Abstract
Periods of caloric deficit substantially attenuate many centrally mediated responses to acute stress, including neural drive to the hypothalamic-pituitary-adrenal (HPA) axis, anxiety-like behavior, and stress-induced suppression of food intake (i.e., stress hypophagia). It is posited that this stress response plasticity supports food foraging and promotes intake during periods of negative energy balance, even in the face of other internal or external threats, thereby increasing the likelihood that energy stores are repleted. The mechanisms by which caloric deficit alters central stress responses, however, remain unclear. The caudal brainstem contains two distinct populations of stress-recruited neurons [i.e., noradrenergic neurons of the A2 cell group that co-express prolactin-releasing peptide (PrRP+ A2 neurons), and glucagon-like peptide 1 (GLP-1) neurons] that also are responsive to interoceptive feedback about feeding and metabolic status. A2/PrRP and GLP-1 neurons have been implicated anatomically and functionally in the central control of the HPA axis, anxiety-like behavior, and stress hypophagia. The current review summarizes a growing body of evidence that caloric deficits attenuate physiological and behavioral responses to acute stress as a consequence of reduced recruitment of PrRP+ A2 and hindbrain GLP-1 neurons, accompanied by reduced signaling to their brainstem, hypothalamic, and limbic forebrain targets.
Despite marked variation in environmental conditions, the internal milieu of mammals is maintained in a state of dynamic equilibrium known as homeostasis. Homeostatic regulation of critical physiological processes such as body temperature, blood pressure, fluid balance, and blood glucose levels is necessary for survival and well-being. However, this physiological balance is frequently challenged by internal and external forces, referred to as stressors, which can be as severe as an attack by a predator or as mild as a slight drop in blood pressure when moving from a seated to a standing position (Sapolsky 2004). In response to acute (i.e., occasional, short-duration) stressors, the central nervous system (CNS) elicits a constellation of neural, neuroendocrine, and behavioral responses that facilitate immediate survival and eventual restoration of homeostatic balance. These responses include activation of the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system, modulation of emotional state to redirect cognitive and attentional resources, and suppression of competing behaviors such as eating and sexual reproduction. These and other responses to acute stressors are complex and multifaceted, and provide a critical means by which to adapt widespread and diverse organismic functions to the frequent homeostatic challenges and threats that pervade daily life. However, stressful challenges also can be chronic and unremitting [e.g., social stress; (Scott et al. 2012; Tamashiro et al. 2007b; Tamashiro et al. 2007a; Tamashiro et al. 2011)], eliciting persistent changes in physiology and behavior that can become maladaptive and compromise health and well-being (Ulrich-Lai et al. 2015). Centrally-mediated stress responses of prolonged duration and/or magnitude have been linked to numerous neuropsychiatric, neurological, and physiological diseases and disorders, emphasizing the need to better understand how the brain coordinates responses to both acute and chronic stress exposure (Chrousos 2009).
Importantly, centrally-mediated responses to acute and chronic stressors are malleable, and can be modified and reorganized based on current physical state and/or prior experience (Bhatnagar and Dallman 1998; Ma and Morilak 2005; Zhang et al. 2010; Myers et al. 2016). As a case in point, caloric deficit (i.e., periods of food restriction or deprivation) substantially attenuates many centrally-mediated responses to acute stress, including stress-induced activation of the neuroendocrine HPA axis and stress-induced suppression of food intake (a.k.a. stress-induced hypophagia). Caloric deficit appears to suppress these stress responses in a coordinated manner, suggesting a process through which their underlying neural substrates are “metabolically tuned” by interoceptive feedback signals reflecting energy balance. These alterations in stress responsiveness can be viewed as adaptive and beneficial during periods of negative energy balance (Bhatnagar and Dallman 1998; Maniscalco et al. 2015); however, the neural mechanisms by which caloric deprivation attenuates stress responsiveness remain unclear. We posit that the apparent “metabolic tuning” of stress responses occurs via modulation of neural activity within a common central circuit node comprising two phenotypically distinct neural populations within the caudal nucleus of the solitary tract (cNTS): glucagon-like peptide 1 (GLP-1) neurons, and prolactin releasing peptide (PrRP)-positive noradrenergic (NA) neurons of the caudal A2 cell group (PrRP+ A2 neurons). As detailed further, below, both neural populations are activated by a wide variety of acute stressors, and both receive robust direct and relayed synaptic input from visceral sensory systems that convey ingestive/metabolic feedback signals from body to brain. Moreover, both neural populations project to multiple stress-related regions of the brainstem, hypothalamus, and limbic forebrain, and both populations contribute to neuroendocrine, emotional, and behavioral stress responses. Considering this, we propose that the ability of caloric deficit to attenuate physiological and behavioral responsiveness to acute stress depends on reduced signaling from hindbrain GLP-1 and PrRP+ A2 neurons. This article will summarize the results of several experiments that we have conducted to test this hypothesis. First, however, we will review the general features of some key neuroendocrine and behavioral stress responses, and the modulation of these responses during states of negative energy balance.
Neuroendocrine stress responses
Hypothalamic-pituitary-adrenal axis
The HPA axis is a metabolic neuroendocrine system that functions continuously to meet hour-by-hour energetic demands of organisms with preditable daily rhythms of behavioral state (i.e., arousal, somnolence, feeding, etc.). However, the typical circadian ebb and flow of HPA axis activity can be “co-opted” by marked activation in response to real or perceived threats, which facilitates meeting the energetic demands of catabolic stress responses. The apex of the HPA axis comprises neurons within the medial parvocellular paraventricular nucleus of the hypothalamus (mpPVN) that synthesize corticotropin-releasing hormone (CRH). Activation of these neurons causes CRH to be released into the hypophyseal portal system, a small system of blood vessels that carry CRH into the anterior lobe of the pituitary gland, where it binds to CRH receptors on corticotropes to induce release of adrenocorticotropic hormone (ACTH) into the systemic circulation. Circulating ACTH then binds to ACTH receptors in the adrenal cortex to increase synthesis of the steroid hormone corticosterone (CORT) (Chrousos 1998). In conjunction with the stress-induced increase in sympathetic outflow, CORT facilitates muscular and hepatic glycogenolysis to mobilize stored energy, and stimulates gluconeogenesis to maintain/elevate circulating glucose. Glucose availability is critical to fuel energy-demanding skeletal muscle contractions occurring as part of the “fight or flight” response to stress. Concurrently, CORT and sympathetic outflow function to suppress energy utilization by physiological maintenance processes such as immune system function, growth, and digestion, instead allocating resources to processes adaptive for immediate survival (Sapolsky 2004). Thus, the recruitment of the HPA axis is necessary for producing a massive physiological shift from a state in which priority is given to processes that are adaptive in the long term (i.e, days to weeks) to a state in which priority is given to functions with more immediate value (i.e., seconds to hours). Activation of the HPA axis is regulated by a complex network of central circuits, all of which converge on hypophysiotropic mpPVN neurons (Ulrich-Lai and Herman 2009), and major changes in the neuroendocrine response can be elicited by influencing individual or multiple components of these circuits.
HPA axis modulation during caloric deficit
In rodents, chronic caloric restriction, complete fasting for 2–4 days, or even a single overnight fast are each sufficient to attenuate central drive to the HPA axis, as evidenced by decreased mpPVN CRH mRNA expression (Brady et al. 1990; Kiss et al. 1994), decreased baseline mpPVN cFos activation (Dallman et al. 1999), and decreased plasma ACTH concentrations at baseline and in response to restraint stress (Akana et al. 1994; Hanson et al. 1994; Chacon et al. 2005). We also have confirmed that a “stressful” systemic dose of cholecystokinin [CCK; 10 microgram/kg body weight (BW)] increases plasma ACTH levels in ad-lib fed rats, but not in rats fasted overnight prior to CCK treatment (Figure 1A). Since central CRH signaling has potent anorexigenic effects (Rothwell 1990) and contributes to hypophagic responses to stress exposure (Krahn et al. 1986), downregulation of central CRH signaling in fasted rats may attenuate not only ACTH secretion, but also centrally-mediated stress hypophagia, thereby limiting the ability of stress to suppress food intake during periods of negative energy balance (discussed further, below).
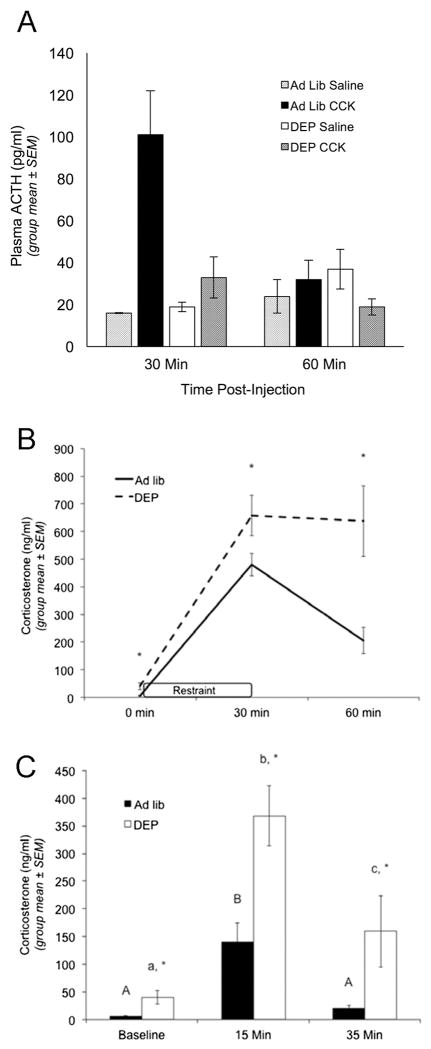
Figure 1. Overnight fasting uncouples plasma adrenocorticotropic hormone from plasma corticosterone.
