Lin E, Morris JS, Gomez H, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Archives Surg. 2009;144(3):216–221. doi: 10.1001/archsurg.2008.580. [DOI] [PubMed] [Google Scholar]
- 7.Benns M, Woodall C, Scoggins C, McMasters K, Martin R. The impact of obesity on outcomes following pancreatectomy for malignancy. Ann Surg Oncol. 2009;16(9):2565–2569. doi: 10.1245/s10434-009-0573-7. [DOI] [PubMed] [Google Scholar]
- 8.Williams TK, Rosato EL, Kennedy EP, Chojnacki KA, Andrel J, Hyslop T, et al. Impact of obesity on perioperative morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2009;208(2):210–217. doi: 10.1016/j.jamcollsurg.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Mathur A, Zyromski NJ, Pitt HA, Al-Azzawi H, Walker JJ, Saxena R, et al. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J Am Coll Surg. 2009;208(5):989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146(2):258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metabol Clin N Am. 2008;37(3):753–768. doi: 10.1016/j.ecl.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 13.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 14.Fain JN, Buehrer B, Bahouth SW, Tichansky DS, Madan AK. Comparison of messenger RNA distribution for 60 proteins in fat cells vs the nonfat cells of human omental adipose tissue. Metabol Clin Exp. 2008;57(7):1005–1015. doi: 10.1016/j.metabol.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metabol. 2008;294(2):E336–E44. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69(12):5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, et al. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97(4):381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 18.Bell LN, Ward JL, Degawa-Yamauchi M, Bovenkerk JE, Jones R, Cacucci BM, et al. Adipose tissue production of hepatocyte growth factor contributes to elevated serum HGF in obesity. Am J Physiol Endocrinol Metabol. 2006;291(4):E843–E8. doi: 10.1152/ajpendo.00174.2006. [DOI] [PubMed] [Google Scholar]
- 19.Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, et al. The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol. 2007;14(9):2600–2607. doi: 10.1245/s10434-007-9435-3. [DOI] [PubMed] [Google Scholar]
- 20.Qian LW, Mizumoto K, Maehara N, Ohuchida K, Inadome N, Saimura M, et al. Co-cultivation of pancreatic cancer cells with orthotopic tumor-derived fibroblasts: fibroblasts stimulate tumor cell invasion via HGF secretion whereas cancer cells exert a minor regulative effect on fibroblasts HGF production. Cancer Lett. 2003;190(1):105–112. doi: 10.1016/s0304-3835(02)00517-7. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita A, Gotze T, Korc M. Hepatocyte growth factor-mediated cell invasion in pancreatic cancer cells is dependent on neuropilin-1. Cancer Res. 2007;67(21):10309–10316. doi: 10.1158/0008-5472.CAN-07-3256. [DOI] [PubMed] [Google Scholar]
- 22.Bauer TW, Somcio RJ, Fan F, Liu W, Johnson M, Lesslie DP, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5(7):1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima Y, Ide T, Ohtsuka T, Miyazaki K. Induction of hepatocyte growth factor activator gene expression under hypoxia activates the hepatocyte growth factor/c-Met system via hypoxia inducible factor-1 in pancreatic cancer. Cancer Sci. 2008;99(7):1341–1347. doi: 10.1111/j.1349-7006.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur A, Pitt HA, Marine M, Saxena R, Schmidt CM, Howard TJ, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246(6):1058–1064. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- 25.Manabe Y, Toda S, Miyazaki K, Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol. 2003;201(2):221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 26.Somasundar P, Frankenberry KA, Skinner H, Vedula G, McFadden DW, Riggs D, et al. Prostate cancer cell proliferation is influenced by leptin. J Surg Res. 2004;118(1):71–82. doi: 10.1016/j.jss.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preziosi G, Oben JA, Fusai G. Obesity and pancreatic cancer. Surg Oncol. 2014;23(2):61–71. doi: 10.1016/j.suronc.2014.02.003. [DOI] [PubMed] [Google Scholar]