Fasting decreases plasma ACTH levels in response to CCK (an acute visceral stressor), but increases plasma corticosterone levels at baseline and in response to acute cognitive stressors. (A) Plasma ACTH levels in ad lib-fed and overnight fasted (DEP) rats. Rats were killed 30 or 60 minutes after i.p. injection of saline or 10 microgram/kg CCK (n = 3–5/group at each time point), and trunk blood collected for ACTH radioimmunoassay. ANOVA confirmed that CCK increased plasma ACTH levels at the 30 min time point in ad lib fed rats, but not in DEP rats (asterisk, p < 0.05). We thank Dr. James Herman (University of Cincinnati) for conducting the ACTH assay. (B) CORT levels in ad lib-fed (solid line, n = 6) and fasted rats (DEP; dashed line, n = 5) prior to restraint stress (0 min), at the end of restraint stress (30 min), and 30 minutes after return to the home cage (60 min). Plasma CORT levels were determined through tail vein sampling followed by CORT enzyme immunoassay. ANOVA indicated that ad lib-fed rats displayed a significant increase in plasma CORT after 30 min of restraint stress, which fell significantly by the 60 min recovery time point. Compared to ad lib-fed rats, fasted rats displayed significantly higher baseline (0 min) CORT levels, and a significantly larger increase in CORT after 30 min of restraint stress. CORT levels remained significantly more elevated at the 60 min recovery time point in fasted vs. ad lib-fed rats. Asterisks at each time point indicate significant differences (p < 0.05) between fed and fasted rats. (C) CORT levels in ad lib-fed rats (solid bars) or fasted rats (DEP; open bars) at baseline (nonhandled; fed n = 6, DEP n = 5), 15 min after the start of a 5-min elevated platform exposure (fed n = 6, DEP n = 5), and 30 min after return to the home cage following elevated platform exposure (fed n = 4, DEP n = 4). ANOVA indicated that ad lib-fed rats sacrificed at the 15 min time point displayed significantly higher plasma CORT levels compared to baseline, whereas ad lib fed rats sacrificed at the 35 min time point had CORT levels no different than baseline. Plasma CORT levels were significantly elevated – as compared to baseline – in fasted rats sacrificed at the 15 min time point, and remained significantly elevated in fasted rats sacrificed at the 35 min time point. Plasma corticosterone levels were significantly higher in fasted vs. fed rats at baseline (nonhandled), 15 min, and 35 min (asterisks, p < 0.05). Within the same feeding status group (i.e., ad lib or DEP), bars with different letters are significantly different (p < 0.05).
Paradoxically, a state of caloric deficit increases plasma CORT levels at baseline and in response to restraint stress, despite suppression of ACTH release (Akana et al. 1994; Chacon et al. 2005), evidence that fasting “uncouples” ACTH release from adrenocortical CORT synthesis. Recent work from our lab further demonstrates that overnight fasting not only increases baseline and peak CORT response to restraint and elevated platform exposure, but also significantly delays CORT “recovery” to baseline levels following stressor termination (Figure 1B&C). There are numerous mechanisms by which fasting could augment stress-induced plasma CORT levels in the face of attenuated pituitary ACTH output (Akana et al. 1994), including factors that can increase adrenocortical sensitivity to ACTH (Bornstein et al. 2008) and thereby potentiate stress-induced CORT synthesis. For example, stressors increase sympathetic drive to the adrenal gland, which can subsequently increase adrenocortical steroidogenic response to ACTH (Engeland and Gann 1989; Edwards and Jones 1993). Thus, it is possible that fasting enhances stress-induced sympathetic drive to the adrenal gland, resulting in elevated circulating CORT levels despite reduced ACTH output. However, while adrenal sympathectomy reduces both circadian and dehydration stress-induced increases in plasma CORT levels (Ulrich-Lai and Engeland 2002; Ulrich-Lai et al. 2006), it does not appear to affect the ability of restraint stress to elevate plasma CORT levels in ad lib-fed rats (Strausbaugh et al. 1999). Nevertheless, it remains possible that adrenal sympathetic innervation is recruited selectively during fasting. Alternatively, adrenal sensitivity to ACTH and adrenal steroidogenic response to stressors may each remain unchanged during overnight fasting, despite increases in circulating CORT. As CORT synthesis occurs within minutes of stressor onset (Sapolsky et al. 2000) and fasting reduces plasma CORT degradation and clearance in rats (Herbst et al. 1960; Woodward et al. 1991; Kiss et al. 1994), it is possible that reduced CORT catabolism could explain higher peak CORT levels as well as reduced post-stress CORT recovery under fasting conditions.
Regardless of the mechanism(s) by which acute caloric deficits enhance circulating CORT levels after stress, there are multiple potential benefits of uncoupling the adrenal CORT response from the ACTH response. First, given that CORT signaling stimulates glycogenolysis, energy substrate mobilization, and gluconeogenesis, increased circulating CORT concentrations during fasting may facilitate acute energy mobilization to support short-term glucose-dependent processes, despite overall negative energy balance (Dallman et al. 1999). Secondly, it is possible that increased CORT levels contribute to the stimulation of feeding in fasted rodents. Substantial evidence indicates that increases in glucocorticoid levels are associated with increased food intake even in the face of normally hypophagic stressful challenges, and that administration of glucocorticoids can elicit increased caloric intake (Maniam and Morris 2012), particularly in the presence of low insulin levels (Dallman et al. 1993). Considering that insulin levels quickly fall at the outset of an overnight fast in rats, and remain significantly decreased through the following morning (Dallman et al. 1999), elevated glucocorticoid signaling may contribute to the adaptive drive for food intake during caloric deficit. Finally, increased CORT levels during fasting may have direct or indirect negative feedback effects to suppress central stress responses that produce consequences that are “maladaptive” in the fasted state, including both anxiety-like behavior and stress-induced hypophagia.
Emotional and behavioral stress responses
In addition to neuroendocrine stress responses, threatening and stressful stimuli frequently alter emotional state and elicit a constellation of adaptive behaviors. In rats and other rodent species, responses to acute stress are generally characterized by a shift from a state of behavioral exploration to a state of behavioral inhibition, which is adaptive within the context of maintaining energy reserves and minimizing exposure to threat. For example, stress suppresses exploratory behavior, serving to decrease the likelihood of predation. Acute stress also promotes hypophagia (i.e., reduced food intake), thereby preserving attentional focus and energy utilization for functions with more immediate survival value.
Behavioral inhibition and anxiety
In rodents, anxiety-like behavior (characterized by behavioral inhibition, avoidance behavior, autonomic and somatic hyperreflexia, and hyper-vigilance) depends on the output of widely distributed neural circuits (Walker et al. 2003). An accumulated literature has implicated the bed nucleus of the stria terminalis (BST) and the central nucleus of the amygdala (CeA), collectively referred to as the “central extended amygdala,” in the coordination of these responses. The CeA and BST receive neural input from the cortex, thalamus, hippocampus, caudal brainstem, and other amygdalar subnuclei (e.g., the basolateral amygdala) that process stimuli associated with stress and fear (Bienkowski and Rinaman 2013). In turn, the BST and CeA convey these integrated signals to widespread target areas that mediate individual components of the anxiety state, such as behavioral arrest, increased vigilance, and augmented startle responses (Dong et al. 2001; Walker et al. 2003; Liang et al. 1992). This connectivity optimally positions the BST and CeA to integrate diverse information regarding stressful/anxiogenic stimuli and produce complex behavioral responses to both internal and external threats.
Behavioral and pharmacological studies substantiate the role of the central extended amygdala in anxiety-like behavior, as lesions or pharmacological inactivation of the CeA or BST produce anxiolytic effects, as measured by behavioral avoidance or freezing/startle responses (Sullivan et al. 2004; Waddell et al. 2006; Sajdyk et al. 2008; Ventura-Silva et al. 2013). Work by Davis and colleagues suggests that the CeA is critical for eliciting short-lasting, stimulus-specific responses to conditioned threat cues (i.e., fear responses), while neurons of the BST are critical for eliciting sustained behavioral responses to innate, unconditioned threatening stimuli, such as those to open spaces, bright light, and predator odor (i.e., anxiety responses) (Lee and Davis 1997; Walker and Davis 1997; Fendt et al. 2003; Walker et al. 2003). Thus, by modulating neural activity in the BST, it is possible to modify the behavioral responses associated with a sustained state of anxiety.
Caloric deficits suppress anxiety-like behavior
Interestingly, chronic food restriction for a period of days to weeks in rodents consistently decreases behavioral measures of anxiety (Heiderstadt et al. 2000; Genn et al. 2003; Levay et al. 2007). One study reported that the anxiolytic effect of caloric deficit is evident within a single day of restricted food access (Inoue et al. 2004), suggesting that the neurophysiological changes mediating the behavioral shift occur rapidly. We recently confirmed that overnight food deprivation reduces anxiety-like behavior in adult male rats, as assessed in the elevated plus maze (i.e., increased open arm time), and in the light-enhanced acoustic startle test (i.e., reduced startle amplitude during both dark and light testing conditions; (Maniscalco et al. 2015). We believe these anxiolytic properties of caloric deficit reflect an adaptive shift in behavioral stress response that increase the likelihood of exploratory and foraging behavior in dangerous environments in order to replenish calorie stores.
Suppression of food intake
Hypophagia can be broadly considered as a decrease in food intake over time, and factors that change intake may do so by altering meal size, meal frequency, or both (Smith 1998; Smith 2000). Hypophagia is a well-documented response to a wide range of acute stressors (Uehara et al. 1989; Rybkin et al. 1997; Liu et al. 2007; Calvez et al. 2011; Maniam and Morris 2012), and is consistent with the overall inhibition of digestive processes that results from stress-induced activation of sympathetic outflow. By inhibiting digestive processes, such as food intake, salivation, pancreatic enzyme secretion, and gastric motility, energy normally utilized in these processes can be redistributed to functions with more immediate survival value during organismic “fight or flight” responses. Despite its clear importance as a component of these stress responses, the neural mechanisms through which stress inhibits food intake are not entirely clear.
A diverse array of acute stressors elicit hypophagia by reducing meal size, including restraint, forced swimming, or systemic administration of CCK or lithium chloride (LiCl) (Dess and Vanderweele 1994; Calvez et al. 2011; Rinaman 1999a; Gibbs et al. 1973). As satiation – the process that determines meal size – is a brainstem-mediated phenomenon (Grill and Norgren 1978; Seeley et al. 1994; Grill and Kaplan 2002), it is likely that stress-induced hypophagia is linked to altered neural activity within brainstem circuits responsible for satiation. After food is consumed, the hormonal and mechanical indices of nutrient and caloric presence in the gut are transmitted to the cNTS, predominantly via direct inputs from vagal afferent sensory neurons (Rinaman 2007). In the cNTS, glutamatergic vagal afferent signaling produces tightly synced, large-amplitude excitatory postsynaptic currents, providing high-fidelity transmission of sensory nerve activity (Appleyard et al. 2007). Activation of cNTS neurons then restricts meal size via regulation of pre-oral motor neurons within the brainstem parvocellular and intermediate reticular formation (Nasse et al. 2008). These reticular neurons maintain efferent synaptic connections with motor neurons innervating muscles of the tongue (Travers and Rinaman 2002; Travers et al. 2005) and other orofacial targets to directly control the motor patterns leading to ingestion or rejection of oral contents (Chen et al. 2001), thus determining meal size. In essence, this network of brainstem neurons produces a complex reflex arc by which ingestion of a meal provides feedback to the motor neurons responsible for the process of food intake. Although neural processing within the brainstem is sufficient for satiation (Grill and Smith 1988), direct descending projections from the cortex, limbic forebrain, and hypothalamus to the cNTS provide a route through which emotional and cognitive events can modulate initiation or avoidance of feeding and other responses to diverse threats, including conditioned responses that are based on past experience (Li et al. 1996; Li and Sawchenko 1998; Woods and Ramsay 2000; Dayas and Day 2001; Taché et al. 2001; Buller et al. 2003; Dayas et al. 2004; Blevins and Baskin 2010; Grill and Hayes 2012). Considered together, it appears likely that stressors elicit hypophagia by altering neural signaling within brainstem satiation circuits.
Caloric deficits blunt stress-induced hypophagia
Chronic caloric restriction blocks the ability of restraint stress to suppress food intake in rats (Youngblood et al. 1997), whereas restraint stress is robustly hypophagic in ad libitum (ad lib)-fed rats (Krahn et al. 1986). Caloric deficit also is sufficient to decrease hypophagic responses to an acute inflammatory event (Lennie et al. 1995) or to systemic administration of CCK (McMinn et al. 2000), indicating that caloric deficit reduces the ability of both cognitive and visceral stressors to suppress food intake. This evidence is consistent with a shift in the hypophagic stress response during periods of food deprivation or fasting, which may function to prioritize caloric ingestion to maintain body energy stores even under conditions of acute stress.
The Role of the cNTS in Central Stress Integration
The collective results of studies localizing cFos expression indicate that GLP-1 and PrRP+ A2 neurons are consistently activated by stimuli that present actual or anticipated threats to bodily homeostasis. As reviewed below, both populations of hindbrain neurons are optimally positioned to integrate information regarding metabolic state into neurally-mediated stress responses, and anatomical/functional evidence clearly demonstrates the capability of these neurons to activate the HPA axis, elicit anxiety-like behavior, and promote hypophagia. Before presenting evidence that signaling from these neural populations is reduced during caloric deficit, we will first review the functional organization of these neural systems, and present evidence supporting their integral role in mediating neuroendocrine and behavioral responses to cognitive and visceral stress.
The cNTS is a key component of the dorsal vagal complex (DVC), which also includes the area postrema (AP) and dorsal motor nucleus of the vagus. The DVC is a critical central node for relaying interoceptive visceral, hormonal, and somatic feedback from body to brain, and regulating glucose homeostasis and other aspects of energy balance (Zagon et al. 1999; Berthoud et al. 2006; Rinaman 2007; Grill and Hayes 2009; Rinaman 2010; Zhang et al. 2010; Rinaman 2011; Grill and Hayes 2012). The AP and a significant portion of the subjacent cNTS contain fenestrated capillaries, and AP neurons innervate the cNTS (Shapiro and Miselis 1985; Kachidian and Pickel 1993; Cunningham-Jr. et al. 1994), allowing blood-borne factors to affect neurons in this region (Yamamoto et al. 2003). Sensitivity to circulating factors, in addition to robust direct and relayed sensory inputs from spinal and cranial nerves, positions the cNTS to integrate multimodal input regarding metabolic state and other features of the internal environment.
Anatomy of PrRP+ A2 and GLP-1 Neurons
The A2 cell group comprises NA neurons located entirely within the cNTS, predominantly within the medial and commissural NTS subnuclei. The A2 cell group extends from the upper cervical spinal cord to just rostral to the AP at the level of the caudal fourth ventricle (Rinaman 2011). These neurons can be identified by immunolabeling for tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis, as well as dopamine-β-hydroxylase (DβH), the enzyme responsible for conversion of dopamine to norepinephrine (NE). A majority of A2 neurons co-localize PrRP (Roland et al. 1999; Maruyama et al. 2001), a neuropeptide originally named for its stimulatory effect on prolactin release from anterior pituicytes in vitro (Hinuma et al. 1998). More recent studies, however, indicate that PrRP+ A2 neurons do not have access to prolactin cells of the anterior pituitary (Morales et al. 2000) and PrRP signaling does not alter prolactin release in vivo (Taylor and Samson 2001).
GLP-1 neurons reside within both the cNTS and subjacent reticular formation (Larsen et al. 1997a; Rinaman 1999a, 2003b), extending from the upper cervical spinal cord to the caudal level of the AP (Vrang and Larsen 2010). Despite the largely overlapping hindbrain distribution of A2 neurons and GLP-1 neurons, the latter are a completely distinct population of non-adrenergic, glutamatergic neurons (Zheng et al. 2015) that expresses mRNA for preproglucagon (PPG), the protein precursor of GLP-1 (Figure 2). Within the brain, PPG mRNA expression is limited to the olfactory bulb, the cNTS, and the caudal medullary reticular formation (Larsen et al. 1997a; Merchenthaler et al. 1999). Since PPG-expressing neurons within the olfactory bulb are interneurons with very short axons, GLP-1 fibers and terminals throughout the rest of the CNS can be assumed to originate from PPG-expressing hindbrain neurons.
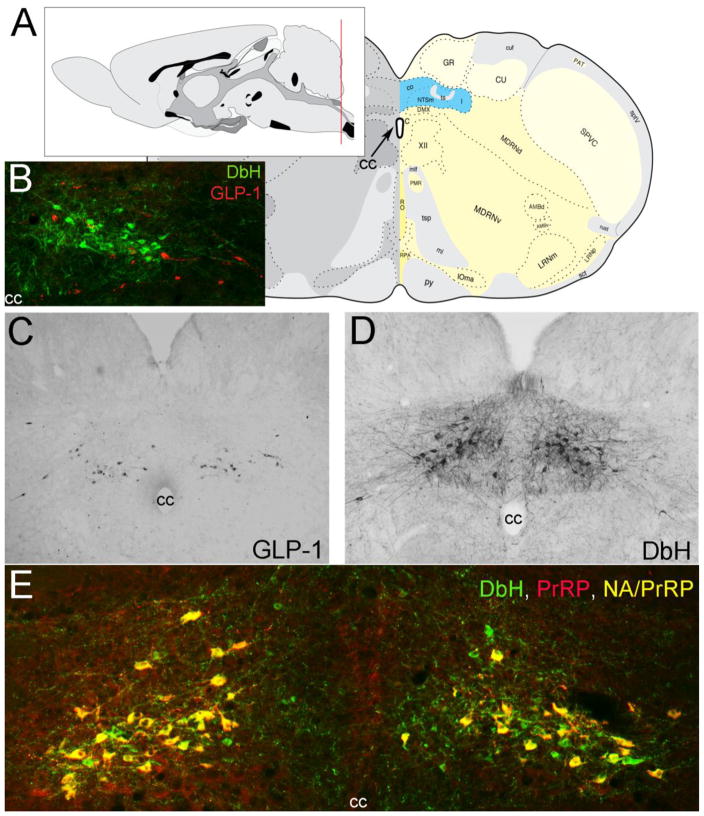
Figure 2.
Location of PrRP and GLP-1 neurons in the rat hindbrain. A, Schematics illustrating the location of the cNST (highlighted in blue), adapted from (Swanson 2004). The red line in the mid-sagittal brain schematic at upper left illustrates the rostrocaudal level of all coronal sections depicted in Figure 1 images. B, In this image, dopamine beta hydroxylase (DbH) immunopositive NA neurons are green, while GLP-1-immunopositive neurons are red. The two intermingled populations are distinct, with no colocalization of immunolabeling. C, GLP-1 immunoperoxidase-labeled neurons. D, DbH immunoperoxidase-positive NA neurons of the A2 cell group. E, In this image, all PrRP-positive neurons are double-labeled for DbH, rendering them yellow/orange (NA/PrRP neurons). Some intermingled NA neurons (green) are PrRP-negative. cc, central canal. [Figure as originally published in (Maniscalco et al. 2013)].
Due to its prominent location within the central stress circuitry (Figure 3), interoceptive modulation of cNTS neural activity is poised to influence centrally-mediated stress responses. As reviewed above, caloric deficit leads to a widespread shift in the stress response, including: 1) decreased anxiety-like behavior, 2) inhibition of stress hypophagia, 3) reduced hypothalamic outflow to the HPA axis, and 4) an uncoupling of CORT response from ACTH output. Together, these alterations represent an adaptive reorganization of stress responses during caloric deficit, promoting foraging and food intake during periods of negative energy balance, while concurrently facilitating the maintenance of glucose-dependent processes (Dallman et al. 1999; Genn et al. 2003). Although the neural mechanisms by which this shift occurs remain unclear, GLP-1 and PrRP+ A2 neurons within the cNTS appear to play major roles in HPA axis activation, anxiety-like behavior, and hypophagia in response to a wide array of acute stressors. The neural circuits by which these stress responses are elicited, however, can vary considerably based on the stimulus attributes of the stressor (Herman and Cullinan 1997), which has led to the categorization of stressors into visceral and cognitive categories. Neurons within the cNTS appear to play an important role in the central organization of physiological and behavioral responses to both categories of stress.
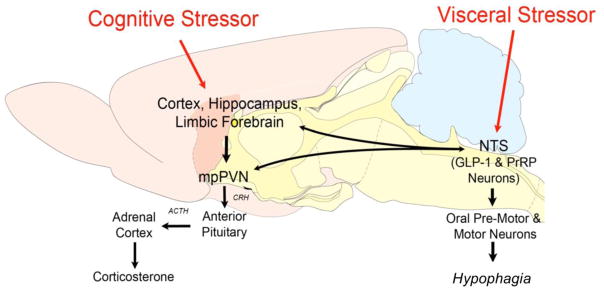
Figure 3.
Schematic representation of selected stress-sensitive neural circuits and their putative relationships with behavioral and neuroendocrine stress responses. cNTS, caudal nucleus of the solitary tract; GLP-1, glucagon-like peptide-1; PrRP+ A2, prolactin-releasing peptide-positive noradrenergic A2 neurons; mpPVN, medial parvocellular paraventricular nucleus of the hypothalamus. Note: “limbic forebrain” includes the bed nucleus of the stria terminalis and the central nucleus of the amygdala.
Visceral stressors and the cNTS
Visceral (a.k.a. interoceptive or systemic) stressors represent a class of stimuli that have already disrupted homeostasis (e.g., infection, hemorrhage, hypoxia) and present an immediate threat to survival. Visceral stressors originate within the body and their signals are conveyed to the cNTS via ascending sensory input. Predominant sources of cNTS sensory input arise from spinal afferents, area postrema (AP) sensory neurons, and the vagus and glossopharyngeal cranial nerves, which ramify throughout a majority of peripheral structures (Rinaman 2007). These inputs are responsive to signals of visceral stress and their axons converge onto cNTS neurons that are positioned to 1) suppress food intake, via influence over brainstem reticular neurons (Grill et al. 2004), 2) activate the HPA axis, via direct innervation of the mpPVN (Rinaman 2010), and 3) elicit behavioral anxiety, via dense projections to the BST (Banihashemi and Rinaman 2006). Thus, adaptive neuroendocrine and behavioral anxiety responses to visceral stressors are contingent upon signaling between afferent sensory inputs, the cNTS, and the forebrain, while visceral stress-induced hypophagia can be mediated by cNTS projections to local brainstem circuits (Figure 3). Numerous studies report that cNTS NA, PrRP, and GLP-1 neurons are activated by a broad array of visceral stressors, including: hemorrhage (Morales and Sawchenko 2003; Uchida et al. 2010), supraphysiological systemic doses of CCK (Rinaman 1999b; Lawrence et al. 2002; Maniscalco and Rinaman 2013), lipopolysaccharide (LPS) (Mera et al. 2006), LiCl (Rinaman 1999a), and interleukin-1 (Li et al. 1996; Sawchenko et al. 2000).
Cognitive stressors and the cNTS
Cognitive (a.k.a. psychogenic or processive) stressors represent a class of stimuli that have not yet produced an actual physical challenge, but rather indicate imminent disruption of homeostasis based on instinctual or learned processes (e.g., the odor of a predator, isolation in a threatening environment, or the sound of a tone predicting an electrical shock). Complex and anticipatory in nature, responses to cognitive stressors require neural processing by regions responsible for integration of multiple sensory modalities (e.g., the cortex and thalamus), executive function (e.g. the medial prefrontal cortex), and memory (e.g. the hippocampus) before eliciting changes in neuroendocrine, autonomic, or behavioral output (Jankord and Herman 2008; Ulrich-Lai and Herman 2009), largely via relays in the medial and baslolateral amygdalar subnuclei (Figure 3) (Dunn and Whitener 1986; Cullinan et al. 1995; Dayas et al. 1999; LeDoux 2000; Dayas et al. 2001). Cognitive stressors also activate neurons within the cNTS (Maniscalco et al. 2015), in part through descending projections from the PVN (Dayas and Day 2001; Dayas et al. 2004). For example, PVN neurons that project to the cNTS are activated by acute restraint stress in rats (Banihashemi et al. 2011), and may include oxytocin-positive PVN neurons that project to the cNTS and suppress vagally-mediated gastric emptying (Holmes et al. 2013). We propose that descending projections from the PVN and limbic forebrain underlie the ability of cognitive stressors to suppress food intake (Figure 3). Notably, activated cNTS target neurons include PrRP+ A2 neurons, which express the immediate-early gene product cFos in rats after cognitive stressor such as restraint (Dayas et al. 2001; Maruyama et al. 2001; Banihashemi et al. 2011), footshock (Li et al. 1996; Morales and Sawchenko 2003), predator odor (Day et al. 2004), and conditioned fear cues (Zhu and Onaka 2003). Interestingly, neuroendocrine responses to cognitive stressors require recruitment of mpPVN-projecting cNTS neurons (Kinzig et al. 2003) and neurons of the cNTS directly innervate the central extended amygdala, positioning this nucleus to influence both visceral and cognitive stress responses (Figure 3). We recently reported that in addition to activating PrRP+ A2 neurons, cognitive stressors (i.e., restraint or exposure to an elevated platform) also robustly activate GLP-1 neurons in rats (Maniscalco et al., 2015), consistent with evidence that central GLP-1 receptor signaling is critical for neuroendocrine and behavioral responses to cognitive stressors (Kinzig et al. 2003).
cNTS modulation of the HPA axis
PrRP+ A2 neurons within the cNTS can directly influence the HPA axis (Plotsky 1987), as indicated by dense NA terminal arborizations within the mpPVN (Cunningham-Jr. and Sawchenko 1988; Morales et al. 2000; Rinaman 2010), asymmetric synapses between catecholaminergic terminals and CRH-positive neurons (Liposits et al. 1986), and the presence of postsynaptic receptors for NA [including 1 and adrenoceptors; (Plotsky et al. 1989)] and PrRP (i.e., GPR10) within the mpPVN (Day et al. 1999; Roland et al. 1999). Functional evidence bolsters these anatomical findings, as norepinephrine (NE) increases excitatory postsynaptic potential frequency in the mpPVN, in part by enhancing glutamate signaling (Daftary et al. 2000), and the presence of NE or PrRP drives mpPVN cellular activity, stimulates CRH synthesis and release, and elevates plasma ACTH and CORT levels (Cole and Sawchenko 2002; Mera et al. 2006; Itoi et al. 2004; Itoi et al. 1999; Plotsky 1987; Seal et al. 2002; Helmreich et al. 2001). Indeed, stimulation of A2 neurons – almost certainly including the PrRP+ subpopulation (Day et al. 1985) – or their ascending fiber tract (Plotsky 1987) is sufficient to drive mpPVN activity and CRH secretion, while selective lesions of A2 NA projections to the mpPVN result in a substantial loss of neuroendocrine response to visceral stressors (Schiltz and Sawchenko 2007; Bienkowski and Rinaman 2008). Furthermore, HPA axis activation is enhanced by co-administration of NE and PrRP (Maruyama et al. 2001), suggesting that these transmitters act in concert to increase hypothalamic outflow.
Hindbrain GLP-1 neurons are also poised to directly influence HPA axis activity, as these neurons densely innervate the mpPVN (Larsen et al. 1997a; Rinaman 1999a; Tauchi et al. 2008) – which expresses GLP-1 receptors (Merchenthaler et al. 1999) – and synapse directly onto CRH neurons (Sarkar et al. 2003). Indeed, central administration of GLP-1 elicits cFos activation in mpPVN CRH neurons, subsequently elevating plasma ACTH and CORT (Larsen et al. 1997b; Kinzig et al. 2003). The strong excitatory effect of GLP-1 on neural drive to the HPA axis has been also been demonstrated electrophysiologically, as in vitro recordings show that GLP-1 receptor activation depolarizes PVN neurons (Cork et al. 2015), and increases PVN neuronal spike frequency via facilitation of presynaptic glutamate release (Acuna-Goycolea and van den Pol 2004). Further, disruption of GLP-1 receptor expression within the PVN and other central regions reduces HPA axis responses to acute and chronic stress in mice (Ghosal et al. 2016). Zheng et al. (2015) recently demonstrated that GLP-1 soma (within the cNTS) express mRNA for vesicular glutamate transporter 2 (vGlut2), and their terminal arbors within the mpPVN co-localize vGLUT2 and GLP-1 immunolabeling. Thus, GLP-1 neurons are glutamatergic, and activation of these neurons may directly drive postsynaptic spiking via glutamate release. Together, this evidence strongly supports a role for PrRP+ A2 neurons and hindbrain GLP-1 neurons in generating neuroendocrine stress responses.
cNTS modulation of anxiety-like behavior
Signaling from hindbrain GLP-1 neurons and PrRP+ A2 neurons can elicit anxiety-like behavior via receptor activation within numerous midbrain and forebrain regions, including the BST and CeA, two highly interconnected nuclei responsible for the coordination of behavioral anxiety responses, as discussed above. The ventrolateral (vl)BST receives particularly dense input from medullary NA and PrRP neurons (Forray et al. 2000; Banihashemi and Rinaman 2006), providing an anatomical substrate by which cNTS neurons can elicit/influence behavioral anxiety. Functional evidence supports this, as immobilization stress results in substantial increases in extracellular NE within the vlBST, while pharmacological antagonism of NA receptors within this region reduces the anxiogenic properties of acute stress (Cecchi et al. 2002). Furthermore, selective lesions that remove NA innervation of the vlBST (almost certainly including input from PrRP+ A2 neurons) prevents the ability of systemic yohimbine, an α2 adrenergic receptor antagonist, from increasing anxiety-like behavior (Zheng and Rinaman 2013).
Both the CeA and vlBST receive axonal input from hindbrain GLP-1 neurons (Goke et al. 1995; Rinaman 2010), providing a route through which GLP-1 neuronal activation can influence anxiety-like behavior. Indeed, antagonism of central GLP-1 receptors reduces anxiety-like behavior, and GLP-1 injection directly into the CeA is sufficient to increase behavioral anxiety (Kinzig et al. 2003). Interestingly, disruption of GLP-1 receptor expression within the PVN (using a genetic approach in which receptor expression remained intact within the CeA and BST) is sufficient to increase the amount of time mice spend on the open (i.e., anxiogenic) arm of the elevated plus maze one day after stress exposure (Ghosal et al. 2016), evidence that GLP-1 signaling within the PVN also contributes to stress-induced modulation of anxiety-like behavior. Despite the presence of GLP-1 terminals within the anterior BST (Rinaman 2010), the specific contribution of BST GLP-1 signaling to anxiety-like behavior has not been determined. Preliminary results in our laboratory indicate that bilateral AAV-mediated knockdown of GLP-1 receptors within the anterior lateral BST is sufficient to attenuate anxiety-like behavior as assessed in the open field, elevated plus maze, acoustic startle, and novelty-suppressed feeding paradigms in rats (unpublished), but additional work is needed to confirm the specificity and magnitude of these effects.
cNTS modulation of stress-induced hypophagia
A2 NA/PrRP+ neurons are activated by stimuli that produce hypophagia, such as cognitive/visceral stressors and intake of a meal (Rinaman et al. 1998; Takayanagi et al. 2008). The strongest evidence that, once activated, these neurons play a causal role in stress-induced hypophagia comes from studies using a DβH-conjugated saporin toxin, which selectively lesions NA neurons. Selective lesions of A2 neurons (presumably including the PrRP+ subset) result in reduction of visceral stress-induced hypophagia after either systemic CCK or LiCl treatment, and the degree to which food intake is suppressed positively correlates with the degree of A2 neuron loss (Rinaman 2003a; Rinaman and Dzmura 2007). These studies, however, cannot differentiate the effects of functional loss of NE signaling from loss of PrRP signaling. Interestingly, DβH knockout mice – which lack NE – continue to display hypophagic responses to systemic CCK (Cannon and Palmiter 2003). In these rodents, NA neurons are not lesioned and maintain the ability to synthesize and release PrRP, suggesting that PrRP signaling may be sufficient for stress-induced hypophagia. In support of this, central administration of PrRP reduces food intake (Lawrence et al. 2000; Lawrence et al. 2002), while knockout of PrRP (Takayanagi et al. 2008) or its receptor (Gu et al. 2004) in mice results in hyperphagia. In addition to their hypothalamic and limbic forebrain projections, varicose axons of NA and PrRP neurons can be found in the brainstem reticular formation (Yano et al. 2001), providing an even more direct potential anatomical route by which these neurons may influence meal size.
Similar to PrRP+ A2 neurons, GLP-1 neurons also are activated by stimuli that inhibit feeding (Rinaman 1999b; Kreisler et al. 2014), and an abundance of pharmacological evidence supports the role of GLP-1 receptor signaling in stress-induced hypophagia. First, lateral ventricular or hypothalamic administration of GLP-1 suppresses food intake (Tang-Christensen et al. 1996; Turton et al. 1996; Rinaman 1999a; Schick et al. 2003). Interestingly, the hypophagic effect is recapitulated with 4th ventricular GLP-1 administration in both intact and chronic supracollicular decerebrate rats (Kinzig et al. 2002; Hayes et al. 2008), indicating that GLP-1 signaling within the brainstem is sufficient to suppress food intake. While beneficial, pharmacological manipulations utilizing administration of agonist have limited physiological relevance, and much stronger evidence comes from studies blocking endogenous function using selective antagonists. For example, rats increase food intake when GLP-1 receptor antagonists are administered into the 4th ventricle or directly into the cNTS (Hayes et al. 2009). Additionally, results from studies study utilizing viral-mediated knockdown of either PPG or GLP-1 receptor within the cNTS indicate that GLP-1 signaling is critical for both short- and long-term regulation of food intake (Barrera et al. 2011; Alhadeff et al. 2016). Together, these results indicate that GLP-1 signaling in the caudal brainstem (and potentially other central target sites) is necessary for normal feeding control, and also is sufficient to suppress intake.
The necessity of GLP-1 signaling for visceral stress-hypophagia has been demonstrated, as i.c.v. administration of GLP-1 receptor antagonist dose-dependently attenuates LiCl-induced hypophagia (Rinaman 1999a; Seeley et al. 2000), and 4th i.c.v. antagonist administration diminishes suppression of food intake following peripheral LPS (Grill et al. 2004). Varicose axons of GLP-1 neurons are present within the reticular formation and other brainstem sites known to control oromotor outflow (e.g., the pontine parabrachial nucleus) (Rinaman 2010; Llewellyn-Smith et al. 2011), indirect evidence that GLP-1 signaling may suppress food intake via modulation of pre-motor brainstem circuits. Together, these findings indicate that PrRP+ A2 neurons and GLP-1 neurons play a critical role in visceral stress-induced hypophagia, and also are well positioned to mediate hypophagic responses to cognitive stress.
Fasting-Induced suppression of cNTS neural responses to acute stress
The preceding sections of this article summarize evidence that GLP-1 and PrRP+ A2 neurons within the cNTS are not only activated by acute stress, including both “cognitive” and “visceral” stress, but that both neural populations play an important functional role in neuroendocrine, emotional, and behavioral (i.e., hypophagic) responses to stress. Furthermore, as summarized above, these stress responses are attenuated in food-deprived/calorically restricted rats. Thus, we set out to test the hypothesis that the ability of caloric deficit to attenuate acute stress responses is due to suppressed activation of GLP-1 and/or PrRP+ A2 neurons.
We first examined whether overnight fasting would attenuate the ability of visceral stress to activate cFos within NA A2 and hindbrain GLP-1 neurons. Since supraphysiological systemic doses of CCK suppress food intake (Gibbs et al. 1973; Gibbs and Smith 1977) and activate hypothalamic endocrine neurons (Parrott et al. 1991; Katsuura et al. 1992; Verbalis et al. 1991; Chen et al. 1993) via ascending vagal input to the cNTS (Monnikes et al. 1997; Smith et al. 1985; Smith et al. 1981; Moran et al. 1990; Raybould et al. 1985; Schwartz et al. 1994), this temporally-discrete “visceral” stressor was used to assess recruitment of the A2 & GLP-1 brainstem neurons. Our results demonstrated that, in ad lib-fed rats, CCK dose-dependently increased cFos activation within A2 neurons (Maniscalco and Rinaman 2013), consistent with previous reports assessing global NTS cFos activation (Monnikes et al. 1997; Zittel et al. 1999). In fasted rats, the ability of i.p. injection and CCK to elicit cFos activation was significantly reduced – but not eliminated – in A2 neurons (Maniscalco and Rinaman 2013). Thus, while A2 neurons are sensitive to feeding status, this neuronal population retains some sensitivity to high doses of CCK in rats after fasting. In contrast to the A2 population, CCK or i.p. saline injection by itself activated the large majority of GLP-1 neurons, indicating a unique sensitivity of GLP-1 neurons to the mild stress of handling and/or i.p. injection. Surprisingly, GLP-1 neural cFos expression after i.p. saline or CCK was nearly eliminated in rats after overnight fasting, evidence that the robust response to i.p. injection displayed by GLP-1 neurons is strongly modulated by interoceptive state (Maniscalco and Rinaman 2013). Overall, these results support our overarching hypothesis, demonstrating that caloric deficit reduces recruitment of stress responsive hindbrain neurons within the central stress circuit (Figure 3).
Considering these results, we next investigated whether overnight fasting also attenuates the ability of cognitive stress to activate cFos within hindbrain GLP-1 and A2 neurons, including the specific population of PrRP+ A2 neurons. Our results indicated that in ad lib-fed rats, the cognitive stress of either a 30-minute restraint or 5-minute elevated platform exposure robustly activated the majority of GLP-1 and PrRP+ A2 neurons (Maniscalco et al. 2015), consistent with previous reports on NTS A2 neurons (including the PrRP+ subpopulation) (Morales and Sawchenko 2003; Zhu and Onaka 2003), and providing novel evidence that cognitive stressors recruit hindbrain GLP-1 neurons. Remarkably, overnight fasting nearly eliminated activation of both GLP-1 and PrRP+ A2 neurons at baseline (i.e., untreated control with no handling or stressor) and in response to cognitive stress (Maniscalco et al. 2015), bolstering our previous report of the metabolic sensitivity of these populations following i.p. injection of saline or CCK.
Since hindbrain GLP-1 and PrRP+ A2 neurons project directly to the vlBST, a forebrain region critical for anxiety-like behavior, we proposed that fasting would also attenuate cFos activation within the vlBST in conjunction with reduced anxiety-like behavior. Indeed, we observed a stress-dependent increase in neural cFos activation within the vlBST following either restraint or elevated platform exposure. In both cases, overnight fasting significantly reduced this vlBST activation, consistent with results observed in the cNTS (Maniscalco et al. 2015). It is likely that reduced neural activation within this anxiogenic site is behaviorally relevant, as overnight fasting also decreased anxiety-like behavior as measured on the elevated plus maze, and also as assessed using acoustic startle and light-enhanced startle responses (Maniscalco et al. 2015). Together, these results provide clear support for our overarching hypothesis that caloric deficit attenuates centrally-mediated stress responses by suppressing stress-induced recruitment of GLP-1 and PrRP+ A2 neurons within the cNTS. The results, however, did not demonstrate a causal link between reduced cNTS neural activation and reduced stress responsiveness.
To better understand the causal role of GLP-1 signaling in stress hypophagia, we centrally administered Exendin-9 (Ex9; 100 g), a specific GLP-1 receptor antagonist, into the lateral ventricle of ad lib-fed rats in order to temporarily block endogenous central GLP-1 signaling. Ex9 by itself did not alter chow intake, but abolished the ability of restraint stress to suppress food intake (Maniscalco et al. 2015), providing the first evidence that central GLP-1 neural signaling pathways are critical for cognitive stress-induced hypophagia. Moreover, central administration of Ex9 attenuated the ability of restraint stress to activate cFos expression by vlBST neurons and PrRP+ A2 neurons, evidence that GLP-1 receptor signaling is necessary for the ability of cognitive stress to recruit these neural populations (Maniscalco et al. 2015). Since GLP-1 and PrRP+ A2 neurons in fasted rats appear to be insensitive to acute stress stimuli, we conclude that reduced central signaling from these cNTS neural populations contributes to fasting-related reductions in stress hypophagia, activation of the HPA axis, and anxiety-like behavior.
Conclusions
Periods of caloric deficit alter neural, neuroendocrine, and behavioral responses to stress, and it is believed that these alterations represent an adaptive shift in stress responses during periods of negative energy balance. While the precise signals and mechanisms underlying this “metabolic tuning” remain to be determined, accumulated evidence supports the hypothesis that metabolic modulation of stress-induced recruitment of GLP-1 and PrRP+ A2 neurons explains why negative energy balance decreases central drive to the HPA axis, promotes anxiolysis, and attenuates hypophagic responses to acute stress. To better understand this phenomenon and its underlying neural mechanisms, it will be critical to address how neurons of the cNTS are modulated by interoceptive state. Overnight fasting elicits a myriad of physiological changes that might directly or indirectly contribute to attenuation of stress-induced activation of GLP-1 and PrRP+ A2 neurons, including: reduced levels of gastric distention, reduced circulating levels of glucose and leptin, and increased circulating levels of ghrelin and CORT. This last metabolic change is of particular interest, as Herman and colleagues have reported that CORT produces potentially relevant “negative-feedback” effects that impact gene expression within the cNTS. In particular, their results indicate that CORT signaling reduces PPG mRNA expression in the cNTS (recall that PPG is cleaved to generate GLP-1) (Zhang et al. 2009; Zhang et al. 2010), and that CORT acts within the cNTS to reduce anxiety-like behavior and HPA axis responses to cognitive stress (Ghosal et al., 2014). Together, these results support the view that CORT signaling can modulate the functionality and stimulus-induced activation GLP-1 neurons (Myers et al., 2016). However, it is not yet clear whether CORT negative-feedback signaling is sufficient to suppress baseline and stimulus-induced cFos activation of hindbrain GLP-1 and PrRP+ A2 neurons in rats after overnight food deprivation. We currently are exploring this potential mechanism – among others – through ongoing research.
Highlights.
Hindbrain neurons help control neuroendocrine and behavioral stress responses.
Brief periods of fasting profoundly suppress acute stress responsiveness.
Fasting suppresses central drive to the HPA axis, and reduces anxiety behavior.
Fasting suppresses stress-induced activation of hindbrain neurons.
The effect of fasting on stress responsiveness is likely due to suppression of hindbrain signaling.
Acknowledgments
Funding: The authors’ research reported in this manuscript was supported by NIH grants MH059911 and DK100685 (to L.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuna-Goycolea C, van den Pol A. Glucagon-like peptide 1 excites hypocretin/orexin neurons by direct and indirect mechanisms: implications for viscera-mediated arousal. J Neurosci. 2004;24(37):8141–8152. doi: 10.1523/JNEUROSCI.1607-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135(3):1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR. Endogenous Glucagon-like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard SM, Marks D, Kobayashi K, Okano H, Low MJ, Andresen MC. Visceral afferents directly activate catecholamine neurons in the solitary tract nucleus. The Journal of Neuroscience. 2007;27:13292–13302. doi: 10.1523/JNEUROSCI.3502-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, O’Neill EJ, Rinaman L. Central neural responses to restraint stress are altered in rats with an early life history of repeated brief maternal separation. Neuroscience. 2011;192:413–428. doi: 10.1016/j.neuroscience.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria teminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. The Journal of Neuroscience. 2006;26(44):11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Jones KR, Herman JP, D’Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci. 2011;31(10):3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–1102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain structure & function. 2013;218(1):187–208. doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Baskin DG. Hypothalamic-brainstem circuits controlling eating. Forum of nutrition. 2010;63:133–140. doi: 10.1159/000264401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein SR, Engeland WC, Ehrhart-Bornstein M, Herman JP. Dissociation of ACTH and glucocorticoids. Trends in endocrinology and metabolism: TEM. 2008;19(5):175–180. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52(5):441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- Buller KM, Dayas CV, Day TA. Descending pathways from the paraventricular nucleus contribute to the recruitment of brainstem nuclei following a systemic immune challenge. Neuroscience. 2003;118:189–203. doi: 10.1016/s0306-4522(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Calvez J, Fromentin G, Nadkarni N, Darcel N, Even P, Tome D, Ballet N, Chaumontet C. Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav. 2011;104(5):675–683. doi: 10.1016/j.physbeh.2011.07.012. S0031-9384(11)00364-7 [pii] [DOI] [PubMed] [Google Scholar]
- Cannon CM, Palmiter RD. Peptides that regulate food intake: norepinephrine is not required for reduction of feeding induced by cholecystokinin. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2003;284(6):R1384–R1388. doi: 10.1152/ajpregu.00689.2002. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Chacon F, Esquifino AI, Perello M, Cardinali DP, Spinedi E, Alvarez MP. 24-hour changes in ACTH, corticosterone, growth hormone, and leptin levels in young male rats subjected to calorie restriction. Chronobiol Int. 2005;22(2):253–265. doi: 10.1081/cbi-200053522. [DOI] [PubMed] [Google Scholar]
- Chen D-Y, Deutsch JA, Gonzalez MF, Gu Y. The induction and suppression of c-fos expression in the rat brain by cholecystokinin and its antagonist L364,718. Neurosci Lett. 1993;149:91–94. doi: 10.1016/0304-3940(93)90355-o. [DOI] [PubMed] [Google Scholar]
- Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2001;280:R1085–R1094. doi: 10.1152/ajpregu.2001.280.4.R1085. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stressors, stress, and neuroendocrine integration of the adaptive response. The 1997 Hans Selye Memorial Lecture. Ann N Y Acad Sci. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature reviews Endocrinology. 2009;5(7):374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. The Journal of Neuroscience. 2002;22(3):959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4(10):718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64(2):477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Miselis RR, Sawchenko PE. The relationship of efferent projections from the area postema to vagal motor and brain stem catecholamine-containing cell groups: an axonal transport and immunohistochemical study in the rat. Neuroscience. 1994;58(3):635–648. doi: 10.1016/0306-4522(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Daftary SS, Boudaba C, Tasker JG. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience. 2000;96(1):743–751. doi: 10.1016/s0306-4522(00)00003-8. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140(9):4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14(4):303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Day HEW, Campeau S, Watson SJJ, Akil H. Expression of a1b adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. The Journal of Neuroscience. 1999;19(22):10098–10106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HEW, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-Trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Day T, Ferguson AV, Renaud L. Noradrenergic afferents facilitate the activity of tuberoinfundibular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1985;41:17. doi: 10.1159/000124148. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–1152. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Hypothalamic paraventricular nucleus neurons regulate medullary catecholamine cell responses to restraint stress. The Journal of Comparative Neurology. 2004;478:22–34. doi: 10.1002/cne.20259. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Day TA. Opposing roles for medial and central amygdala in the initiation of noradrenergic cell responses to a psychological stressor. Eur J Neurosci. 2001;15:1712–1718. doi: 10.1046/j.1460-9568.2001.02011.x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Vanderweele DA. Lithium chloride and inescapable, unsignaled tail shock differentially affect meal patterns of rats. Physiol Behav. 1994;56(1):203–207. doi: 10.1016/0031-9384(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. The Journal of Comparative Neurology. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dunn JD, Whitener J. Plasma corticosterone responses to electrical stimulation of the amygdaloid complex: cytoarchitectural specificity. Neuroendocrinology. 1986;42(3):211–217. doi: 10.1159/000124442. [DOI] [PubMed] [Google Scholar]
- Edwards AV, Jones CT. Autonomic control of adrenal function. J Anat. 1993;183( Pt 2):291–307. [PMC free article] [PubMed] [Google Scholar]
- Engeland WC, Gann DS. Splanchnic nerve stimulation modulates steroid secretion in hypophysectomized dogs. Neuroendocrinology. 1989;50(2):124–131. doi: 10.1159/000125211. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23(1):23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Gysling K, Andres ME, Bustos G, Araneda S. Medullary noradrenergic neurons projecting to the bed nucleus of the stria terminalis express mRNA for the NMDA-NR1 receptor. Brain Res Bull. 2000;52(3):163–169. doi: 10.1016/s0361-9230(00)00229-x. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci SA, Thomas A, Edwards JE, File SE. Age-associated sex differences in response to food deprivation in two animal tests of anxiety. Neurosci Biobehav Rev. 2003;27(1–2):155–161. doi: 10.1016/s0149-7634(03)00017-4. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Packard AE, Mahbod P, McKlveen JM, Seeley RJ, Myers B, Ulrich-Lai Y, Smith EP, D’Alessio DA, Herman JP. Disruption of glucagon-like peptide 1 signaling in Sim1 neurons reduces physiological and behavioral reactivity to acute and chronic stress. J Neurosci. 2016 doi: 10.1523/JNEUROSCI.1104-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Smith GP. Cholecystokinin and satiety in rats and rhesus monkeys. Am J Clin Nutr. 1977;30(5):758–761. doi: 10.1093/ajcn/30.5.758. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7(11):2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Carmody JS, Sadacca LA, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brainstem but not forebrain GLP-1 receptors. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2004 doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33(Suppl 1):S11–15. doi: 10.1038/ijo.2009.10. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201(4352):267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol. 1988;253:R853–R856. doi: 10.1152/ajpregu.1988.254.6.R853. (Regulatory Integrative Comp. Physiol.) [DOI] [PubMed] [Google Scholar]
- Gu W, Geddes BJ, Zhang C, Foley KP, Stricker-Krongrad A. The prolactin-releasing peptide receptor (GPR10) regulates body weight homeostasis in mice. J Mol Neurosci. 2004;22(1–2):93–103. doi: 10.1385/JMN:22:1-2:93. JMN:22:1–2:93 [pii] [DOI] [PubMed] [Google Scholar]
- Hanson ES, Bradbury MJ, Akana SF, Scribner KS, Strack AM, Dallman MF. The diurnal rhythm in adrenocorticotropin responses to restraint in adrenalectomized rats is determined by caloric intake. Endocrinology. 1994;134(5):2214–2220. doi: 10.1210/endo.134.5.8156924. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150(6):2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149(8):4059–4068. doi: 10.1210/en.2007-1743. en.2007-1743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiderstadt KM, McLaughlin RM, Wright DC, Walker SE, Gomez-Sanchez CE. The effect of chronic food and water restriction on open-field behaviour and serum corticosterone levels in rats. Lab Anim. 2000;34(1):20–28. doi: 10.1258/002367700780578028. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Itoi K, Lopez-Figueroa MO, Akil H, Watson SJ. Norepinephrine-induced CRH and AVP gene transcription within the hypothalamus: differential regulation by corticosterone. Brain Res Mol Brain Res. 2001;88(1–2):62–73. doi: 10.1016/s0169-328x(01)00018-3. S0169328X01000183 [pii] [DOI] [PubMed] [Google Scholar]
- Herbst AL, Yates FE, Glenister DW, Urquhart J. Variations in hepatic inactivation of corticosterone with changes in food intake: an explanation of impaired corticosteroid metabolism following noxious stimuli. Endocrinology. 1960;67:222–238. doi: 10.1210/endo-67-2-222. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hinuma S, Habata Y, Fujii R, Kawamata Y, Hosoya M, Fukusumi S, Kitada C, Masuo Y, Asano T, Matsumoto H, Sekiguchi M, Kurokawa T, Nishimura O, Onda H, Fujino M. A prolactin-releasing peptide in the brain. Nature. 1998;393(6682):272–276. doi: 10.1038/30515. [DOI] [PubMed] [Google Scholar]
- Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol. 2013;591(12):3081–3100. doi: 10.1113/jphysiol.2013.253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Zorrilla EP, Tabarin A, Valdez GR, Iwasaki S, Kiriike N, Koob GF. Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol Psychiatry. 2004;55(11):1075–1081. doi: 10.1016/j.biopsych.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Itoi K, Helmreich DL, Lopez-Figueroa MO, Watson SJ. Differential regulation of corticotropin-releasing hormone and vasopressin gene transcription in the hypothalamus by norepinephrine. The Journal of Neuroscience. 1999;19(13):5464–5472. doi: 10.1523/JNEUROSCI.19-13-05464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Jiang YQ, Iwasaki Y, Watson SJ. Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004;16(4):348–355. doi: 10.1111/j.0953-8194.2004.01172.x. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachidian P, Pickel VM. Localization of tyrosine hydroxylase in neuronal targets and efferents of the area postrema in the nucleus tractus solitarii of the rat. The Journal Of Comparative Neurology. 1993;329(3):337–353. doi: 10.1002/cne.903290305. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Ibii N, Matsushita A. Activation of CCK-A receptors induces elevation of plasma corticosterone in rats. Peptides. 1992;13(1):203–205. doi: 10.1016/0196-9781(92)90163-w. 0196-9781(92)90163-W [pii] [DOI] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23(15):6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. The Journal of Neuroscience. 2002;22(23):10470–10475. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A, Jezova D, Aguilera G. Activity of the hypothalamic pituitary adrenal axis and sympathoadrenal system during food and water deprivation in the rat. Brain Res. 1994;663(1):84–92. doi: 10.1016/0006-8993(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Grace M, Levine AS. CRF antagonist partially reverses CRF- and stress-induced effects on feeding. Brain Res Bull. 1986;17(3):285–289. doi: 10.1016/0361-9230(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Kreisler AD, Davis EA, Rinaman L. Differential activation of chemically identified neurons in the caudal nucleus of the solitary tract in non-entrained rats after intake of satiating vs. non-satiating meals. Physiol Behav. 2014 doi: 10.1016/j.physbeh.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in rat hypothalamus and brainstem. Neuroscience. 1997a;77(1):257–270. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997b;138(10):4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Celsi F, Brennand J, Luckman SM. Alternative role for prolactin-releasing peptide in the regulation of food intake. Nature. 2000;3(7):645–646. doi: 10.1038/76597. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KLJ, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002;143(2):360–367. doi: 10.1210/endo.143.2.8609. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Reviews in Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. The Journal of Neuroscience. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie TA, McCarthy DO, Keesey RE. Body energy status and the metabolic response to acute inflammation. Am J Physiol. 1995;269(5 Pt 2):R1024–1031. doi: 10.1152/ajpregu.1995.269.5.R1024. [DOI] [PubMed] [Google Scholar]
- Levay EA, Govic A, Penman J, Paolini AG, Kent S. Effects of adult-onset calorie restriction on anxiety-like behavior in rats. Physiol Behav. 2007;92(5):889–896. doi: 10.1016/j.physbeh.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Li H-Y, Ericsson A, Sawchenko PE. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences, USA. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-Y, Sawchenko PE. Hypothalamic effector neurons and extended circuitries activated in “neurogenic” stress: A comparison of footshock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. The Journal of Comparative Neurology. 1998;393:244–266. [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJD, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. The Journal of Neuroscience. 1992;12(6):2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liposits Z, Sherman D, Phelix C, Paull WK. A combined light and electron microscopic immunocytochemical method for the simultaneous localization of multiple tissue antigens. Tyrosine hydroxylase immunoreactive innervation of corticotropin releasing factor synthesizing neurons in the paraventricular nucleus of the rat. Histochemistry. 1986;85(2):95–106. doi: 10.1007/BF00491754. [DOI] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology. 2007;148(11):5531–5540. doi: 10.1210/en.2007-0745. en.2007-0745 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 2011;180:111–121. doi: 10.1016/j.neuroscience.2011.02.023. S0306-4522(11)00164-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17(11):761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012;63(1):97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing Peptide or glucagon-like Peptide 1. Frontiers in neuroscience. 2013;6:199. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Rinaman L. Overnight food deprivation markedly attenuates hindbrain noradrenergic, glucagon-like peptide-1, and hypothalamic neural responses to exogenous cholecystokinin in male rats. Physiol Behav. 2013;121:35–42. doi: 10.1016/j.physbeh.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco JW, Zheng H, Gordon PJ, Rinaman L. Negative energy balance blocks neural and behavioral responses to acute stress by “silencing” central glucagon-like peptide 1 signaling in rats. J Neurosci. 2015;35(30):10701–10714. doi: 10.1523/JNEUROSCI.3464-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M, Matsumoto H, Fujiwara K, Noguchi J, Kitada C, Fujino M, Inoue K. Prolactin-releasing peptide as a novel stress mediator in the central nervous system. Endocrinology. 2001;142(5):2032–2038. doi: 10.1210/endo.142.5.8118. [DOI] [PubMed] [Google Scholar]
- McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141(12):4442–4448. doi: 10.1210/endo.141.12.7815. [DOI] [PubMed] [Google Scholar]
- Mera T, Fujihara H, Kawasaki M, Hashimoto H, Saito T, Shibata M, Saito J, Oka T, Tsuji S, Onaka T, Ueta Y. Prolactin-releasing peptide is a potent mediator of stress responses in the brain through the hypothalamic paraventricular nucleus. Neuroscience. 2006;141:1069–1086. doi: 10.1016/j.neuroscience.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770(1–2):277–288. doi: 10.1016/s0006-8993(97)00865-2. S0006-8993(97)00865-2 [pii] [DOI] [PubMed] [Google Scholar]
- Morales T, Hinuma S, Sawchenko PE. Prolactin-releasing peptide is expressed in afferents to the endocrine hypothalamus, but not in neurosecretory neurones. J Neuroendocrinol. 2000;12:131–140. doi: 10.1046/j.1365-2826.2000.00428.x. [DOI] [PubMed] [Google Scholar]
- Morales T, Sawchenko PE. Brainstem prolactin-releasing peptide neurons are sensitive to stress and lactation. Neuroscience. 2003;121(3):771–778. doi: 10.1016/s0306-4522(03)00522-0. S0306452203005220 [pii] [DOI] [PubMed] [Google Scholar]
- Moran TH, Norgren R, Crosby RJ, McHugh PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res. 1990;526(1):95–102. doi: 10.1016/0006-8993(90)90253-8. 0006-8993(90)90253-8 [pii] [DOI] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasse J, Terman D, Venugopal S, Hermann G, Rogers R, Travers JB. Local circuit input to the medullary reticular formation from the rostral nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295(5):R1391–1408. doi: 10.1152/ajpregu.90457.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott RF, Ebenezer IS, Baldwin BA, Forsling ML. Central and peripheral doses of cholecystokinin that inhibit feeding in pigs also stimulate vasopressin and cortisol release. Exp Physiol. 1991;76(4):525–531. doi: 10.1113/expphysiol.1991.sp003518. [DOI] [PubMed] [Google Scholar]
- Plotsky P. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–930. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10(4):437–458. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Gayton RJ, Dockray GJ. CNS effects of circulating CCK8: involvement of brainstem neurones responding to gastric distension. Brain Res. 1985;342:187–190. doi: 10.1016/0006-8993(85)91373-3. [DOI] [PubMed] [Google Scholar]
- Rinaman L. A functional role for central glucagon-like peptide-1 receptors in lithium chloride-induced anorexia. Am J Physiol. 1999a;277:R1537–R1540. doi: 10.1152/ajpregu.1999.277.5.R1537. (Reg. Int. Comp. Physiol. 46) [DOI] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999b;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. (Reg. Int. Comp. Physiol. 46) [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. The Journal of Neuroscience. 2003a;23(31):10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Postnatal development of hypothalamic inputs to the dorsal vagal complex in rats. Physiol Behav. 2003b;79:65–70. doi: 10.1016/s0031-9384(03)00105-7. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010;1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R222–235. doi: 10.1152/ajpregu.00556.2010. ajpregu.00556.2010 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Baker EA, Hoffman GE, Stricker EM, Verbalis JG. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. (Regulatory Integrative Comp. Physiol. 44) [DOI] [PubMed] [Google Scholar]
- Rinaman L, Dzmura V. Experimental dissociation of neural circuits underlying conditioned avoidance and hypophagic responses to lithium chloride. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2007;293:R1495–R1503. doi: 10.1152/ajpregu.00393.2007. [DOI] [PubMed] [Google Scholar]
- Roland BL, Sutton SW, Wilson SJ, Luo L, Pyati J, Huvar R, Erlander MG, Lovenberg TW. Anatomical distribution of prolactin-releasing peptide and its receptor suggests additional functions in the central nervous system and periphery. Endocrinology. 1999;140:5736–5745. doi: 10.1210/endo.140.12.7211. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Central effects of CRF on metabolism and energy balance. Neurosci Biobehav Rev. 1990;14(3):263–271. doi: 10.1016/s0149-7634(05)80037-5. [DOI] [PubMed] [Google Scholar]
- Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol. 1997;273(5 Pt 2):R1612–1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22(6):633–641. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. Why Zebras Don’t Get Ulcers. Henry Holt and Company, LLC; New York, NY: 2004. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fekete C, Legradi G, Lechan RM. Glucagon like peptide-1 (7–36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985:163–168. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li H-Y, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. In: Mayer EA, Saper CB, editors. Progress in Brain Research. Vol. 122. Elsevier Science BV; 2000. pp. 61–78. [DOI] [PubMed] [Google Scholar]
- Schick RR, Zimmermann JP, Walde Tv, Schusdziarra V. Glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regulatory Integrative Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. The Journal of Comparative Neurology. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- Schwartz GJ, McHugh PR, Moran TH. Pharmacological dissociation of responses to CCK and gastric loads in rat mechanosensitive vagal afferents. Am J Physiol. 1994;267:R303–R308. doi: 10.1152/ajpregu.1994.267.1.R303. (Reg. Int. Comp. Physiol. 36) [DOI] [PubMed] [Google Scholar]
- Scott KA, Melhorn SJ, Sakai RR. Effects of Chronic Social Stress on Obesity. Curr Obes Rep. 2012;1(1):16–25. doi: 10.1007/s13679-011-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal LJ, Small CJ, Dhillo WS, Kennedy AR, Ghatei MA, Bloom SR. Prolactin-releasing peptide releases corticotropin-releasing hormone and increases plasma adrenocorticotropin via the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2002;76(2):70–78. doi: 10.1159/000064427. 64427 [pii]. 64427. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D’Alessio D. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. The Journal of Neuroscience. 2000;20(4):1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci. 1994;108(2):347–352. [PubMed] [Google Scholar]
- Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. The Journal of Comparative Neurology. 1985;234:344–364. doi: 10.1002/cne.902340306. [DOI] [PubMed] [Google Scholar]
- Smith GP. Satiation: From Gut to Brain. Oxford University Press; New York: 1998. [Google Scholar]
- Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213(4511):1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249(5 Pt 2):R638–641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- Strausbaugh HJ, Dallman MF, Levine JD. Repeated, but not acute, stress suppresses inflammatory plasma extravasation. Proc Natl Acad Sci U S A. 1999;96(25):14629–14634. doi: 10.1073/pnas.96.25.14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swanson L. Structure of the Rat Brain. Elsevier; Amsterdam: 2004. Brain Maps III. [Google Scholar]
- Taché Y, Martinez V, Million M, Wang L. Stress and the Gastrointestinal Tract. III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. American Journal of Physiology Gastrointestinal and Liver Physiology. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Matsumoto H, Nakata M, Mera T, Fukusumi S, Hinuma S, Ueta Y, Yada T, Leng G, Onaka T. Endogenous prolactin-releasing peptide regulates food intake in rodents. J Clin Invest. 2008;118(12):4014–4024. doi: 10.1172/JCI34682. 34682 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007a;91(4):440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, Sakai RR. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007b;293(5):R1864–1874. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14(5):468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. (Reg. Int. Comp. Physiol. 40) [DOI] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008;36(3–4):144–149. doi: 10.1016/j.jchemneu.2008.07.009. S0891-0618(08)00097-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. The prolactin releasing peptides: RF-amide peptides. Cell Mol Life Sci. 2001;58(9):1206–1215. doi: 10.1007/PL00000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Rinaman L. Identification of lingual motor control circuits using two strains of pseudorabies virus. Neuroscience. 2002;115(4):1139–1151. doi: 10.1016/s0306-4522(02)00489-x. [DOI] [PubMed] [Google Scholar]
- Travers JB, Yoo JE, Chandran R, Herman K, Travers SP. Neurotransmitter phenotypes of intermediate zone reticular formation projections to the motor trigeminal and hypoglossal nuclei in the rat. J Comp Neurol. 2005;488(1):28–47. doi: 10.1002/cne.20604. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kobayashi D, Das G, Onaka T, Inoue K, Itoi K. Participation of the prolactin-releasing peptide-containing neurones in caudal medulla in conveying haemorrhagic stress-induced signals to the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2010;22:33–42. doi: 10.1111/j.1365-2826.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- Uehara A, Sekiya C, Takasugi Y, Namiki M, Arimura A. Anorexia induced by interleukin 1: involvement of corticotropin-releasing factor. Am J Physiol. 1989;257(3 Pt 2):R613–617. doi: 10.1152/ajpregu.1989.257.3.R613. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1128–1135. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76(2):79–92. doi: 10.1159/000064426. 64426. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Fulton S, Wilson M, Petrovich G, Rinaman L. Stress exposure, food intake and emotional state. Stress. 2015;18(4):381–399. doi: 10.3109/10253890.2015.1062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews - Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pego JM. Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci. 2013;7:32. doi: 10.3389/fnbeh.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbalis JG, Stricker EM, Robinson AG, Hoffman GE. Cholecystokinin activates cFos expression in hypothalamic oxytocin and corticotropin-releasing hormone neurons. J Neuroendocrinol. 1991;3:205–213. doi: 10.1111/j.1365-2826.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: role of peripherally secreted and centrally produced peptides. Prog Neurobiol. 2010;92(3):442–462. doi: 10.1016/j.pneurobio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120(2):324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110(1–2):175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Woodward CJ, Hervey GR, Oakey RE, Whitaker EM. The effects of fasting on plasma corticosterone kinetics in rats. Br J Nutr. 1991;66(1):117–127. doi: 10.1079/bjn19910015. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, Drucker DJ, Elmquist JK. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. The Journal of Neuroscience. 2003;23(7):2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Iijima N, Kataoka Y, Hinuma S, Tanaka M, Ibata Y. Developmental expression of prolactin releasing peptide in the rat brain: localization of messenger ribonucleic acid and immunoreactive neurons. Developmental Brain Research. 2001;128(2):101–111. doi: 10.1016/s0165-3806(01)00148-1. [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Ryan DH, Harris RB. Appetitive operant behavior and free-feeding in rats exposed to acute stress. Physiol Behav. 1997;62(4):827–830. doi: 10.1016/s0031-9384(97)00245-x. [DOI] [PubMed] [Google Scholar]
- Zagon A, Rocha I, Ishizuka K, Spyer KM. Vagal modulation of responses elicited by stimulation of the aortic depressor nerve in neurons of the rostral ventrolateral medulla oblongata in the rat. Neuroscience. 1999;92(3):889–899. doi: 10.1016/s0306-4522(99)00041-x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30(44):14907–14914. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proceedings of the National Academy of Sciences. 2009;106(14):5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Rinaman L. Yohimbine anxiogenesis in the elevated plus maze requires hindbrain noradrenergic neurons that target the anterior ventrolateral bed nucleus of the stria terminalis. Eur J Neurosci. 2013;37(8):1340–1349. doi: 10.1111/ejn.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain structure & function. 2015;220(5):3011–3022. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Onaka T. Facilitative role of prolactin-releasing peptide neurons in oxytocin cell activation after conditioned-fear stimuli. Neuroscience. 2003;118(4):1045–1053. doi: 10.1016/s0306-4522(03)00059-9. S0306452203000599 [pii] [DOI] [PubMed] [Google Scholar]
- Zittel TT, Glatzle J, Kreis ME, Starlinger M, Eichner M, Raybould HE, Becker HD, Jehle EC. C-fos protein expression in the nucleus of the solitary tract correlates with cholecystokinin dose injected and food intake in rats. Brain Res. 1999;846(1):1–11. doi: 10.1016/s0006-8993(99)01842-9. S0006-8993(99)01842-9 [pii] [DOI] [PubMed] [Google Scholar]